Stem cell-compatible eumelanin biointerface fabricated by chemically controlled solid state polymerization†
Alessandro
Pezzella
*a,
Mario
Barra
b,
Anna
Musto
cd,
Angelica
Navarra
cd,
Michela
Alfè
e,
Paola
Manini
a,
Silvia
Parisi
cd,
Antonio
Cassinese
b,
Valeria
Criscuolo
a and
Marco
d'Ischia
a
aDepartment of Chemical Sciences, University of Naples “Federico II”, Via Cintia 4, I-80126 Naples, Italy. E-mail: alessandro.pezzella@unina.it
bCNR-SPIN and Department of Physics, University of Naples Federico II, Piazzale Tecchio 80, Naples 80125, Italy
cDepartment of Molecular medicine and Medical Biotechnology, University of Naples “Federico II”, Italy
dCeinge Biotecnologie Avanzate Via Gaetano Salvatore 486, 80145 Naples, Italy
eCNR, Istituto di Ricerche sulla Combustione, Naples, Italy
First published on 15th October 2014
Abstract
Relying on the water-dependent hybrid conductor properties of eumelanin biopolymers, a structurally controlled melanin thin film that can be reversibly switched-on and off by hydration–dehydration cycles and used as a biocompatible platform for stem cell growth and differentiation up to 11 days is reported. A key feature of the new eumelanin-based bioelectronic interface is its remarkable structural regularity and integrity, as the result of an ad hoc fabrication protocol involving ammonia-induced solid state polymerization (AISSP) technology of a 5,6-dihydroxyindole thin film. The AISSP technology is operationally simple and versatile, enabling the preparation of device-quality thin films (AFM and MALDI-MS evidence) on various substrates with efficient chemical control over molecular complexity. Overall the results of this study pave the way for the implementation of tailored eumelanin thin films for bioelectronic devices, e.g., in organic electrochemical transistors (OECTs).
Conceptual insightsThis work discloses a pioneering eumelanin thin film as an operational platform for stem cell growth and differentiation. Key elements of the technology that allow exploitation of the natural pigment as a bioinspired and biocompatible semiconductor thin film are the solvent-free fabrication protocol, mild reaction conditions and the chemical control of the polymer structure. The resulting films feature remarkable adhesion properties responsible for the film stability to water and the consequent reversibility in water-induced eumelanin electrical property switching. Further, the solid state procedure resulted in the retention of the pigment backbone integrity, avoiding oxidative ring fission and associated loss of aromatic stacking units. The convergence of all these elements provides the groundwork for the actual exploitation of eumelanin based devices in organic electronics. |
Introduction
The implementation of organic interfaces for electrical stimulation of cell-tissue constructs is a central goal in the rapidly expanding fields of bioelectronics and nanomedicine, and is contingent upon the development of biocompatible, easily accessible and tailored organic semiconductors. Among the various nature-inspired materials that hold promise for the design of bioelectronic interfaces, eumelanins, the black insoluble photoprotective biopolymers of human skin and eyes,1–3 occupy a prominent position. Both natural and synthetic eumelanins display peculiar optoelectronic properties,4,5 which are exemplified by water-dependent semiconductor-like behavior,6–8 broad band absorption,9,10 permanent paramagnetism11,12 and efficient non-radiative UV-dissipation.13,14 These features were originally attributed to an amorphous semiconductor structure6,9 but, more recently, they have been explained in terms of a chemical disorder model15 integrated to include a dynamic component of reversible intermolecular interactions perturbing π-electron systems.10,16 Structural disorder in eumelanins derives from their complex biosynthetic origin from the tyrosinase-catalyzed oxidation of tyrosine and/or dopa (Scheme 1). In this process, copolymerization of the various intermediates produced from dopa to 5,6-dihydroxyindole (DHI) metabolites and the post-synthetic oxidative breakdown of monomer units3 generate an entire array of chemically different species.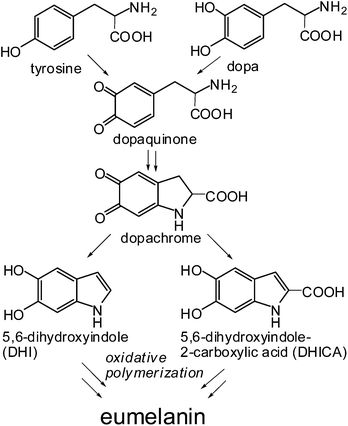 | ||
| Scheme 1 Schematic view of eumelanin synthesis from tyrosine or dopa. Representative intermediates in the process are highlighted. | ||
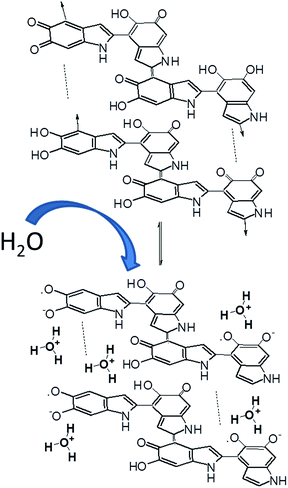
In line with the dynamic chemical disorder model, humidity-dependent electrical properties of eumelanins have been attributed to the peculiar electronic–ionic hybrid conductor behavior,7 in which thermodynamically favorable, water-driven redox equilibria between catechol and quinone species would generate semiquinone moieties17 doping electrons and protons into the system.
In the framework of a basic model to identify eumelanin in its solid-state form as an amorphous semiconductor, it was suggested that the eumelanin dielectric constant increases in the presence of water, thus lowering the activation energy which rules the charge carrier hopping phenomenon.6,7,18
This effect was also considered to justify the occurrence of electrical switchings in eumelanin pellets and films, with the conductivity values changing by many orders of magnitude when the applied electrical field exceeds a threshold value.6,19 More recently, an alternative model was proposed pointing out that eumelanin hydration conditions modify the comproportionation equilibrium, in such a way that the densities of both electrons and protons are considerably increased in the system, thus justifying the observed electrical conductivity change.7
Although further tests are required, it may be conjectured that the role of water is to mediate the formation of hydrogen bonds between parallel sheets of stacked oligomers favoring dynamic equilibration of tautomeric molecular components and charge movement across the film. Water may also affect the local dielectric constant favoring ionization of acid groups within eumelanin constituents (Scheme 2).
Despite a burst of interest in the use of synthetic eumelanins and related biopolymers20–22 for organic electronics and bioelectronics,1,23–26 the implementation of competitive eumelanin-based technology has so far been hindered by several drawbacks. The drawbacks include complete insolubility in all solvents, preventing the development of standardized and reproducible synthetic procedures, and the lack of a solid conceptual frame of structure–property–function relationships. In consequence, eumelanin-based thin films are commonly prepared from structurally ill-defined commercially available materials23,26 or by prolonged alkaline oxidation of dopa27 or dopamine,22 causing severe alkali-induced post-synthetic degradation which adds to the intrinsic structural disorder.20 Although the impact of high molecular heterogeneity on the electrical performances of eumelanins has not been systematically assessed, several critical drawbacks can be anticipated, for example, the lack of well-defined HOMO–LUMO gaps. A heterogeneous composition in terms of structural units is obviously detrimental for effective tailoring of chemical properties and for film quality, an important requisite for cell growth. As a matter of fact, the usual translation of structure–property relationships into rational design rules and effective chemical manipulation strategies to engineer high quality materials has remained a most difficult task in the case of eumelanins.
In this paper, we report remarkable advancements in eumelanin chemistry that surmount most of the abovementioned issues. Disclosed herein are: (a) an original strategy that allowed us to overcome most of the drawbacks limiting the competitiveness in eumelanin-based technology and leading for the first time to high quality thin films with efficient chemical control of structural heterogeneity and complexity; (b) the ability of the new bio-inspired material to be reversibly switched on and off by controlled hydration–dehydration steps; and (c) the efficient growth and differentiation of stem cells seeded on the novel eumelanin thin films.
Eumelanin thin film fabrication
Typical eumelanin thin film fabrication protocols involve direct deposition of the pigment. Because of the unfavorable properties of eumelanin (chiefly insolubility in any solvent3), film fabrication requires a pretreatment of dopa or tyrosine derived eumelanin to gain at least partial solubilization.28 The most commonly adopted aqueous alkaline treatment results in serious pigment backbone modification associated with aromatic ring fission29 and consequent loss of chemically defined properties with the increase of chemical disorder.20Such a scenario appears unwanted in order to achieve chemically defined films, which is a fundamental goal because of the impact of the chemical structure on functional properties.30 Structural control at the molecular scale level, jointly with the knowledge of the chemical–physical properties of the film material, is thus mandatory for rational design of eumelanin-based devices.
The approach we devised allows for turning the intractable nature of eumelanin into advantages.
The rationale that inspired this approach was the use of DHI as the eumelanin precursor in place of commonly used dopa or dopamine, for the following reasons: (1) DHI is soluble in organic solvents and is the ultimate monomer precursor in the pathways of natural and synthetic eumelanins, ensuring the generation of homopolymers rather than copolymers of various intermediates, as in the case of dopa and dopamine melanins;3,11,21 (2) the mode of polymerization of DHI and its dimers and oligomers,17,31–35 the mechanisms of aggregation underlying particle growth,36 and the optical and free radical properties of DHI melanin11,16 have all been clarified in detail; (3) the electrical properties of DHI melanin suspensions have been characterized using an organic-electrochemical transistor;37 (4) DHI melanin can be used to prepare thin films by a variety of methodologies, although their morphological properties are not always of high quality.38,39
The manifold problems associated with the limited processability of insoluble eumelanins were then overcome by rational development of a procedure, referred to as ammonia-induced solid state polymerization (AISSP), which is based on the uniform deposition by spin coating of the soluble DHI monomer as highly homogeneous thin films, followed by solid state polymerization induced by exposure to gaseous ammonia in an air-equilibrated atmosphere (Scheme 3).
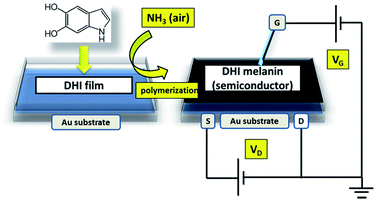 | ||
| Scheme 3 Schematic illustration of the AISSP procedure for DHI melanin film deposition and the FET device setup for the determination of electrical properties of the eumelanin film. | ||
Film fabrication on suitable quartz substrates allowed us to easily follow the polymerization process by UV-vis spectroscopy, observing the spectral evolution with time.
In Fig. 1 the film absorption spectra are reported at several times after AISSP was started. Here, the spectrum of the DHI film is also reported for comparison. From the absorption profile at 60 min reaction time, it is clearly evident how the DHI spectrum has completely evolved into the typical eumelanin profile.10,16,28 Moreover, the clear occurrence of the melanochromic intermediate phase is noteworthy, characterized by a broad, but distinctive, maximum around 540 nm (ref. 40) in the UV-vis plots in the range of 10–30 min.
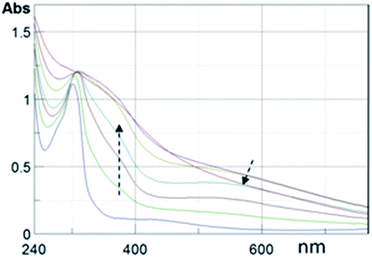 | ||
| Fig. 1 UV-vis profiles of a DHI thin film fabricated by AISSP. Plots are taken at 5, 15, 30, 45, 60 and 180 min after exposition to a polymerization inducing atmosphere. Arrows denote evolution. The UV-vis profile of the DHI film (bottom line) is also reported. See Fig. S1 of ESI† for corresponding images of the films on quartz. | ||
SEM images were also taken in order to check the film compactness and the quality of the adhesion on the substrates. These images (see Fig. S2 of ESI†) demonstrate definitely that eumelanin films obtained by the AISSP procedure are structurally compact, being able to homogeneously cover the substrate surface.
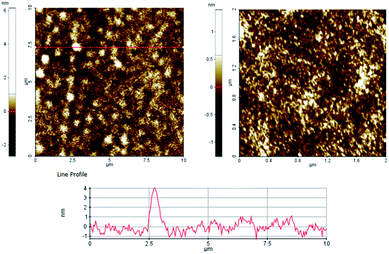
More insights into the morphology of the eumelanin films were gained by acquiring AFM images on different length scales, going from 2 μm × 2 μm to 30 μm × 30 μm (see Fig. 2 and S3 of ESI†). From the AFM images, it becomes clear that the film surface is characterized by a very smooth matrix where the only specific morphological feature is the presence of some columnar structures having heights of a few nm. On a 2 μm × 2 μm scale, the average roughness is found to be lower than 0.3 nm, while it increases up to about 1 nm, when larger scales are considered.
The chemical nature of the DHI melanin films obtained by the AISSP methodology was investigated by matrix-assisted laser desorption ionization (MALDI) mass spectrometry in comparison with a dopa melanin sample, prepared by auto-oxidation in an alkaline medium according to a reported protocol (Fig. 3).7
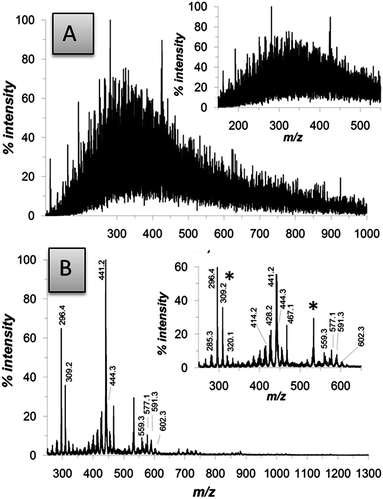 | ||
| Fig. 3 MALDI-MS spectra of eumelanin thin films on a standard MALDI plate. (A) Dopa melanin prepared by alkaline autoxidation.22 (B) DHI melanin prepared by the AISSP methodology. Asterisks denote impurities. See Fig. S2 of ESI† for further details on structural identification. | ||
MALDI-MS data allowed us to appreciate several important chemical and technological advances associated with the AISSP technology. From the chemical viewpoint, when compared with representative films obtained by deposition of preformed eumelanins, such as tyrosinase-catalyzed DHI melanin and autoxidative dopa melanin, AISSP DHI films exhibited greater structural integrity, as evidenced by the regular clusters of peaks centered around the expected pseudomolecular ion peak, and a limited degree of polymerization (the highest oligomer detected at the hexamer–eptamer stage, to be compared with the 30-mer identified in the polymerization mixture of DHI).35
The main structural components accounting for the mass spectrum of the DHI melanin produced by AISSP are illustrated in Fig. S3 of ESI.†
Apparently, ammonia is not incorporated into the chemical structure of the DHI polymer. Both structural integrity (i.e. preservation of indole units and avoiding aromatic ring fission29) and control of polymerization can be attributed to the specific constraints and the lack of water associated with the solid state conditions, limiting water and/or hydroperoxyl anion induced oxidative cleavage of indole units29 and uncontrolled oligomer chain growth.
In contrast, preformed eumelanins are shown by mass spectrometry to be highly heterogeneous as a consequence of disordered polymerization of different units, higher molecular weight dispersion and extensive post-synthetic degradation under the solution conditions of the polymerization processes.
From the technological viewpoint, AISSP thin films proved to be more adhesive (over 3 days in aqueous media without detectable detachment) and smooth. By contrast, films obtained by previously reported techniques exhibited marked surface roughness38 and, most importantly, could be easily detached from the supporting surface by an aqueous medium (not shown). Such worse features can be attributed to the generation of a range of morphologies and to degradative fission of structural units of preformed eumelanins during the harsh synthetic process, decreasing adhesive functionalities of the biopolymer. Indeed, low adhesion and tendency to detach on water exposition was also observed with a smooth film obtained from dopa fabricated according to a reported procedure28 that has been known to provide very smooth surfaces. This was expected, again, on the basis of the structural modification induced by aqueous alkaline treatment and the associated backbone fragmentation and carboxyl group generation with consequent loss in adhesion to water resistance.
Finally, avoiding water and polar solvents in the film fabrication combined with the pigments' backbone structural integrity preservation also allowed us to virtually eliminate the known morphological modifications associated with prolonged electrical biasing.41
Effect of sample hydration on film electrical properties
As experimentally demonstrated in several studies reported in the recent past, the electrical properties of eumelanin films or pellets are largely dominated by the material hydration state.7,12Independent of the physical origins, the possibility to exploit such a hydration dependence in the development of eumelanin-based devices appears quite appealing in technological terms and, undoubtedly, worthy of further investigation.
In our work, the electrical tests were conducted for DHI melanin thin films deposited on multilayer structures composed of a thick (500 μm) highly doped (Si++) substrate, a 200 thin SiO2 insulating barrier and gold pre-patterned electrodes with interdigitated layout (see Fig. S5A and B of ESI†). These Si++/SiO2/Au structures were considered for investigating the possibility to further modulate the charge transport through eumelanin channels connecting the gold electrodes (source–drain contacts) by applying a gate voltage (VG) to the Si++ substrate, as has been commonly done in basic field-effect transistors (FETs). Our measurements, however, indicated clearly the lack of any detectable field-effect response and, hence, the current IDS discussed hereafter is referred to the basic condition with VG = 0 V.
I DS curves were recorded by sweeping the related VDS voltage first between 0 and 50 V and then in the backward direction (from 50 to 0 V), with the aim to highlight the presence of hysteresis phenomena. The exemplificative measurement reported in Fig. S5C† confirms the presence of hysteretic behavior with the IDS values measured in the backward scan being lower than those measured in the forward cycle (clockwise hysteresis). This phenomenon is quite typical of electrically active materials characterized by poor structural order and gives indications of the occurrence of charge trapping phenomena.
For eumelanin layers, in any case, this effect should be associated also with the presence of proton space charge accumulation effects in the regions close to the electrodes.
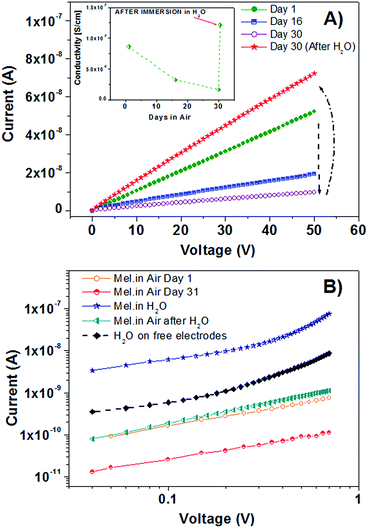
This specific feature will be discussed in detail elsewhere, where a set of dynamic electrical measurements will be reported. Here, indeed, the attention was basically focused on the general capability of AISSP-deposited eumelanin films to carry electrical charges. In this regard, Fig. 4A reports the IDS curves measured only during the forward VDS scan for a sample stored in air within a prolonged period.
From this plot, it is possible to appreciate that the measurements carried out on freshly prepared moisture-equilibrated films exhibited well-defined ohmic behavior (i.e. IDS is a linear function of VDS) with a conductivity (σ) value of about 10−7 S cm−1.
The conducting properties of a pristine eumelanin film were also assessed by the application of continuously repeated IV measurements (Fig. S6 of ESI†). Under this condition, the current flow between the two gold electrodes displayed only a limited (by a few percent) decrease over time. This effect should be basically ascribed to the same charge trapping processes invoked above to justify the hysteretic behavior of these channels. It is worth mentioning, however, that all the repeated IV curves preserve the basic ohmic response.
On the other hand, Fig. 4A shows that, after a prolonged (30 days) standing in air, a gradual drop by about one order of magnitude in conductivity of the same film has occurred. Apparently, the σ lowering was related to the drying process of eumelanin films during the storage in air. In order to confirm this conclusion, eumelanin channels were re-hydrated by covering them with de-ionized water which was left to evaporate for 30 minutes. A final drying with nitrogen gas was carried out to complete the procedure.
As shown, the subsequent IV curve recorded in air confirmed the eumelanin channel capability to recover completely the initial conductivity state. In particular, for these films, several desiccation–hydration cycles (not shown) could be performed, which states the fully reversible character of the charge doping/de-doping process.
After the electrical characterization in air, specific measurements were carried out by keeping the eumelanin channels directly immersed in water. In this case, IV curves were recorded by sweeping VDS voltages between 0 and 0.7 V in order to minimize the occurrence of electrical currents related to the water electrolysis effect.
As reported in Fig. 4B, the IV curve measured for the eumelanin film covered with water displayed considerably higher values than those of the corresponding measurements recorded in air at different times (namely, under different hydration conditions). Moreover, the semi-log plot allows us to clarify that the electrical current flowing through the water-covered eumelanin channel exceeds by about one order of magnitude that flowing between two free gold electrodes immersed in the same amount of de-ionized water.
Investigation of film biocompatibility
In a final series of experiments, we investigated the biocompatibility of the DHI melanin films for potential use as bio-interface for stem cell (Murine Embryonic Stem Cell – ESC) growth.The only previous paper in the field reported the ability of thin films from commercial eumelanin to enhance Schwann cell growth and neurite extension compared to collagen film growth,42 but the compatibility with stem cell43 growth has not yet been reported. This cell population is of particular interest for tissue engineering application and is under scrutiny for compatibility with classical electro-active materials.44
In a series of experiments we addressed the survival and proliferation of undifferentiated ESCs grown on eumelanin films, the preservation of ESC morphology and the occurrence of differentiation.
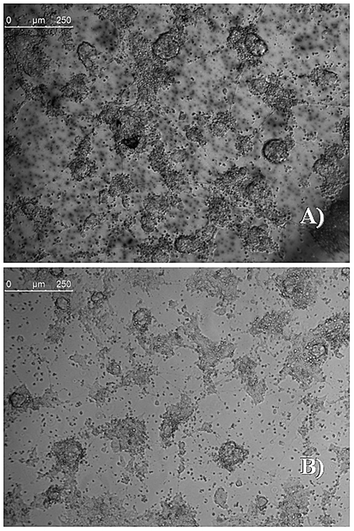
To assess the ability of eumelanin to support the survival of ESCs, undifferentiated ESCs were trypsinized to obtain a single-cell suspension and 6 × 104 cells per cm2 were plated on a 100 mm melanin-coated dish in ESC medium. The medium was changed every day for three days. As shown in Fig. 5 eumelanin coating has supported adhesion and colony formation of ESCs. ESC proliferation was evaluated by counting cells after trypsinization and dissociation at 2 and 4 days in colonies grown on dishes coated with eumelanin or with gelatin (as control). As reported in Fig. S7 of ESI,† the growth curves of ESCs plated on eumelanin show a trend comparable with the same cells plated on gelatin indicating that eumelanin supports ESC proliferation.
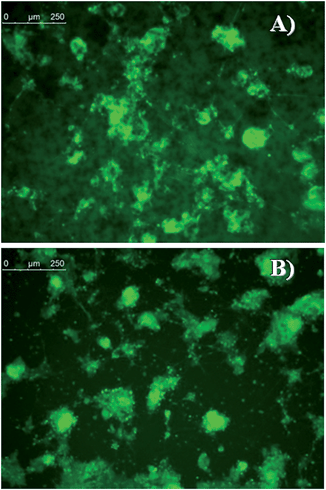
We have also verified that eumelanin does not impair the ESC morphology by staining F-actin. Fig. 6 shows that the structural arrangement of ESCs grown on eumelanin is indistinguishable from those conventionally grown on gelatin. To confirm that the presence of healthy colonies highlighted by the morphological analysis corresponds to the absence of abnormal cell death we have analyzed the level of active caspase-3, an apoptosis hallmark. As shown in Fig. 7, western blot analysis revealed that there is no accumulation of cleaved caspase-3 to the detriment of the uncleaved one that is normally present in healthy cells.
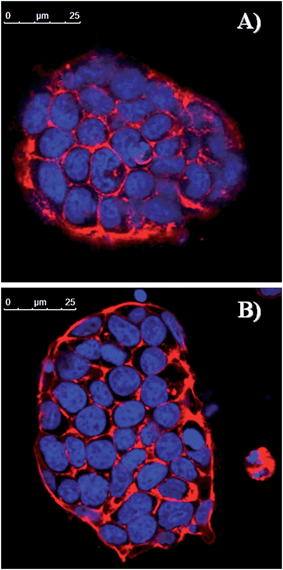 | ||
| Fig. 6 Confocal analysis of undifferentiated ESCs grown for 2 days on (A) melanin or (B) gelatin-coated plates and stained with TRITC-labeled phalloidin that binds to F-actin. Nuclei were counterstained with DRQ5. Scale bars: 25 μm. For single channels see Fig. S8 of ESI.† | ||
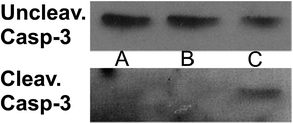 | ||
| Fig. 7 Western blot analysis of uncleaved and cleaved caspase-3. ESCs were grown on (A) gelatin or (B) melanin-coated dishes and collected after 48 hours. The positive control (C) is ESCs exposed to X-rays (15 Gy) to induce apoptosis. See Fig. S9 of ESI† for bar graphs depicting the densitometric analysis. | ||
Since we planned to exploit eumelanin films not only to support ESC growth but also to apply, in future, these substrates to direct the differentiation of pluripotent stem cells into a specific cell fate such as specific neuronal subpopulations (i.e. dopaminergic neurons, motor neurons, etc.), we have tested the ability of melanin to allow neuron formation from undifferentiated ESCs.
To this aim, we have used a reporter cell line bearing Green Fluorescent Protein (GFP) under the control of α1-tubulin promoter.45 Thus, the GFP is expressed only when the ESCs differentiate into neuronal precursor or mature neurons. We have employed two differentiation protocols to verify the versatility of the melanin film.
In the first protocol, we have allowed neuronal differentiation of ESCs by plating them on gelatin or melanin at low density in a chemically defined medium (see Materials and methods in the ESI† and ref. 46). As shown in Fig. 8, after 4 days of differentiation we have found that the differentiation yield reached on eumelanin is comparable to that on gelatin. The presence of both neural precursors and mature neurons with their axonal extensions is visible in both conditions.
In the second differentiation method, we have induced the formation of serum-free embryoid bodies in suspension SFEBs (see Materials and methods and ref. 47) that allow the formation of neuronal precursors and then, upon adhesion to an appropriate substrate, the formation of mature neurons. Fig. S10 of ESI† shows that melanin supports the development of mature neurons from neural precursors derived from SFEBs after 11 days of differentiation.
All together these results suggest that eumelanin is able not only to support normal growth of ESCs without impairing proliferation and survival but, importantly, this substrate is also able to allow the formation of neuronal precursors and neurons starting from undifferentiated ESCs (first differentiation method) and the development of mature neurons from already established neural precursors (second differentiation method).
Conclusions
In light of chemical properties of 5,6-dihydroxyindole, chiefly its high oxidizability, we speculated on the possibility to access eumelanin films circumventing typical difficulties connected with the intractable nature of this pigment and, at the same time, gain a chemical control of the pigment backbone.We have reported herein high quality eumelanin thin films displaying appealing features, such as reversible conductivity by controlled hydration–dehydration steps, excellent biocompatibility with stem cells and water-resistant adhesion, for bioelectronic applications.
The morphology and the chemical uniformity and nature of the eumelanin films may be expected to play a key role in allowing cell cultures. Stem cells are highly sensitive to the environment, and their adhesion and healthy growth on eumelanin films reported here denote not only generic biocompatibility of these films but also their identification as a safe substrate that does not induce apoptosis and capable of supporting differentiation up to 11 days.
Important advantages of the AISSP procedure with respect to previously reported protocols include: (1) efficient chemical control of structural disorder and film morphology, due to the facile deposition of the soluble DHI monomer opening the way to more efficient chemical manipulation and tailoring; (2) robustness and strong adhesion of the resulting film to a variety of substrates, from glass to silicon and plastic polymers; (3) the lack of artifacts associated with post-synthetic work-up procedures; (4) synthetic versatility for controlled polymerization and copolymerization with other co-substrates or for engineering wafer architectures; (5) low environmental impact due to the solvent-free, oxygen-based protocol. Very significantly, the AISSP procedure is of general relevance by being applicable to any easily oxidizable and filmable polyphenolic substrate.
Moreover, in this work, the AISSP technique was applied to various types of substrates. In particular, eumelanin films were deposited on quartz (see Fig. S1 and S11 of ESI†), SiO2, and ITO (indium-tin oxide), and exhibited substantially the same structural and morphological properties. In principle, the AISSP technique has no limitation with respect to the substrate. Indeed eumelanin coating via the AISSP technique is achievable for any substrate (organic as well as inorganic) featuring a low contact angle (below 30°) with a solvent capable of sustaining a DHI concentration of up to 30 mg ml−1.
The properties of the eumelanin films described herein may pave the way for the design and implementation of organic electrochemical transistors (OECT)48,49 featuring full biocompatibility even with highly sensitive stem cell cultures and capable of translating cellular activity into electrical signals.
Acknowledgements
This work was supported by a grant from Italian MIUR (PRIN 2010–2011, 010PFLRJR “PROxi” project), a grant to SP from the Ministry of University and Research for the projects: Futuro in Ricerca 2013 (RBFR13YZ2Y), and was carried out in the frame of the EuMelaNet program (http://www.espcr.org/eumelanet) and the PolyMed project within the FP7-PEOPLE-2013-IRSES framework, PIRSES-GA-2013-612538.Notes and references
- M. d'Ischia, A. Napolitano, A. Pezzella, P. Meredith and T. Sarna, Angew. Chem., Int. Ed., 2009, 48, 3914–3921 CrossRef PubMed.
- P. Meredith, C. J. Bettinger, M. Irimia-Vladu, A. B. Mostert and P. E. Schwenn, Rep. Prog. Phys., 2013, 76, 034501 CrossRef CAS PubMed.
- M. d'Ischia, K. Wakamatsu, A. Napolitano, S. Briganti, J. C. Garcia-Borron, D. Kovacs, P. Meredith, A. Pezzella, M. Picardo, T. Sarna, J. D. Simon and S. Ito, Pigm. Cell Melanoma Res., 2013, 26, 616–633 CrossRef PubMed.
- S. Ito and K. Wakamatsu, J. Eur. Acad. Dermatol. Venereol., 2011, 25, 1369–1380 CrossRef CAS PubMed.
- J. D. Simon, D. Peles, K. Wakamatsu and S. Ito, Pigm. Cell Melanoma Res., 2009, 22, 563–579 CrossRef CAS PubMed.
- J. McGinness, P. Corry and P. Proctor, Science, 1974, 183, 853–855 CAS.
- A. B. Mostert, B. J. Powell, F. L. Pratt, G. R. Hanson, T. Sarna, I. R. Gentle and P. Meredith, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 8943–8947 CrossRef CAS PubMed.
- J. Wunsche, F. Cicoira, C. F. O. Graeff and C. Santato, J. Mater. Chem. B, 2013, 1, 3836–3842 RSC.
- P. Meredith and T. Sarna, Pigm. Cell Res., 2006, 19, 572–594 CrossRef CAS PubMed.
- A. Pezzella, A. Iadonisi, S. Valerio, L. Panzella, A. Napolitano, M. Adinolfi and M. d'Ischia, J. Am. Chem. Soc., 2009, 131, 15270–15275 CrossRef CAS PubMed.
- L. Panzella, G. Gentile, G. D'Errico, N. F. Della Vecchia, M. E. Errico, A. Napolitano, C. Carfagna and M. d'Ischia, Angew. Chem., Int. Ed., 2013, 52, 12684–12687 CrossRef CAS PubMed.
- A. B. Mostert, G. R. Hanson, T. Sarna, I. R. Gentle, B. J. Powell and P. Meredith, J. Phys. Chem. B, 2013, 117, 4965–4972 CrossRef CAS PubMed.
- M. Gauden, A. Pezzella, L. Panzella, M. T. Neves-Petersen, E. Skovsen, S. B. Petersen, K. M. Mullen, A. Napolitano, M. d'Ischia and V. Sundstrom, J. Am. Chem. Soc., 2008, 130, 17038–17043 CrossRef CAS PubMed.
- A. Corani, A. Huijser, A. Iadonisi, A. Pezzella, V. Sundstrom and M. d'Ischia, J. Phys. Chem. B, 2012, 116, 13151–13158 CrossRef CAS PubMed.
- M. L. Tran, B. J. Powell and P. Meredith, Biophys. J., 2006, 90, 743–752 CrossRef CAS PubMed.
- L. Ascione, A. Pezzella, V. Ambrogi, C. Carfagna and M. d'Ischia, Photochem. Photobiol., 2013, 89, 314–318 CrossRef CAS PubMed.
- A. Pezzella, O. Crescenzi, L. Panzella, A. Napolitano, E. J. Land, V. Barone and M. d'Ischia, J. Am. Chem. Soc., 2013, 135, 12142–12149 CrossRef CAS PubMed.
- M. Jastrzebska, A. Kocot, J. K. Vij, J. Zalewska-Rejdak and T. Witecki, J. Mol. Struct., 2002, 606, 205–210 CrossRef CAS.
- P. J. Goncalves, O. Baffa and C. F. O. Graeff, J. Appl. Phys., 2006, 99, 104701–104705 CrossRef.
- N. F. Della Vecchia, R. Avolio, M. Alfe, M. E. Errico, A. Napolitano and M. d'Ischia, Adv. Funct. Mater., 2013, 23, 1331–1340 CrossRef CAS.
- J. Liebscher, R. Mrowczynski, H. A. Scheidt, C. Filip, N. D. Hadade, R. Turcu, A. Bende and S. Beck, Langmuir, 2013, 29, 10539–10548 CrossRef CAS PubMed.
- C. T. Chen, V. Ball, J. J. de Almeida Gracio, M. K. Singh, V. Toniazzo, D. Ruch and M. J. Buehler, ACS Nano, 2013, 7, 1524–1532 CrossRef CAS PubMed.
- M. Ambrico, P. F. Ambrico, A. Cardone, T. Ligonzo, S. R. Cicco, R. Di Mundo, V. Augelli and G. M. Farinola, Adv. Mater., 2011, 23, 3332–3336 CrossRef CAS PubMed.
- M. Ambrico, P. F. Ambrico, A. Cardone, N. F. Della Vecchia, T. Ligonzo, S. R. Cicco, M. M. Talamo, A. Napolitano, V. Augelli, G. M. Farinola and M. d'Ischia, J. Mater. Chem. C, 2013, 1, 1018–1028 RSC.
- M. Ambrico, P. F. Ambrico, A. Cardone, S. R. Cicco, F. Palumbo, T. Ligonzo, R. Di Mundo, V. Petta, V. Augelli, P. Favia and G. M. Farinola, J. Mater. Chem. C, 2014, 2, 573–582 RSC.
- M. Abbas, M. Ali, S. K. Shah, F. D'Amico, P. Postorino, S. Mangialardo, M. Cestelli Guidi, A. Cricenti and R. Gunnella, J. Phys. Chem. B, 2011, 115, 11199–11207 CrossRef CAS PubMed.
- A. B. Mostert, K. J. Davy, J. L. Ruggles, B. J. Powell, I. R. Gentle and P. Meredith, Langmuir, 2010, 26, 412–416 CrossRef CAS PubMed.
- J. P. Bothma, J. de Boor, U. Divakar, P. E. Schwenn and P. Meredith, Adv. Mater., 2008, 20, 3539–3542 CrossRef CAS.
- A. Napolitano, A. Pezzella, M. R. Vincensi and G. Prota, Tetrahedron, 1995, 51, 5913–5920 CrossRef CAS.
- R. Noriega, J. Rivnay, K. Vandewal, F. P. V. Koch, N. Stingelin, P. Smith, M. F. Toney and A. Salleo, Nat. Mater., 2013, 12, 1037–1043 CrossRef PubMed.
- A. Pezzella, L. Panzella, A. Natangelo, M. Arzillo, A. Napolitano and M. d'Ischia, J. Org. Chem., 2007, 72, 9225–9230 CrossRef CAS PubMed.
- A. Pezzella, L. Panzella, O. Crescenzi, A. Napolitano, S. Navaratman, R. Edge, E. J. Land, V. Barone and M. d'Ischia, J. Am. Chem. Soc., 2006, 128, 15490–15498 CrossRef CAS PubMed.
- M. D'Ischia, A. Napolitano, A. Pezzella, E. J. Land, C. A. Ramsden and P. A. Riley, Adv. Heterocycl. Chem., 2005, 89, 1–63 CrossRef.
- L. Panzella, A. Pezzella, A. Napolitano and M. d'Ischia, Org. Lett., 2007, 9, 1411–1414 CrossRef CAS PubMed.
- S. Reale, M. Crucianelli, A. Pezzella, M. d'Ischia and F. De Angelis, J. Mass Spectrom., 2012, 47, 49–53 CrossRef CAS PubMed.
- M. Arzillo, G. Mangiapia, A. Pezzella, R. K. Heenan, A. Radulescu, L. Paduano and M. d'Ischia, Biomacromolecules, 2012, 13, 2379–2390 CrossRef CAS PubMed.
- G. Tarabella, A. Pezzella, A. Romeo, P. D'Angelo, N. Coppede, M. Calicchio, M. d'Ischia, R. Mosca and S. Iannotta, J. Mater. Chem. B, 2013, 1, 3843–3849 RSC.
- F. Bloisi, A. Pezzella, M. Barra, M. Alfe, F. Chiarella, A. Cassinese and L. Vicari, Appl. Phys. A: Mater. Sci. Process., 2011, 105, 619–627 CrossRef CAS.
- F. Bloisi, A. Pezzella, M. Barra, F. Chiarella, A. Cassinese and L. Vicari, J. Appl. Phys., 2011, 110, 26105–26108 CrossRef.
- G. Prota, Med. Res. Rev., 1988, 8, 525–556 CrossRef CAS PubMed.
- J. Wunsche, L. Cardenas, F. Rosei, F. Cicoira, R. Gauvin, C. F. O. Graeff, S. Poulin, A. Pezzella and C. Santato, Adv. Funct. Mater., 2013, 23, 5591–5598 CrossRef.
- C. J. Bettinger, P. P. Bruggeman, A. Misra, J. T. Borenstein and R. Langer, Biomaterials, 2009, 30, 3050–3057 CrossRef CAS PubMed.
- M. R. Alison, R. Poulsom, S. Forbes and N. A. Wright, J. Pathol., 2002, 197, 419–423 CrossRef PubMed.
- I. N. Wang, J. T. Robinson, G. Do, G. Hong, D. R. Gould, H. Dai and P. C. Yang, Small, 2014, 10, 1479–1484 CrossRef CAS PubMed.
- S. Parisi, F. Passaro, L. Aloia, I. Manabe, R. Nagai, L. Pastore and T. Russo, J. Cell Sci., 2008, 121, 2629–2634 CrossRef CAS PubMed.
- Q. L. Ying, M. Stavridis, D. Griffiths, M. Li and A. Smith, Nat. Biotechnol., 2003, 21, 183–186 CrossRef CAS PubMed.
- S. Parisi, M. Battista, A. Musto, A. Navarra, C. Tarantino and T. Russo, FASEB J., 2012, 26, 3957–3968 CrossRef CAS PubMed.
- D. Khodagholy, J. Rivnay, M. Sessolo, M. Gurfinkel, P. Leleux, L. H. Jimison, E. Stavrinidou, T. Herve, S. Sanaur, R. M. Owens and G. G. Malliaras, Nat. Commun., 2013, 4, 2133 Search PubMed.
- M. Bongo, O. Winther-Jensen, S. Himmelberger, X. Strakosas, M. Ramuz, A. Hama, E. Stavrinidou, G. G. Malliaras, A. Salleo, B. Winther-Jensen and R. M. Owens, J. Mater. Chem. B, 2013, 1, 3860–3867 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental methods and film characterization. See DOI: 10.1039/c4mh00097h |
| This journal is © The Royal Society of Chemistry 2015 |