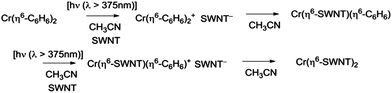Photochemical generation of bis-hexahapto chromium interconnects between the graphene surfaces of single-walled carbon nanotubes
Aron
Pekker
ab,
Mingguang
Chen
ac,
Elena
Bekyarova
ab and
Robert C.
Haddon
*abcd
aCenter for Nanoscale Science and Engineering, University of California, Riverside, California 92521, USA. E-mail: haddon@ucr.edu
bDepartment of Chemistry, University of California, Riverside, California 92521, USA
cDepartment of Chemical and Environmental Engineering, University of California, Riverside, California 92521, USA
dDepartment of Physics, King Abdulaziz University, Jeddah 21589, Saudi Arabia
First published on 17th November 2014
Abstract
The electrical conductivity of single-walled carbon nanotube (SWNT) networks is strongly enhanced by the high vacuum e-beam deposition of transition metals. In the present communication we demonstrate that it is possible to accomplish the same chemical functionalization reactions at room temperature beginning with simple organometallic precursors. We show that the photochemically induced reactions of solutions of Cr(CO)6, Cr(η6-benzene)(CO)3, and Cr(η6-benzene)2 with thin films of semiconducting, metallic and non-separated SWNT films all lead to strongly enhanced conductivities which produce consistent results for each SWNT type among the three organometallic reagents. We conclude that all three of these reactions lead to the generation of covalent (η6-SWNT)Cr(η6-SWNT) interconnects which provide conducting pathways in the SWNT films and our results broaden the applicability of the transition metal bis-hexahapto-bond as an electronically conjugating linkage between graphene surfaces.
Conceptual insightsThe building block of carbon nanotubes and graphene is the graphitic surface of sp2 hybridized carbon atoms which are incorporated in continuous, periodic poly-benzenoid structures. Due to their high degree of perfection there is no simple way to covalently interconnect such structures without disrupting the electronic band structure and introducing sp3 carbon atoms into the lattice. The introduction of rehybridized carbon atoms into these graphitic structures has been shown to decrease the electrical conductivity and the mobility of the carriers and to lead to localized structures. In this report we extend our use of the covalent organometallic hexahapto-bond in the conjugation of these delocalized graphene surfaces by exploring photochemical routes for the preparation of (η6-SWNT)Cr(η6-SWNT) junctions (SWNT = single-walled carbon nanotubes), in random networks of SWNT thin films. We show that standard organometallic chromium precursors may be utilized to improve the conductivity of metallic, semiconducting, and non-separated SWNT thin films by the use of simple photochemical reactions utilizing the appropriate wavelength of light. The work supports the idea that the minimal rehybrization which is implicit in the formation of organometallic hexahapto-linkages to conjugated graphene surfaces is the only form of covalent bonding which is capable increasing the conductivity of these networks. |
Introduction
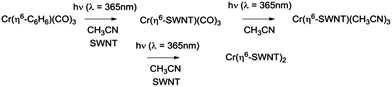
The electrical interconnection of the graphene surfaces of carbon nanomaterials remains a challenging problem in carbon electronics1,2 and it is now well known that chemical functionalization processes in which the conjugated carbon atoms are fully pyramidalized to the geometry required for sp3 hybridization, lead to a loss of conductivity and mobility in these systems.3 However the formation of hexahapto-organometallic bonds to the graphene surface largely preserves the electronic structure of these polybenzenoid carbon structures,4–8 and the deposition of chromium atoms on thin films of SWNTs exerts a dramatic effect on the percolation threshold of SWNT networks which we have attributed to the formation of organometallic bonds of the form Cr(η6-SWNT)2.9The preparation of hexahapto-bis(arene) metal complexes may be accomplished by a number of different routes, but only two have found general application:10 the metal vapor synthesis (MVS) technique of Timms11 and the solution-based Fischer–Hafner process.12 The preparation of hexahapto-arene-chromium-tricarbonyls can be accomplished by the solution reaction of Cr(CO)6 with the appropriate arene under thermal or photochemical conditions,13,14 although neither process proceeds to the fully substituted Cr(η6-arene)2 complexes.15 We have found that variants of some of these gas phase MVS and solution-based thermal reactions are capable of realizing the organometallic functionalization of extended, periodic π-electrons systems such as single-walled carbon nanotubes (SWNT) and graphene.8,9,16–18 The MVS reactions are accomplished within the high vacuum chamber of an e-beam evaporation system and this has allowed us to show that the formation of these hexahapto-bis(η6-SWNT) metal complexes dramatically increases the conductivity of thin films of SWNTs.
In the present communication we introduce facile solution-based routes for the ambient temperature introduction of organometallic atomic interconnects between the graphene surfaces of SWNTs which obviate the need for high vacuum reaction vessels. We show that photochemical reactions of reagents such as Cr(CO)6 and Cr(η6-benzene)(CO)3 are very effective in forming Cr(η6-SWNT)2 complexes which act as interconnects between the graphitic surfaces, as evidenced by an abrupt increase in conductivity on irradiation of thin films of these materials in the presence of the appropriate reagents. Surprisingly we find that room temperature treatment of the SWNT films with solutions of Cr(η6-benzene)2 spontaneously produces the same effect apparently as a result of incidental exposure to room light.
Experimental
Nanotube sample preparation
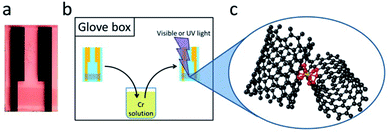
The single-walled carbon nanotube thin films were prepared by vacuum filtration using aqueous suspensions of semiconducting (SC, Nanointegris Inc. – IsoNanotubes-S 99%), metallic (MT, Nanointegris Inc. – IsoNanotubes-M 99%) and non-separated purified nanotubes (NS, Nanointegris Inc. – PureTubes). The volume of suspension for vacuum filtration using a mixed cellulose ester membrane (Millipore VCWP), was calculated on the basis of the concentration of the SWNT dispersion and the measured film density so as to obtain a film of thickness, t = 8 nm.19 The SWNT film and filter membrane was cut into 6 mm × 2 mm pieces and the SWNT film was transferred onto a glass substrate with pre-patterned gold electrodes (Fig. 2) using a hot acetone vapor process described in the literature.20 The films for the conductivity experiments were measured under a microscope in order to determine the film geometry for the final film conductivity calculation. The samples were vacuum annealed (8 h at 300 °C, 10−6 torr), immediately before use in order to remove atmospheric dopants and transferred into a glove box (O2, H2O < 1 ppm) without further air exposure.Reaction conditions
Approximately 1 × 10−3 M solutions of the chromium compounds (Cr(CO)6 – 3.5 mg, Aldrich 98%, Cr(η6-C6H6)(CO)3 – 2.4 mg, Aldrich 98%, Cr(η6-C6H6)2 – 2 mg, Strem 97%) were prepared in degassed acetonitrile (10 ml, Aldrich anhydrous, 99.7%); the samples were dipped into the reagent solutions and irradiated inside the glove box. The light sources for the photochemistry consisted of a handheld dual UV source (Spectroline ENF-260C), making use of the UVC (254 nm) light in the case of Cr(CO)6 and UVA (365 nm) light for the Cr(η6-C6H6)(CO)3 samples, whereas for the Cr(η6-C6H6)2 experiments the ambient room light was sufficient.Characterization
The resistance of the samples was measured using a Keithley 2400 Source Meter unit driven with custom LabVIEW software with sample contacts made via feed throughs into the glove box. The UV-VIS spectroscopy of the solutions was performed with a CARY 5000 grating spectrometer using 1 mm quartz cuvettes.Results and discussion
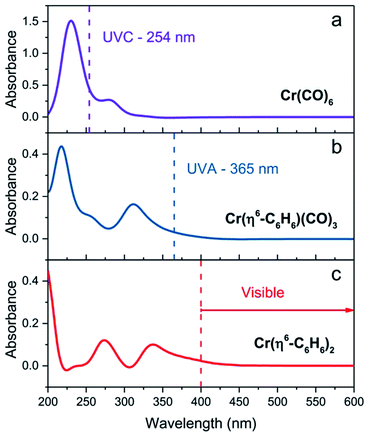
The experiments we describe are most closely related to our MVS conductivity studies which were conducted in a Temescal BJD 1800 cryo-pumped high vacuum e-beam evaporator that allows the exposure of appropriately wired SWNT thin films to a flux of metal vapors.9,18,19,21 We initially transferred this experiment to a 1′′ pyrex tube which was maintained under an argon atmosphere and was therefore amenable to heating in a tube furnace or irradiation by an external light source (in these studies we used a handheld UV lamp). However when we discovered the room temperature photochemical routes we were able to carry out the experiments in a glove box which allowed the whole process to be conducted under inert atmosphere conditions, including the treatment of the films with the organometallic reagents. The absorption spectra of these reagents in acetonitrile solution are shown in Fig. 1 together with the wavelength of light which was found to be successful in inducing the photochemical reactions.Typically we first measured the pristine film conductivities on overnight annealing under argon in order to remove residual solvent and atmospheric contaminants and to provide a baseline for the organometallic reactions. The protocol for the experiments is shown in Fig. 2 and consists of the exposure of a suitably wired SWNT thin film (thickness, t = 8 nm), to a solution of the organometallic precursor by dipping the film in an acetonitrile solution of the reagent (concentration ≈ 1 × 10−3 M) in a glove box followed by the measurement of the conductivity of the SWNT thin film at ambient temperature. For these experiments we included three types of SWNT thin films, as described in the experimental section: semiconducting (SC-), metallic (MT-), and non-separated (NS-) SWNTs; the transport properties of these films have been previously characterized in connection with our MVS experiments.9,22 The conductivities of the pristine films are sensitive to atmospheric doping (particularly SC-SWNTs), but representative room temperature conductivities of the annealed films are as follows: σ ≈ 3 S cm−1 (SC-SWNT), 350 (MT-SWNT), 300 (NS-SWNT).
The irradiation of matrices containing Cr(CO)6 with UV light is known to lead to the successive dissociation of the CO ligands which are replaced with molecules from the surrounding matrix;23,24 the high reactivity of these intermediates leads to complexation with inert ligands such as acetonitrile,25 alkanes,26 dihydrogen,27 and inert gases (Ne, Ar, Kr, Xe).23,24
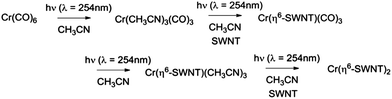
We found that SWNT films in the presence of Cr(CO)6, in which the solvent had been removed by evaporation, did not experience a discernible change in conductivity on irradiation and thus we conclude that acetonitrile complexes25 such as Cr(CO)3(CH3CN)3 must be implicated in these reactions and we have previously shown that this complex reacts readily with exfoliated graphene samples to give (η6-graphene)Cr(CO)3.7 The subsequent reactions with Cr(CO)6 were conducted without removal of the acetonitrile and we postulate the following reaction pathway (Scheme 1).
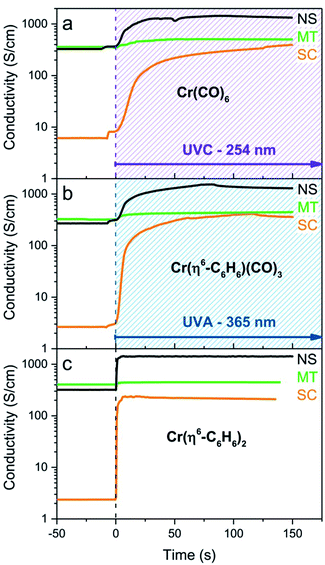
As may be seen in Fig. 3a, the response of the conductivity is essentially complete in the first 25 s of UVC irradiation (λ = 254 nm) and we were unable to detect differences in the reactivity of the three SWNT films toward the species generated from the photolysis of Cr(CO)6; the use of UVA light (λ = 365 nm) produced no discernible change in the film conductivities. The changes in conductivity are discussed in detail later, but they show the same qualitative behavior as was found previously in the MVS experiments,9 with the SC-SWNT films experiencing the strongest enhancement of the conductivity (by a factor of more than two orders of magnitude in the present experiments).
We found that SWNT films previously exposed to an acetonitrile solution of Cr(η6-benzene)(CO)3 when irradiated with UVA light (λ = 365 nm) led to a virtually identical set of conductivity responses (Fig. 3b), although on a slightly shorter time scale and in this case the use of UVC light (λ = 254 nm) produced no discernible effect on the film conductivities. The photochemistry of Cr(η6-benzene)(CO)3 is known to be complex,26,28 but based on the wavelength of the light found to initiate reaction, we postulate the following reaction mechanism (Scheme 2), as Cr(η6-benzene)(CO)3 is known to undergo both arene exchange and CO loss under irradiation with light.29
The exposure of the SWNT films to a solution of Cr(η6-benzene)2 in acetonitrile in the presence of room light (filtered through the plexiglass of the glove box), lead to immediate reaction as evidenced by the instantaneous response of the conductivity (Fig. 3c).
The benzene ring is usually regarded as kinetically inert toward arene exchange reactions in Cr(η6-benzene)2 and benzene is noted for its ability to replace other arenes under appropriate conditions.10,15,30,31 Furthermore Cr(η6-benzene)2 is known to be quite stable to irradiation and it does not normally undergo light-induced arene exchange.29 However, based on the redox potentials of Cr(η6-C6H6)2 (reduction potential of Cr(η6-C6H6)2+, E1/2 = −0.69 V against SCE in DME)15 and SC-SWNTs (reduction potential of electric arc SWNTs, E1/2 ≈ −0.5 V against SCE in DMSO),32 it seems likely that the reaction may proceed by a series of electron transfer processes as the metallocene cations generally undergo facile arene exchange reactions.10,31
In Scheme 3 we show the reaction as a photochemical process which requires visible light for its initiation, but as may be seen in Fig. 4 it seems the reaction is able to proceed in the absence of light, but at a much slower rate in accord with the redox potentials discussed above which are thermodynamically sufficient for spontaneous electron transfer (Scheme 3).
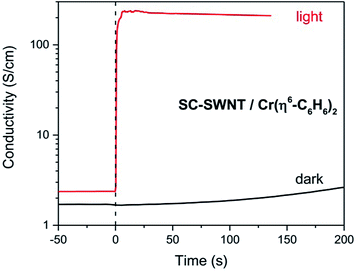 | ||
| Fig. 4 Evolution of the conductivity of semiconducting (SC-) SWNT films after dipping in Cr(η6-benzene)2 solution, in the presence of room light and in the dark. | ||
It is apparent from Fig. 3 that the photochemical reactions of all three organometallic species lead to the same modification of the electronic structure of the SWNT films and the final conductivities show good agreement after chromium functionalization: σ ≈ 300 S cm−1 (SC-SWNT), 500 S cm−1 (MT-SWNT), 1250 S cm−1 (NS-SWNT), which gives the following conductivity enhancements: (σ − σ0)/σ0 ≈ 100 (SC-SWNT), 1 (MT-SWNT), 4 (NS-SWNT), where σ is SWNT film conductivity after functionalization and σ0 is conductivity of the pristine SWNT film. These values exceed the previously determined MVS results which gave values of: (σ − σ0)/σ0 ≈ 70 (SC-SWNT) and 0.6 (MT-SWNT), for films of thickness, t = 8 nm.9 We assign the slightly improved conductivities of the solution processed films to the ability of the solvent to penetrate and uniformly disperse the reagents throughout the films thereby allowing contact with SWNT junctions within the film that are not accessible to the line-of-sight flux of metal atoms that is characteristic of high vacuum evaporation processes. For films of thickness, t = 2 nm the MVS experiments gave values of (σ − σ0)/σ0 ≈ 130 (SC-SWNT) and 0.8 (MT-SWNT).9 Previous experiments show that the stoichiometry of Cr atoms necessary to bring about these conductivity enhancements is very low.19,21
As noted above, the reaction rates estimated from the conductivities, suggest the following order of reagent reactivities under the specified conditions: Cr(η6-benzene)2 > Cr(η6-benzene)(CO)3 > Cr(CO)6, whereas under most circumstances the reverse order might be expected in reactions of these reagents with free arenes.10,15 Nevertheless the high reactivities shown in Fig. 3 are in accord with our frontier molecular orbital theory (FMO) analysis of the electronic structure of graphene, in which the high lying HOMOs and low lying LUMOs together with the presence of appropriate FMOs suggests that graphene and related small band gap nanomaterials such as SWNTs might be very reactive in certain circumstances, including organometallic functionalization chemistry.8,33
The conductivity enhancements of the MT-SWNTs are less than in the case of the SC-SWNTs which may be related to the different symmetries of the FMOs in the case of arm chair and semiconducting SWNTs which will affect the interaction with the transition metal d-orbitals;34 furthermore two-terminal conductances measured across MT-MT junctions of pristine SWNTs are comparable to the two-terminal conductances of the individual SWNTs.35
Conclusions
In conclusion, we have discovered a series of facile routes for the photochemical solution-based, ambient temperature introduction of transition metal interconnects between the surfaces of semi-conducting, metallic and non-separated single-walled carbon nanotubes (SWNT)s that substantially increase the conductivity of thin SWNT networks. We show that the reagents exhibit the following order of reactivities Cr(η6-benzene)2 > Cr(η6-benzene)(CO)3 > Cr(CO)6 toward the SWNT networks based on the conductivity responses, although all reagents lead to the same final conductivities and involve the Cr(η6-SWNT)2 linkages as the common feature. The broad scope of these organometallic reactions suggest that the present work will allow the preparation of the full complement of organometallic complexes of any carbon-based nanomaterial containing the poly-benzenoid electronic structure, particularly SWNTs and graphene structures thereby allowing the further development of atomtronics.17Acknowledgements
The study of the organometallic reactions with carbon nanotubes was supported by NSF under contract DMR-1305724. The carbon nanotube thin film preparation was sponsored by the Defense Microelectronics Activity (DMEA) under agreement number H94003-10-2-1003. The United States Government is authorized to reproduce and distribute reprints for Government purposes, notwithstanding any copyright notation thereon.Notes and references
- M. A. Topinka, M. W. Rowell, D. Goldhaber-Gordon, M. D. McGehee, D. S. Hecht and G. Gruner, Nano Lett., 2009, 9, 1866–1871 CrossRef CAS PubMed.
- P. N. Nirmalraj, P. E. Lyons, S. De, J. N. Coleman and J. J. Boland, Nano Lett., 2009, 9, 3890–3895 CrossRef CAS PubMed.
- H. Zhang, E. Bekyarova, J.-W. Huang, Z. Zhao, W. Bao, F. Wang, R. C. Haddon and C. N. Lau, Nano Lett., 2011, 11, 4047–4051 CrossRef CAS PubMed.
- E. Y. Li and N. Marzari, ACS Nano, 2011, 5, 9726–9736 CrossRef CAS PubMed.
- S. M. Avdoshenko, I. N. Ioffe, G. Cuniberti, L. Dunsch and A. A. Popov, ACS Nano, 2011, 5, 9939–9949 CrossRef CAS PubMed.
- J. Dai, Y. Zhao, X. Wu, X. C. Zeng and J. Yang, J. Phys. Chem. C, 2013, 117, 22156–22161 CAS.
- S. Sarkar, H. Zhang, J.-W. Huang, F. Wang, E. Bekyarova, C. N. Lau and R. C. Haddon, Adv. Mater., 2013, 25, 1131–1136 CrossRef CAS PubMed.
- E. Bekyarova, S. Sarkar, F. Wang, M. E. Itkis, I. Kalinina, X. Tian and R. C. Haddon, Acc. Chem. Res., 2013, 46, 65–76 CrossRef CAS PubMed.
- X. Tian, M. L. Moser, A. Pekker, S. Sarkar, J. Ramirez, E. Bekyarova, M. E. Itkis and R. C. Haddon, Nano Lett., 2014, 14, 3930–3937 CrossRef CAS PubMed.
- G. Pampaloni, Coord. Chem. Rev., 2010, 254, 402–419 CrossRef CAS PubMed.
- P. L. Timms, Chem. Commun., 1969, 1033 RSC.
- E. O. Fischer and W. Hafner, Z. Naturforsch., B: Anorg. Chem., Org. Chem., Biochem., Biophys., Biol., 1955, 10, 665–668 Search PubMed.
- E. P. Kundig, Top. Organomet. Chem., 2004, 7, 3–20 CrossRef.
- G. B. M. Kostermans, M. Bobeldijk, W. H. d. W. Kwakman and F. Bickelhaupt, J. Organomet. Chem., 1989, 363, 291–296 CrossRef CAS.
- C. Elschenbroich, Organometallics, Wiley-VCH, Weinheim, 3rd edn, 2006 Search PubMed.
- S. Sarkar, S. Niyogi, E. Bekyarova and R. C. Haddon, Chem. Sci., 2011, 2, 1326–1333 RSC.
- S. Sarkar, M. L. Moser, X. Tian, X. J. Zhang, Y. F. Al-Hadeethi and R. C. Haddon, Chem. Mater., 2014, 26, 184–195 CrossRef CAS.
- M. L. Moser, X. Tian, A. Pekker, S. Sarkar, E. Bekyarova, M. E. Itkis and R. C. Haddon, Dalton Trans., 2014, 7379–7382 RSC.
- F. Wang, M. E. Itkis, E. Bekyarova, X. Tian, S. Sarkar, A. Pekker, I. Kalinina, M. Moser and R. C. Haddon, Appl. Phys. Lett., 2012, 100, 223111 CrossRef PubMed.
- Z. Wu, Z. Chen, X. Du, J. M. Logan, J. Sippel, M. Nikolou, K. Kamaras, J. R. Reynolds, D. B. Tanner, A. F. Hebard and A. G. Rinzler, Science, 2004, 305, 1273–1276 CrossRef CAS PubMed.
- F. Wang, M. E. Itkis, E. Bekyarova, S. Sarkar, X. Tian and R. C. Haddon, J. Phys. Org. Chem., 2012, 25, 607–610 CrossRef CAS.
- I. Kalinina, E. Bekyarova, S. Sarkar, F. Wang, M. E. Itkis, X. Tian, S. Niyogi, N. Jha and R. C. Haddon, Macromol. Chem. Phys., 2012, 213, 1001–1019 CrossRef CAS.
- R. N. Perutz and J. J. Turner, J. Am. Chem. Soc., 1975, 97, 4791–4800 CrossRef CAS.
- R. N. Perutz and J. J. Turner, J. Am. Chem. Soc., 1975, 97, 4800–4804 CrossRef CAS.
- G. R. Dobson, M. A. El-Sayed, I. W. Stolz and R. K. Sheline, Inorg. Chem., 1962, 1, 526–530 CrossRef CAS.
- M. A. H. Alamiry, N. M. Boyle, C. M. Brookes, M. W. George, C. Long, P. Portius, M. T. Pryce, K. L. Ronanyne, X. Z. Sun, M. Towrie and K. Q. Vuong, Organometallics, 2009, 28, 1461–1468 CrossRef CAS.
- J. D. Egbert and D. M. Heinekey, Organometallics, 2010, 29, 3387–3391 CrossRef CAS.
- M. W. George, C. Long, M. T. Pryce, X. Z. Sun and K. Q. Vuong, Organometallics, 2012, 31, 268–272 CrossRef CAS.
- A. Gilbert, J. M. Kelly, M. Budzwait and E. Koerner von Gustorf, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1976, 31, 1091–1095 Search PubMed.
- J. A. Connor, J. A. Martinhosimoes, H. A. Skinner and M. T. Zafaranimoattar, J. Organomet. Chem., 1979, 179, 331–356 CrossRef CAS.
- G. Wilkinson, F. G. A. Stone and E. W. Abel, Comprehensive Organometallic Chemistry, Pergamon Press, Oxford, 1982 Search PubMed.
- D. Paolucci, M. M. Franco, M. Iurlo, M. Marcaccio, M. Prato, F. Zerbetto, A. Penicaud and F. Paolucci, J. Am. Chem. Soc., 2008, 130, 7393–7399 CrossRef CAS PubMed.
- S. Sarkar, E. Bekyarova and R. C. Haddon, Acc. Chem. Res., 2012, 45, 673–682 CrossRef CAS PubMed.
- M. S. Dresselhaus, G. Dresselhaus and P. C. Eklund, Science of Fullerenes and Carbon Nanotubes, Academic, San Diego, 1996 Search PubMed.
- M. S. Fuhrer, J. Nygard, L. Shih, M. Forero, Y.-G. Yoon, M. S. C. Mazzoni, H. J. Choi, J. Ihm, S. G. Louie, A. Zettl and P. L. McEuen, Science, 2000, 288, 494–497 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |