Metal oxynitrides as emerging materials with photocatalytic and electronic properties
Amparo
Fuertes
Institut de Ciència de Materials de Barcelona (ICMAB-CSIC). Campus UAB, 08193 Bellaterra, Spain. E-mail: amparo.fuertes@icmab.es; Fax: +34 93 5805729; Tel: +34 93 5801853, ext. 277
First published on 19th May 2015
Abstract
Oxynitrides of transition metals, alkaline earth metals and rare earth metals are intensively investigated as a group of materials to expand and tune the properties of oxides. The differences in polarizability, electronegativity and anion charge of nitrogen and oxygen induce changes in the physical and chemical properties of oxides by nitrogen introduction. The effects on properties arise from the higher covalency of the metal–nitrogen bond and the changes in the energies of electronic levels, and are important in slightly doped nitrogen metal oxides as in stoichiometric oxynitrides. More intense recent progress in oxynitride research has been made in some specific fields such as photocatalysis in water splitting and other processes as the observed small band gaps lead to activity in the visible light range. The stabilization of new perovskite oxynitrides, with the oxidation states of cations tuned by N/O stoichiometry, has led to new magnetic and dielectric materials. The lower electronegativity of nitrogen and larger crystal field splitting induced by N3− shifts the emission wavelengths of phosphors to the red, and oxynitridosilicates have been investigated as components of white LEDs.
1. Introduction
Research on metal oxynitrides has increased significantly in the last decade because of their emerging applications as photocatalysts, pigments, phosphors, dielectrics and magnetic materials.1–6 The introduction of nitrogen in an oxidic compound expands the possibilities of tuning properties with respect to the most used strategy of cation substitution. Nitrogen is less electronegative and more polarizable than oxygen and this increases the covalency of bonding. The interelectronic repulsion decreases and the nephelauxetic effect (expansion of the electron cloud) increases. The crystal field splitting is larger as a consequence of the higher charge of nitride. The −3 charge of the nitride anion may allow the formation of new compounds where cations show higher oxidation states than in oxides. These effects induce important changes in properties. For instance in an insulating oxide the band gap decreases with nitride introduction and the optical properties may change drastically. As a result of the band gap reduction the photocatalytic activity of semiconductors may be shifted from the UV to the visible light range. The emission wavelengths of rare earths are shifted to the red because of lowering of the energy of 5d orbitals, which is a consequence of the larger nephelauxetic effect and crystal field splitting (Fig. 1a). Additional effects such as symmetry distortions or anion order may further modify the luminescence properties. The Fermi level in a late transition metal nitride in the charge transfer regime7 is placed at higher energies than in the corresponding oxide (Fig. 1b). This decreases the operating voltage in lithium or sodium batteries making the transition metal (oxy)nitrides attractive as electrode materials.8,9 The higher covalency and larger polarization of bonds with metals affect the electronic properties leading to new magnetic, conducting and dielectric materials. This review focuses on recent developments in significant applications of metal oxynitrides as electronic and photocatalytic materials. These include nitrogen doped binary oxides (N-TiO2 and N-CeO2) and ternary oxynitrides (TaON) to complex quaternary and higher oxynitrides of transition metals, alkaline earth metals and rare earth metals (perovskites and oxynitridosilicates), showing a variety of optical, magneto/electric (dielectric, thermoelectric, ferromagnetic, spin glass or magnetoresistance) and photocatalytic (in water splitting and organic reactions) properties.2. Synthesis: reactions in NH3 and N2 at high temperature
The two general approaches for the synthesis of oxynitrides are the treatment at high temperatures of reactants in NH3 and N2. Ammonolysis reactions are used starting with precursor oxides or mixtures of binary oxides, nitrides and carbonates. Ammonia dissociates appreciably into N2 and H2 at temperatures higher than 500 °C (Fig. 2a). The kinetics of the dissociation is slow and its extent can be controlled by the flow rate. For instance, at 700 °C the % of decomposition varies from ca. 10% for a flow rate of 150 cm3 min−1 to ca. 45% for 50 cm3 min−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Fig. 2b). Under high flow rate conditions and at high temperature, NH3 is very reactive and highly nitriding. Typical flow rates are between 180 and 1000 cm3 min−1. The key parameters of temperature, flow rate, time and sample placement in the reaction tube among other factors determine the gas composition (NH3, N2 and H2) around the sample and should be considered to optimize the purity of the oxynitride phase.11 The reducing power of NH3 together with the presence of H2 makes more difficult the stabilization of ionocovalent oxynitrides of the metals more electronegative (e.g. late transition metals) which form interstitial nitrides with the cations in low oxidation states.12,13 On the other hand in ammonolysis reactions some cations may be oxidized under the same conditions. For instance the rare earth vanadium oxynitrides RVO3−xNx (R = La, Pr, Nd) are prepared in NH3 between 650 and 800 °C starting with the precursors RVO4 (monazite or zircon).14 The ammonolysis reaction proceeds through an initial reduction of RVO4 precursors to the perovskites RVO3 that are progressively nitrided and reoxidised to RVO3−xNx materials with varying proportions of V4+/V3+. A similar oxidation reaction takes place for rare earth molybdenum pyrochlores R2Mo2O7 which under ammonia lead topotactically to oxynitrides R2Mo2O7−xNx with molybdenum in oxidation states between +4 and +6.15
10 (Fig. 2b). Under high flow rate conditions and at high temperature, NH3 is very reactive and highly nitriding. Typical flow rates are between 180 and 1000 cm3 min−1. The key parameters of temperature, flow rate, time and sample placement in the reaction tube among other factors determine the gas composition (NH3, N2 and H2) around the sample and should be considered to optimize the purity of the oxynitride phase.11 The reducing power of NH3 together with the presence of H2 makes more difficult the stabilization of ionocovalent oxynitrides of the metals more electronegative (e.g. late transition metals) which form interstitial nitrides with the cations in low oxidation states.12,13 On the other hand in ammonolysis reactions some cations may be oxidized under the same conditions. For instance the rare earth vanadium oxynitrides RVO3−xNx (R = La, Pr, Nd) are prepared in NH3 between 650 and 800 °C starting with the precursors RVO4 (monazite or zircon).14 The ammonolysis reaction proceeds through an initial reduction of RVO4 precursors to the perovskites RVO3 that are progressively nitrided and reoxidised to RVO3−xNx materials with varying proportions of V4+/V3+. A similar oxidation reaction takes place for rare earth molybdenum pyrochlores R2Mo2O7 which under ammonia lead topotactically to oxynitrides R2Mo2O7−xNx with molybdenum in oxidation states between +4 and +6.15
 | ||
Fig. 2 (a) Equilibrium constant of dissociation of NH3 as a function of temperature using the equation26 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp = 2095/T − 2.7884 log Kp = 2095/T − 2.7884 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T + 0.0003986T + 2.6253 where Kp is the equilibrium constant of the formation of NH3, and (b) % of decomposition of NH3 as a function of flow rate at different temperatures (produced with data of ref. 10). T + 0.0003986T + 2.6253 where Kp is the equilibrium constant of the formation of NH3, and (b) % of decomposition of NH3 as a function of flow rate at different temperatures (produced with data of ref. 10). | ||
Because of the moderate temperatures during the synthesis of oxynitrides under NH3 the sintering of samples is difficult. Dense ceramics are needed for different applications and for the measurement of electrical properties. High pressure with and without sample heating has been used for producing densities above 85%.16 The homogeneity of the samples in terms of N stoichiometry (and then of the oxidation states of cations) is important and in most cases several treatments are needed to accomplish it.
More homogeneous and sintered samples are obtained in the synthesis under N2, which is performed at higher temperatures, typically between 1300 and 1500 °C. This requires a strict control of H2O and O2 impurities around the sample to avoid the formation of oxides. The use of carbonates as reactants is common but they constitute an additional source of oxygen. CO2 decomposes into CO and O2 at temperatures above ca. 1300 °C and also it may react with nitrides at lower temperatures oxidizing N3− to N2 and giving rise to oxides.17 Amides,18 imides,19 urea20 and azides21 have also been used as nitrogen sources.
High pressure has been used in few examples to stabilize quaternary oxynitrides containing small cations. The perovskites LnZrO2N can be prepared at room pressure only for Ln![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) La and are synthesized at 2–3 GPa and 1200–1500 °C for smaller rare earth metals such as Pr, Sm or Nd.22 More recently MnTaO2N with LiNbO3 type structure has been synthesized at 6 GPa and 1400 °C.23
La and are synthesized at 2–3 GPa and 1200–1500 °C for smaller rare earth metals such as Pr, Sm or Nd.22 More recently MnTaO2N with LiNbO3 type structure has been synthesized at 6 GPa and 1400 °C.23
3. N-doped TiO2 and CeO2
The introduction of nitrogen into TiO2 and CeO2 takes place with concomitant creation of anion vacancies as the charge compensation mechanism.24,25 The oxynitrides TiO2−x−yNx and CeO2−x−yNx with anatase and fluorite structures, respectively, are prepared under NH3 from the corresponding oxides. Under these conditions both systems behave similarly chemically. Using a constant reaction time of several hours the nitrogen content of the samples increases to a maximum of ca. 0.1 atoms per formula unit at a temperature between 550 and 700 °C. At higher temperatures the reduction of Ti4+ to Ti3+ or that of Ce4+ to Ce3+ competes with the formation of the oxynitride and the nitrogen content decreases. For powder samples of CeO2 the solid solution Ce1–yIVCeyIIIO2−3x/2−y/2Nx (x ≤ 0.09, y ≤ 0.06) is formed between 600 and 680 °C, with the cell parameter of the cubic fluorite structure increasing with N content.25 At temperatures higher than 800 °C the reduced oxides CeO1.78 and Ce7O12 are formed. In the ammonolysis of anatase TiN is produced at temperatures higher than 800 °C, and N doped rutile is also observed at high temperature.24N-doped TiO2 (anatase) has been extensively investigated since in 2001 Asahi et al. reported the visible light induced photooxidation of acetaldehyde (CH3CHO) to CO2 on this material.27 Nitrogen doping shifts the photocatalytic activity of TiO2 from UV to the visible range. DFT calculations show that there is no shift of both the top and bottom positions of the O 2p valence band, as well as of the conduction band, with respect to the undoped material. The red shift of the absorption edge is caused by N 2p localized states just above the top of the O 2p valence band.28 Visible light photocatalytic activity has also been reported in oxidation of other compounds (carbon monoxide, formaldehyde, acetic acid among many others) and for water splitting.29 Antibacterial, antiviral and antiallergen properties under visible or indoor lighting have also been reported.
N-doped CeO2 has shown photocatalytic activity under visible light in the decomposition of acetaldehyde and other organic compounds30–32 (Fig. 3). The shift of the band gap in powder samples has been estimated by diffuse reflectance spectroscopy from 2.96 eV for CeO2 to 2.65 eV for CeO2−x−yNx with x = 0.084.30 DFT calculations suggest that in contrast to N doped TiO2, the band edges in N-CeO2 are lowered with respect to CeO2 on both sides of the energy gap.31 Nitrogen introduction into CeO2 has also been reported to remarkably improve scavenging of free radical oxygen species in polymer electrolyte membranes of fuel cells.33
 | ||
| Fig. 3 Dependence of the CO2 evolution rate for the decomposition of acetaldehyde over a film of CeO2−x−yNx with x = 0.05 on the cut-off wavelength of the irradiated light. (Reproduced from ref. 30 with permission of The Royal Society of Chemistry.) | ||
4. TaON
The stable polymorph of tantalum oxynitride at room pressure is monoclinic, isostructural to baddeleyite ZrO2 (β-TaON). Tantalum is heptacoordinated and there are two crystallographically independent sites for anions where N and O order.34 As in many oxynitrides and other mixed anion compounds this order can be rationalised and predicted by using Pauling's second crystal rule.35,36 This oxynitride is an important visible active photocatalyst for water splitting.37–39 As Ta3N5, TaON is a semiconductor with a band gap of 2.4 eV allowing visible light absorption, and has band edge potentials suitable for H2 and O2 production (from the reduction and oxidation of H2O respectively). This requires that the minimum of the conduction band and the maximum of the valence band of the photocatalyst would be lower and higher than the oxidation–reduction potential of H+/H2 and O2/H2O respectively.6 If no co-catalyst is used the oxidation of water on TaON is efficient but the activity in the reduction is not high.37 Efficient reduction and oxidation of water has been achieved in two steps,40,41 and more recently in one step by using ZrO2 as a co-catalyst.42 Both Ta3N5 and β-TaON have also been reported as photocatalysts in oxidation of organic molecules.43The synthesis of β-TaON is usually done by ammonolysis of Ta2O5 at 800–850 °C, using either low flow rates of NH3 (typically 20–50 cm3 min−1) or some PH2O (bubbling NH3 in water) to prevent the formation of the nitride Ta3N5.44 Nanoparticles of β-TaON are prepared by a Ca-assisted urea route45 and efficient photoanodes have been prepared by electrophoretic deposition.46,47
γ-TaON is a metastable polymorph that can be prepared starting with Ta2O5 using lower ammonia flow rates than for TaON and shorter reaction times.48 It shows a crystal structure related to VO2(B)49 consisting of sharing edge octahedra of tantalum in layers that are connected through the Ta(O,N)6 octahedra corners. δ-TaON with anatase structure is another metastable polymorph that has been recently obtained in powder and thin film forms.50,51 As powder it forms by ammonolysis of amorphous citrate precursors at 760 °C, and transforms into the more stable β-TaON between 800 and 850 °C. A cotunnite polymorph of TaON was predicted at high pressure52 and it has been recently stabilized at 33 GPa at room temperature.53 The cotunnite phase shows a very high bulk modulus Ko ≈ 370 GPa. γ-TaON has been reported to show efficient visible-light photocatalytic activity in water splitting54 whereas δ-TaON films show high mobility of electron carriers and a band gap of 2.37 eV.51
5. Perovskite oxynitrides
Oxynitrides with a perovskite structure have been reported for alkaline earth or rare earth cations in the A site and the transition metals Ti, Zr, V, Nb, Ta, Mo, W and Fe in the B sites and nitrogen contents up to a maximum of 2.16 atoms per formula shown for A = La and B = W.2,3,55–64 TaThN3 is one of the few reported examples of fully nitrided perovskites65 whereas many antiperovskite nitrides (with one nitrogen per formula) have been reported.66 Ruddlesden–Popper oxynitrides are known for the series (SrO)(SrNbO2N)n (n = 1, 2),67 A2TaO3N (A = alkaline earth metal)68 and the rare earth aluminates R2AlO3N.69,70 The tolerance factors of existing perovskite oxynitrides range from 0.878 to 1.03871 as calculated from ionic radii72 (0.827 to 1.042 from observed interatomic distances), in contrast to those of oxides, from 0.74 to 1.00 (0.822 to 1.139 from bond valence parameters).71The synthesis of perovskite oxynitrides can be done under NH3 starting with binary metal oxides and carbonates or with oxide precursor phases. The use of precursors is needed for rare earth perovskites, as the rare earth oxides are poorly reactive at the usual synthesis temperatures of ammonolysis reactions (up to 1100 °C). The synthesis in N2 has been performed at temperatures between 1100 and 1500 °C starting with binary nitrides, oxynitrides, oxides or carbonates.17,73 The oxide and nitride anions order in many reported perovskite oxynitrides.73–77 In layered Sr2TaO3N and Sr2NbO3N with K2NiF4-type structures the nitrogen atoms occupy the equatorial sites of the octahedra,75,78 which show larger bond strength sums, in agreement with Pauling's second crystal rule.36 In pseudo cubic perovskites all the anion sites show the same bond strength sums and the anion order cannot be rationalized based on this rule. In the perovskites SrTaO2N, SrNbO2N and more recently in RVO2N (R = La, Pr, Nd), a local cis arrangement of nitrides has been suggested from electron diffraction and high resolution neutron diffraction studies.14,79,80 Long-range disorder of this arrangement results in 4 sites with N/O mixed occupancies close to 50/50. The existence of zig-zag N–M–N chains confined in planes that are prone to disorder has been suggested to explain the observed results (Fig. 4a). The local cis order has also been suggested for the cubic perovskite BaTaO2N from neutron pair distribution function analysis81 and has been found energetically favoured from DFT calculations.82–84 It can be explained in terms of maximum utilization of d orbitals in π bonding with the lone pairs of the anions (Fig. 4b).85 In temperature dependent neutron diffraction studies of ATaO2N and ATaON2 perovskites the cis order has been observed up to 1100 °C (the maximum temperature investigated).86 The combination of the four most common types of octahedral tilting and anion order (considering the cis and trans configurations of N in AMO2N or O in AMON2) results in 14 possible space groups.77,87
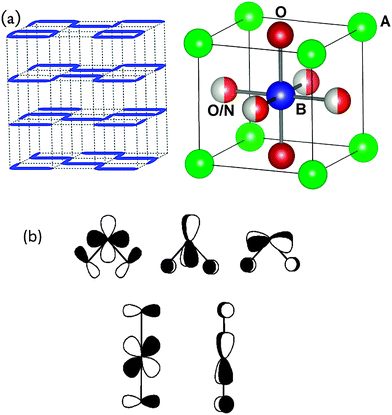 | ||
| Fig. 4 (a) N/O order in ABO2N perovskite oxynitrides showing N–M–N zig-zag chains (blue line) confined in planes.79 (b) π bonding of d orbitals with ON lone pairs showing three and two interactions in the cis and trans configurations respectively.85 | ||
5.1 Photocatalytic properties
Tantalum(V), niobium(V) and titanium(IV) perovskite oxynitrides have been reported as photocatalysts in water splitting. The band gaps and band edge positions of CaTaO2N, SrTaO2N, BaTaO2N, LaTaON2, LaTiO2N and BaNbO2N88–90 are suitable for both water oxidation and reduction under visible light. For efficient conversion of solar energy, band gaps smaller than 2 eV (equivalent to an absorption edge of λ > 600 nm) are convenient, and the band gaps of this group of compounds range from 1.8 eV (for LaTaO2N) to 2.5 eV (for CaTaO2N). Co-catalysts like transition metal oxides and sacrificial agents are used to enhance the photocatalytic activity as they provide active centres for the oxidation/reduction reactions and may improve charge carrier separation and lifetime. The tantalum perovskites evolve H2 from water (λ > 420 nm) but they are not active in O2 production and show efficiencies in water reduction up to 20% (for LaTaON2).91,92 Overall water splitting with visible light up to 600 nm has been recently reported for the double tantalum/magnesium perovskites with formula LaMgxTa1−xO1+3xN2−3x (x ≥ 1/3).93,94 Among the niobium perovskite oxynitrides ANbO2N (A = Ca, Sr, Ba) and LaNbON2, the compound CaNbO2N showed the highest activity for both H2 and O2 production (λ > 420 nm), SrNbO2N showed activity only for O2 evolution and the remaining perovskites were not active or showed poor activity.95,96 The lower activities with respect to the tantalum materials are likely due to the presence of reduced oxidation states of niobium. LaTiO2N has a band gap of 2.1 eV and is active for O2 and H2 production in the presence of sacrificial agents.97 The co-catalyst CoOx improves the efficiency of LaTiO2N to 27.1% at 440 nm which is the highest reported for oxynitrides with absorption edges near to 600 nm.985.2. Electrical and magnetic properties
Tantalum perovskite oxynitrides have been investigated as dielectric materials since relative permittivities at room temperature of 4900 and 2900 were reported for SrTaO2N and BaTaO2N, respectively, with a relaxor-type ferroelectric behaviour.99 As both compounds crystallize in centrosymmetric space groups the local Ta-O,N dipoles counterbalance by each other and different possibilities for the origin of the dielectric properties have been discussed. Local cis ordering of nitrides in the TaO4N279,81 octahedra together with polar cooperative disorder100 of N–M–N chains101 have been suggested. Classical ferroelectricity has been recently observed in compressively strained SrTaO2N epitaxial thin films grown on SrTiO3 substrates.102 DFT calculations under epitaxial strain of this material suggest that small domains of the trans N ordered ferroelectric phase are responsible for this behaviour, and microstructural analysis suggests the coexistence of ferroelectric (trans type) and relaxor ferroelectric (cis type) domains.103 Recently the new structurally related oxynitride MnTaO2N with a polar LiNbO3-type structure has been reported. (Fig. 5)23 It is insulating, and dielectric measurements have not been reported, but the calculated spontaneous polarization is similar to multiferroic Mn oxides with a LiNbO3 structure. It shows a long range, helical order of Mn2+ spins and strong frustration interpreted as a consequence of octahedral tilting and N/O disorder. | ||
| Fig. 5 Polar LiNbO3 type structure of MnTaO2N.23 | ||
The dielectric properties of thin films of LaTiO2N104 and N-doped ATiO3 (A = Sr, Ba) have also been investigated.105 The dielectric constant of LaTiO2N films was 325 whereas in N doped BaxSr1−xTiO3 films it improved by a factor of 1.44 with respect to the undoped material.
Molybdenum perovskite oxynitrides with composition AMoO3−xNx (A = alkaline earth) have been reported as thermoelectric materials. They show low electrical conductivities and Seebeck coefficients from 15 to 30 μV K−1.106
Tantalum, niobium or tungsten perovskite oxynitrides with europium at A sites and formula EuMO3−xNx are ferromagnetic because of the ordering of Eu(II) S = 7/2 spins, with Curie temperatures of 5.2, 5.1 and 12 K for M = Nb, Ta and W respectively.59,107,108 When mixed oxidation states of the transition metals near to stochiometric Eu2+Nb+5O2N and Eu2+W+6ON2 are present (Nb4+ or W5+ doping) these perovskites show colossal magnetoresistance below Tc because of coupling between the Eu2+ spins and d-band carriers of Nb or W. In EuWO1+xN2−x the electrical conductivity is tuned by the nitrogen content and the doping (p or n) mechanism.
Lanthanum or praseodymium vanadium oxynitride perovskites with formula RVO2N show spin freezing transitions at low temperatures14 and the oxynitride perovskite Sr2FeMoO5N shows a broad ferromagnetic transition with Tc in the temperature range of 150–200 K.64
6. Rare earth activated (oxy)nitridosilicates with luminescence properties
Oxynitridosilicates of alkaline earth metals and rare earth metals have been widely investigated as hosts for Eu2+ or Ce3+ to produce luminescent materials with long wavelengths and broad emission bands.4,5 Within pure nitrides the compounds A2Si5N8 (A = Sr, Ba) doped with Eu2+ have found applications as phosphors in white LEDs as they show high quantum efficiencies, strong absorptions in the blue light region and a range of emission wavelengths from 580 to 680 nm.109,110 These nitridosilicates show a condensed and rigid structure formed by corrugated layers of corner sharing [SiN4] tetrahedra which are three dimensionally connected.5 Remarkable long emission wavelengths (up to 650 nm) and high efficiency are shown also by Eu2+ doped CaAlSiN3111 and the nitridoaluminate SrLiAl3N4, structurally related to nitridosilicates.112 In cerium doped CaSiN2 wavelength emissions up to 630 nm under excitation at around 460 nm and quantum efficiencies up to 40% have been reported.113The synthesis of oxynitridosilicates is frequently performed by treatment of mixtures of Si3N4, SiO2, alkaline earth carbonates and rare earth oxides in N2 or N2/H2 at high temperatures (typically 1400–1600 °C). Silicon diimide has also been used as a source of silicon and nitrogen.19 Ammonolysis of precursor silicates may produce oxynitridosilicates at lower temperatures (below 1050 °C).114
The oxynitridosilicates (Sr,Ca,Ba)Si2O2N2 doped with Eu2+ are highly efficient phosphors showing yellow-green broad emissions (with λemiss from 492 to 563 nm) under activation in the UV-blue range.115 They show layered structures where sheets of the alkaline earth cations alternate with double layers of [SiO2N2]2− corner shared tetrahedra and composition [Si2N2O]. More recently the layered oxynitrides Ba3Si6O12N2 doped with Eu2+ have been reported to show green emission with a maximum at 527 nm.116
The structure of recently discovered compounds A3Si2O4N2 (A = Sr, Ca) are formed by rings of 12 corner-sharing [SiO2N2]2− tetrahedra where the alkaline earth cations are placed.117,118 When activated with Eu2+ they show emission wavelengths up to 600 nm under excitation in the UV-blue light range.
The new oxynitride orthosilicates LaMSiO3N (M = Sr, Ba)119 are isotypic to α′-M2SiO4 (M = Sr, Ba) (Fig. 6a) with a β-K2SO4 structure and can be activated either with Ce3+ or Eu2+. Under excitation with UV-blue light the emission wavelengths are red shifted with respect to the highly efficient phosphors Sr2SiO4:Eu or Ba2SiO4:Eu,120 from ca. 550 nm to 650–700 nm (Fig. 6b).
 | ||
| Fig. 6 (a) Crystal structure of LaMSiO3N compounds (M = Sr, Ba) showing polyhedra of the two cation sites of the β-K2SO4 structure and the isolated [SiO3N]5− tetrahedra. Anions are represented by red (oxygen) and light blue (mixed oxygen–nitrogen) spheres. (b) Excitation (λem = 550 nm, dotted line) and emission (λex = 405 nm, solid line) spectra of LaSr0.95Eu0.05SiO3N and Sr1.95Eu0.05SiO4.119 | ||
7. Conclusions and future prospects
The precedent sections have shown that recent research on metal oxynitrides has led to important new materials. The consequences of introducing nitride into an oxidic network are relevant on physical and chemical properties because of differences in charge, electronegativity and polarizability of both anions. The higher charge of nitride allows the stabilization of phases with higher oxidation states of cations with respect to the analogous oxides. On the other hand the synthesis conditions of oxynitrides under NH3 or N2 at high temperatures are reducing and may allow the stabilization of low oxidation states. The diversity of new materials increases with the possibility to tune the degree of anion order which may affect the properties. For electric/magnetic applications the sinterability and chemical homogeneity of ceramics are still issues because of the possibility of domains with a different N/O ratio and the limited thermal stability of some transition metal oxynitrides. High pressure methods have been used to solve this problem and have been useful to isolate new quaternary or multinary phases of late transition metals.23 These are still scarce because the higher electronegativity of metals like Fe, Mn or Co promotes the formation of interstitial binary nitrides under the reducing conditions of N2 or under NH3 at high temperatures. The development of new methods of preparation of thin films with defined compositions and nano/microstructures is also a challenge.The developments in the last few years have been more active in the reviewed applications in photocatalysis, dielectrics, magnetic materials and solid-state lighting, but the results in other important fields are promising. For instance the introduction of nitrogen into the cathode material Li2FeSiO4 has been predicted to decrease the lithium de-insertion voltage associated with the Fe3+/Fe4+ redox couple,9 and the oxynitrido phosphates Na2Fe2P3O9N and Li2Fe2P3O9N have been recently suggested as cathode materials for sodium and lithium batteries.121 The growth of new groups in the field and the improvement of synthetic approaches are expected to fuel new applications and materials in the near future.
Acknowledgements
This work was supported by the Spanish Ministerio de Economía y Competitividad, Spain (Project MAT2011-24757 and MAT2014-53500-R).Notes and references
- A. Fuertes, Dalton Trans., 2010, 39, 5942 RSC.
- S. G. Ebbinghaus, H.-P. Abicht, R. Dronskowski, T. Müller, A. Reller and A. Weidenkaff, Prog. Solid State Chem., 2009, 37, 173 CrossRef CAS PubMed.
- A. Fuertes, J. Mater. Chem., 2012, 22, 3293 RSC.
- R.-J. Xie and T. B. Hintzen, J. Am. Ceram. Soc., 2013, 96, 665 CrossRef CAS PubMed.
- M. Zeuner, S. Pagano and W. Schnick, Angew. Chem., Int. Ed., 2011, 50, 7754 CrossRef CAS PubMed.
- Y. Moriya, T. Takata and K. Domen, Coord. Chem. Rev., 2013, 257, 1957 CrossRef CAS PubMed.
- S. Suzuki and T. Shodai, Solid State Ionics, 1999, 116, 1 CrossRef CAS.
- J. Cabana, G. Rousse, A. Fuertes and M. R. Palacín, J. Mater. Chem., 2003, 13, 2402 RSC.
- M. Armand and M. E. Arroyo, J. Mater. Chem., 2011, 21, 10026 RSC.
- A. H. White and W. M. Melville, J. Am. Chem. Soc., 1905, 27, 373 CrossRef CAS.
- M. R. Brophy, S. M. Pilgrim and W. A. Schulze, J. Am. Ceram. Soc., 2011, 94, 4263 CrossRef CAS PubMed.
- R. Juza, Adv. Inorg. Chem. Radiochem., 1966, 9, 81 CAS.
- D. H. Gregory, J. Chem. Soc., Dalton Trans., 1999, 259 RSC.
- J. Oró-Solé, L. Clark, N. Kumar, W. Bonin, A. Sundaresan, J. P. Attfield, C. N. Rao and A. Fuertes, J. Mater. Chem. C, 2014, 2, 2212 RSC.
- M. Yang, J. Oró-Solé, A. Fuertes and J. P. Attfield, Chem. Mater., 2010, 22, 4132 CrossRef CAS.
- Y. Masubuchi, F. Kawamura, T. Taniguchi and S. Kikkawa, J. Eur. Ceram. Soc., 2015, 35, 1191 CrossRef CAS PubMed.
- S.-K. Sun, T. Motohashi, Y. Masubuchi and S. Kikkawa, J. Eur. Ceram. Soc., 2014, 34, 4451 CrossRef CAS PubMed.
- D. Ostermann, H. Jacobs and B. Harbrecht, Z. Anorg. Allg. Chem., 1993, 619, 1277 CrossRef CAS PubMed.
- F. Stadler, R. Kraut, O. Oeckler, S. Schmid and W. Schnick, Z. Anorg. Allg. Chem., 2005, 631, 1773 CrossRef CAS PubMed.
- A. Gomathi, S. Reshma and C. N. R. Rao, J. Solid State Chem., 2009, 182, 72 CrossRef CAS PubMed.
- N. Arumugam, A. Hönnerscheid and M. Jansen, Z. Anorg. Allg. Chem., 2003, 629, 939 CrossRef CAS PubMed.
- M. Yang, J. A. Rodgers, L. C. Middler, J. Oró-Solé, A. B. Jorge, A. Fuertes and J. P. Attfield, Inorg. Chem., 2009, 48, 11498 CrossRef CAS PubMed.
- C. Tassel, Y. Kuno, Y. Goto, T. Yamamoto, C. M. Brown, J. Hester, K. Fujita, M. Higashi, R. Abe, K. Tanaka, Y. Kobayashi and H. Kageyama, Angew. Chem., Int. Ed., 2015, 54, 516 CAS.
- E. Martínez-Ferrero, Y. Sakatani, C. Boissière, D. Grosso, A. Fuertes, J. Fraxedas and C. Sanchez, Adv. Funct. Mater., 2007, 17, 3348 CrossRef PubMed.
- A. B. Jorge, J. Fraxedas, A. Cantarero, A. J. Williams, J. Rodgers, J. P. Attfield and A. Fuertes, Chem. Mater., 2008, 20, 1628 CrossRef.
- E. B. Maxted, Ammonia and the nitrides, J. & A. Churchill, London, 1921 Search PubMed.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS PubMed.
- C. Di Valentin, G. Pacchioni and A. Selloni, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 085116 CrossRef.
- R. Asahi, T. Morikawa, H. Irie and T. Ohwaki, Chem. Rev., 2014, 114, 9824 CrossRef CAS PubMed.
- A. B. Jorge, Y. Sakatani, C. Boissière, C. Laberty-Roberts, G. Sauthier, J. Fraxedas, C. Sanchez and A. Fuertes, J. Mater. Chem., 2012, 22, 3220 RSC.
- C. Mao, Y. Zhao, X. Qiu, J. Zhu and C. Burda, Phys. Chem. Chem. Phys., 2008, 10, 5633 RSC.
- D. Sung, M. Gu, R. Li, S. Yin, Z. X. Song, B. Zhao, C. Li, J. Li, Z. Feng and T. Sato, Appl. Surf. Sci., 2013, 280, 693 CrossRef PubMed.
- V. Prabhakaran and V. Ramani, J. Electrochem. Soc., 2014, 161, F1 CrossRef CAS PubMed.
- D. Armytage and B. E. F. Fender, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1974, 30, 809 CrossRef CAS.
- L. Pauling, J. Am. Chem. Soc., 1929, 51, 1010 CrossRef CAS.
- A. Fuertes, Inorg. Chem., 2006, 45, 9640 CrossRef CAS PubMed.
- G. Hitoki, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, Chem. Commun., 2002, 1698 RSC.
- P. Zhang, J. Zhang and J. Gong, Chem. Soc. Rev., 2014, 43, 4395 RSC.
- J. Gan, X. Lu and Y. Tong, Nanoscale, 2014, 6, 7142 RSC.
- R. Abe, M. Higashi and K. Domen, ChemSusChem, 2011, 4, 228 CrossRef CAS PubMed.
- K. Maeda, M. Higashi, D. Lu, R. Abe and K. Domen, J. Am. Chem. Soc., 2010, 132, 5858 CrossRef CAS PubMed.
- K. Maeda, D. Lu and K. Domen, Chem. – Eur. J., 2013, 19, 4986 CrossRef CAS PubMed.
- Q. Gao, A. Wang, Y. Ma, C. Giordano and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 961 CrossRef CAS PubMed.
- E. Orhan, F. Tessier and R. Marchand, Solid State Sci., 2002, 4, 1071 CrossRef CAS.
- Q. Gao, C. Giordano and M. Antonietti, Small, 2011, 7, 3334 CrossRef CAS PubMed.
- R. Abe, M. Higashi and K. Domen, J. Am. Chem. Soc., 2010, 132, 11828 CrossRef CAS PubMed.
- M. Higashi, K. Domen and R. Abe, Energy Environ. Sci., 2011, 4, 4138 CAS.
- H. Schilling, A. Stork, E. Irran, H. Wolff, T. Bredow, R. Dronskowski and M. Lerch, Angew. Chem., Int. Ed., 2007, 46, 2931 CrossRef CAS PubMed.
- R. Marchand, L. Brohan and M. Tournoux, Mater. Res. Bull., 1980, 15, 1129 CrossRef CAS.
- T. Lüdtke, A. Schmidt, C. Göbel, A. Fischer, N. Becker, C. Reimann, T. Bredow, R. Dronskowski and M. Lerch, Inorg. Chem., 2014, 53, 11691 CrossRef PubMed.
- A. Suzuki, Y. Hirose, D. Oka, S. Nakao, T. Fukumura, S. Ishii, K. Sasa, H. Matsuzaki and T. Hasegawa, Chem. Mater., 2014, 26, 976 CrossRef CAS.
- M.-W. Lumey and R. Dronskowski, Z. Anorg. Allg. Chem., 2005, 631, 887 CrossRef CAS PubMed.
- K. Woodhead, S. Pascarelli, A. L. Hector, R. Briggs, N. Alderman and P. F. McMillan, Dalton Trans., 2014, 43, 9647 RSC.
- Z. Wang, J. Hou, C. Yang, S. Jiao, K. Huang and H. Zhu, Energy Environ. Sci., 2013, 6, 2134 CAS.
- R. Marchand, F. Pors and Y. Laurent, Ann. Chim., 1991, 16, 553 CAS.
- P. Bacher, P. Antoine, R. Marchand, P. L'Haridon, Y. Laurent and G. Roult, J. Solid State Chem., 1988, 77, 67 CrossRef CAS.
- G. Liu, X. Zhao and H. A. Eick, J. Alloys Compd., 1992, 187, 145 CrossRef CAS.
- P. Antoine, R. Assabaa, P. L'Haridon, R. Marchand, Y. Laurent, C. Michel and B. Raveau, Mater. Sci. Eng., B, 1989, 5, 43 CrossRef.
- A. B. Jorge, J. Oró-Solé, A. M. Bea, N. Mufti, T. T. M. Palstra, J. A. Rodgers, J. P. Attfield and A. Fuertes, J. Am. Chem. Soc., 2008, 130, 12572 CrossRef CAS PubMed.
- S. J. Clarke, B. P. Guinot, C. W. Michie, M. J. C. Calmont and M. J. Rosseinsky, Chem. Mater., 2002, 14, 288 CrossRef CAS.
- F. Pors, R. Marchand and Y. Laurent, Mater. Res. Bull., 1988, 23, 1447 CrossRef CAS.
- P. Bacher, P. Antoine, R. Marchand, P. L'Haridon, Y. Laurent and G. Roult, J. Solid State Chem., 1988, 77, 67 CrossRef CAS.
- G. Liu, X. Zhao and H. A. Eick, J. Alloys Compd., 1992, 187, 145 CrossRef CAS.
- M. Retuerto, C. De la Calle, M. J. Martínez-Lope, F. Porcher, K. Krezhov, N. Menéndez and J. A. Alonso, J. Solid State Chem., 2012, 185, 18 CrossRef CAS PubMed.
- N. E. Brese and F. J. DiSalvo, J. Solid State Chem., 1995, 120, 378 CrossRef CAS.
- R. Niewa, Z. Anorg. Allg. Chem., 2013, 639, 1699 CrossRef CAS PubMed.
- G. Tobías, J. Oró-Solé, D. Beltrán-Porter and A. Fuertes, Inorg. Chem., 2001, 40, 6867 CrossRef.
- F. Pors, R. Marchand and Y. Laurent, Ann. Chim., 1991, 16, 547 CAS.
- R. Marchand, C. R. Acad. Sci. Paris, 1976, 282, 329 CAS.
- F. Cheviré, E. Pallu, E. Ray and F. Tessier, J. Alloys Compd., 2011, 509, 5839 CrossRef PubMed.
- W. Li, E. Ionescu, R. Riedel and A. Gurlo, J. Mater. Chem. A, 2013, 1, 12239 CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- S. J. Clarke, K. A. Hardstone, C. W. Michie and M. J. Rosseinsky, Chem. Mater., 2002, 14, 2664 CrossRef CAS.
- S. G. Ebbinghaus, A. Weidenkaff, A. Rachel and A. Reller, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2004, 60, i91 Search PubMed.
- G. Tobías, D. Beltrán-Porter, O. Lebedev, G. Van Tendeloo, J. Oró-Solé, J. Rodríguez-Carvajal and A. Fuertes, Inorg. Chem., 2004, 43, 8010 CrossRef PubMed.
- E. Gunther, R. Hagenmayer and M. Jansen, Z. Anorg. Allg. Chem., 2000, 626, 1519 CrossRef CAS.
- S. H. Porter, Z. Huang and P. M. Woodward, Cryst. Growth Des., 2014, 14, 117 CAS.
- N. Diot, R. Marchand, J. Haines, J. M. Léger, P. Macaudière and S. Hull, J. Solid State Chem., 1999, 146, 390 CrossRef CAS.
- M. Yang, J. Oró-Solé, J. A. Rodgers, A. B. Jorge, A. Fuertes and J. P. Attfield, Nat. Chem., 2011, 3, 47 CrossRef CAS PubMed.
- J. Oró-Solé, L. Clark, W. Bonin, J. P. Attfield and A. Fuertes, Chem. Commun., 2013, 49, 2430 RSC.
- K. Page, M. W. Stoltzfus, Y.-I. Kim, T. Proffen, P. M. Woodward, A. K. Cheetham and R. Seshadri, Chem. Mater., 2007, 19, 4037 CrossRef CAS.
- H. Wolff and R. Drosnkowski, J. Comput. Chem., 2008, 29, 2260 CrossRef CAS PubMed.
- R. Poloni, J. Iñiguez, A. Garcia and E. Canadell, J. Phys.: Condens. Matter, 2010, 22, 415401 CrossRef PubMed.
- Y. Hinuma, H. Moriwake, Y.-R. Zhang, T. Motohashi, S. Kikkawa and I. Tanaka, Chem. Mater., 2012, 24, 4343 CrossRef CAS.
- K. Tatsumi and R. Hoffmann, Inorg. Chem., 1980, 19, 2656 CrossRef CAS.
- L. Clark, J. Oró-Solé, K. S. Knight, A. Fuertes and J. P. Attfield, Chem. Mater., 2013, 25, 5004 CrossRef CAS.
- J. P. Attfield, Cryst. Growth Des., 2013, 13, 4623 CAS.
- I. E. Castelli, T. Olsen, S. Datta, D. D. Landis, S. Dahl, K. S. Thyegesen and K. W. Jacobsen, Energy Environ. Sci., 2012, 5, 5814 CAS.
- Y. Wu, P. Lazic, G. Hautier, K. Persson and G. Ceder, Energy Environ. Sci., 2013, 6, 157 CAS.
- S. Balaz, S. H. Porter, P. M. Woodward and L. J. Brillson, Chem. Mater., 2013, 25, 3337 CrossRef CAS.
- D. Yamasita, T. Takata, M. Hara, J. N. Kondo and K. Domen, Solid State Ionics, 2004, 172, 591 CrossRef CAS PubMed.
- K. Maeda and K. Domen, J. Phys. Chem. C, 2007, 111, 7851 CAS.
- Y.-I. Kim and P. M. Woodward, J. Solid State Chem., 2007, 180, 3224 CrossRef CAS PubMed.
- C. Pan, T. Takata, M. Nakabayashi, T. Matsumoto, N. Shibata, Y. Ikuhara and K. Domen, Angew. Chem., Int. Ed., 2015, 54, 2955 CrossRef CAS PubMed.
- B. Siritanaratkul, K. Maeda, T. Hisatomi and K. Domen, ChemSusChem, 2011, 4, 74 CrossRef CAS PubMed.
- T. Hisatomi, C. Katayama, K. Teramura, T. Takata, Y. Moriya, T. Minegishi, M. Katayama, H. Nishiyama, T. Yamada and K. Domen, ChemSusChem, 2014, 7, 2016 CrossRef CAS PubMed.
- A. Kasahara, K. Nukumizu, G. Hitoki, T. Takata, J. N. Kondo, M. Hara, H. Kobayashi and K. Domen, J. Phys. Chem. A, 2002, 106, 6750 CrossRef CAS.
- F. Zhang, A. Yamakata, K. Maeda, Y. Moriya, T. Takata, J. Kubota, K. Teshima, S. Oishi and K. Domen, J. Am. Chem. Soc., 2012, 134, 8348 CrossRef CAS PubMed.
- Y.-I. Kim, P. M. Woodward, K. Z. Baba-Kishi and C. W. Tai, Chem. Mater., 2004, 16, 1267 CrossRef CAS.
- R. L. Withers, Y. Liu, P. Woodward and Y.-I. Kim, Appl. Phys. Lett., 2008, 92, 102907 CrossRef PubMed.
- Y. Hinuma, H. Moriwake, Y.-R. Zhang, T. Motohashi and S. Kikawa, Chem. Mater., 2012, 24, 4343 CrossRef CAS.
- D. Oka, Y. Hirose, H. Kamisaka, T. Fukumura, K. Sasa, S. Ishii, H. Matsuzaki, Y. Sato, Y. Ikuhara and T. Hasegawa, Sci. Rep., 2014, 4, 4987 CAS.
- W. Zhu, H. Kamisaka, D. Oka, Y. Hirose, A. Leto, T. Hasegawa and G. Pezzoti, J. Appl. Phys., 2014, 116, 053505 CrossRef PubMed.
- Y. Lu, C. Le Paven, H. V. Nguyen, R. Benzerga, L. Le Gendre, S. Rioual, F. Tessier, F. Cheviré, A. Sharaiha, C. Delaveaud and X. Castel, Cryst. Growth Des., 2013, 13, 4852 CAS.
- A. David, S. Guérin, B. E. Hayden, R. Noble, J.-P. Soulié, C. Vian, I. P. Koustaroff, S. Higai, N. Tanaka, T. Konoike, A. Ando, H. Tagaki, T. Yamamoto, T. Fukura and H. Ieki, Cryst. Growth Des., 2014, 14, 523 CAS.
- D. Loginovich, J. Hetmánek, K. Knizek, M. Marysko, N. Homazava, P. Tomes, R. Aguiar, S. G. Ebbinghaus and A. Widenkaff, J. Appl. Phys., 2009, 105, 023522 CrossRef PubMed.
- M. Yang, J. Oró-Solé, A. Kusmartseva, A. Fuertes and J. P. Attfield, J. Am. Chem. Soc., 2010, 132, 4822 CrossRef CAS PubMed.
- R. Pastrana-Fábregas, J. Isasi-Marín, C. Cascales and R. Sáez-Puche, J. Solid State Chem., 2007, 180, 92 CrossRef PubMed.
- H. A. Hoppe, H. Lutz, P. Morys, W. Schnick and A. J. Seilmeier, J. Phys. Chem. Solids, 2000, 61, 2001 CrossRef CAS.
- Y. Q. Li, J. E. J. van Steen, J. W. H. van Krevel, G. Botty, A. C. A. Delsing, F. J. DiSalvo, G. de With and H. T. Hintzen, J. Alloys Compd., 2006, 417, 273 CrossRef CAS PubMed.
- K. Uheda, N. Hirosaki, Y. Yamamoto, A. Naito, T. Nakajima and H. Yamamoto, Electrochem. Solid-State Lett., 2006, 9, H22 CrossRef CAS PubMed.
- P. Pust, V. Weiler, C. Hecht, A. Tücks, A. S. Wochnick, A.-K. Henß, D. Wiechert, C. Scheu, P. J. Schmidt and W. Schnick, Nat. Mater., 2014, 13, 891 CrossRef CAS PubMed.
- R. Le Toquin and A. K. Cheetham, Chem. Phys. Lett., 2006, 423, 352 CrossRef CAS PubMed.
- S. Thomas, J. Oró-Solé, B. Glorieux, V. Jubera, V. Buissette, T. Le Mercier, A. Garcia and A. Fuertes, J. Mater. Chem., 2012, 22, 23913 RSC.
- Y. Q. Li, A. C. A. Delsing, G. de With and H. T. Hintzen, Chem. Mater., 2005, 17, 3242 CrossRef CAS.
- C. Braun, M. Seibald, S. L. Börger, O. Oeckler, T. D. Boyko, A. Moewes, G. Miehe, A. Tücks and W. Schnick, Chem. – Eur. J., 2010, 16, 9646 CrossRef CAS PubMed.
- X.-M. Wang, C.-H. Wang, X.-J. Kuang, R.-Q. Zou, Y.-X. Wang and X.-P. Jing, Inorg. Chem., 2012, 51, 3540 CrossRef CAS PubMed.
- Y.-C. Chiu, C.-H. Huang, T.-J. Lee, W.-R. Liu, Y.-T. Yeh, A.-M. Jang and R.-S. Liu, Opt. Express, 2011, 19, A331 CrossRef PubMed.
- A. P. Black, K. A. Denault, J. Oró-Solé, A. R. Goñi and A. Fuertes, Chem. Commun., 2015, 51, 2166 RSC.
- T. L. Barry, J. Electrochem. Soc., 1968, 115, 1181 CrossRef CAS PubMed.
- J. Liu, X. Yu, E. Hu, K.-W. Nam, X.-Q. Yang and P. G. Khalifah, Chem. Mater., 2013, 25, 3929 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |


