DOI:
10.1039/C5MD00079C
(Concise Article)
Med. Chem. Commun., 2015,
6, 1093-1103
Received
23rd February 2015
, Accepted 14th April 2015
First published on 20th April 2015
1. Introduction
Two subtypes of sigma (σ) receptors, termed σ1 and σ2, have been identified.1 Both subtypes display different distributions in the central nervous system and peripheral organs. The σ1 receptor contains 223 amino acids with two transmembrane regions.2,3 It functions as “ligand-operated receptor chaperone” and regulates various ion channels, G protein-coupled receptors, lipids, and other signalingproteins.4,5 In contrast, the σ2 receptor has not been cloned so far, and its molecular weight was estimated to be 21.5 kD. Recently, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSprogesterone receptor membrane component 1 (PGRMC1) was reported as the putative σ2receptor binding site.6
It is interesting that both subtypes are expressed in a variety of human and rodent tumor cell lines.7,8 However, the expression of the σ2 receptor was found to be higher than that of the σ1 receptor. In proliferating tumor cells, the density of the σ2 receptor was about 8- to 10-fold higher than that in quiescent tumor cells.9–11 Moreover, σ2 receptor ligands can rapidly internalize into tumor cells and activate apoptosis via multiple pathways.12–15 Thus, the σ2 receptor may both serve as a receptor-based biomarker to distinguish different proliferative states of solid tumors and as a promising target for the treatment of cancer.16
In the past decades, morphans, indoles (siramesine analogues), granatanes, flexible benzamides and N-cyclohexylpiperazines have been reported to serve as selective σ2 receptor ligands.17 Among these ligands, siramesine (also known as Lu-28-179) and its analogues, conformationally flexible amines such as RHM-1, and PB28 analogues were more extensively investigated.17 Their structures are presented in Fig. 1. Although clinical trials of siramesine for the treatment of depression and anxiety were paused in 2002, it proved to be non-toxic and well tolerated in humans. Most importantly, siramesine was demonstrated to induce cell death in many tumorigenic and immortalized cells via different apoptotic pathways.12–14,18 To obtain selective σ2 receptor ligands with antiproliferative activity, we used siramesine as the lead compound to design a series of novel COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSindole-based compounds. It was reported that the indole residue and the butyl chain between the indole and the spirocyclic piperidine moieties were important to maintain the σ2 receptor selectivity for siramesine derivatives.17 We introduced different functional groups to develop new σ2 receptor ligands. Moreover, we also introduced the substituents with fluorineatom to find PET radiotracers for σ2 receptor tumor imaging. The design concept is shown in Fig. 2. First, by keeping the 4-fluorophenyl ring at the indole N-atom and the butyl chain constant, 3H-spiro(2-benzofuran-1,4′-piperidinyl) moiety was replaced by different pharmacophores including σ1 preferred group C and σ2 preferred group A, B, or D (1). Secondly, the 4-fluorophenyl ring at the indole N-atom was replaced by a 2-fluoroalkyl group (2). As a third approach, both the 4-fluorophenyl ring at the indole N-atom and the 3H-spiro(2-benzofuran-1,4′-piperidinyl) moiety were modified (3). Fourth, 4-fluorophenyl was replaced by the 4-iodophenyl group, while the 3H-spiro(2-benzofuran-1,4′-piperidinyl) moiety was replaced by C (4). Finally, the indole core was replaced by 4-fluoro-benzophenone (5). Moreover, the structure–affinity relationships (SAR) of these analogues for σ2 receptors were analyzed. The 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to investigate the antiproliferative activity of the most potent ligands. In order to further support this, cell cycle analysis was carried out to examine the effects of these potent compounds on the cell cycle progression using flow cytometry in DU145 cells.
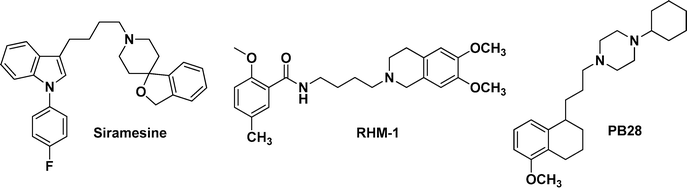 |
| | Fig. 1 The structures of siramesine, RHM-1 and PB28. | |
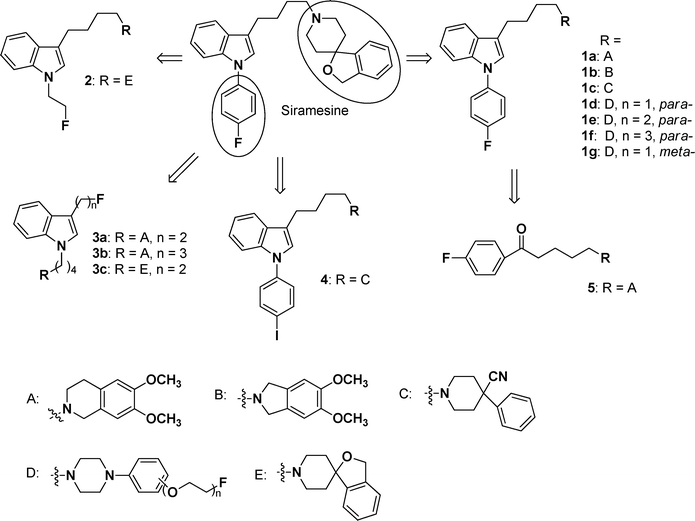 |
| | Fig. 2 Design concept of the indole-based compounds. | |
2. Results and discussion
2.1 Chemistry
The synthetic routes of fluorophenylindole derivatives 1a–1g are depicted in Scheme 1. All compounds in this series were prepared from the key bromobutyl derivative 6. Compounds 6,1912,201520 and 1921 were synthesized according to the method reported in the literature. Compound 7 or 8 reacted with intermediates 9–11 under basic conditions, followed by deprotection to obtain compounds 12–15 with yields of 56–95%. N-Alkylation of 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (16), 5,6-dimethoxyisoindoline (17), 4-phenylpiperidine-4-carbonitrile (18) or 12–15 with compound 6 provided compounds 1a, 1b, 1c, or 1d–1g, respectively, with yields of 29–84%.
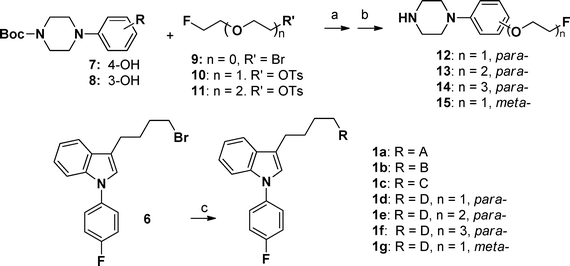 |
| | Scheme 1
Synthesis of fluorophenylindole derivatives 1a–1g. Reagents and conditions: (a) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3, NaI, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF, 105 °C, overnight, 64–92%; (b) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTFA, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2, 0 °C, 2 h; (c) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3, NaI, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN, 80 °C, 4 h, for 1a, 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (16), 52%; for 1b, 5,6-dimethoxyisoindoline (17), 52%; for 1c, 4-phenylpiperidine-4-carbonitrile (18), 42%; for 1d–1g, 12–15, 16–83%. | |
The synthetic route of compound 2 is presented in Scheme 2. Protection of compound 19 with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTBDMS chloride, followed by N-alkylation of compound 20 with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS2-bromoethanol and tosylation of compound 21 with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSp-TsCl provided compound 22. Deprotection of TBDMS and fluorination of compound 22 with TBAF by a one-pot reaction afforded compound 23 with yield of 56%. Tosylation of compound 23 led to compound 24, which reacted with 3H-spiro[2-benzofuran-1,4′-piperidine] (25) to obtain target compound 2.
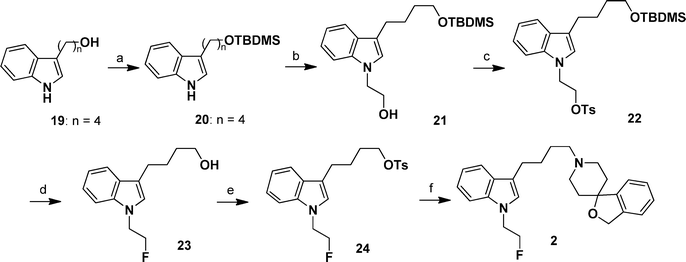 |
| | Scheme 2 Synthetic route of compound 2. Reagents and conditions: (a) TBDMSCl, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSimidazole, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2, r.t., 2 h, 84%; (b) Ar atmosphere, 110 °C, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS2-bromoethanol, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNaH, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF, overnight, 37%; (c) p-TsCl, DIPEA, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMAP, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTHF, r.t., 2 h, 18%; (d) TBAF, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTHF, r.t., overnight, 56%; (e) p-TsCl, DIPEA, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMAP, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTHF, r.t., 2 h, 44%; (f) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3, NaI, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN, 3H-spiro[2-benzofuran-1,4′-piperidine] (25), 80 °C, 4 h, 38%. | |
The synthetic routes of compounds 3a–3c are depicted in Scheme 3. Reduction of indole-3-acetic acid (26) and indole-3-propanoic acid (27) with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSLiAlH4 gave the corresponding alcohols28 and 29 in 86% and 79% yield, respectively. Alkylation with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS1,4-dibromobutane yielded 30 and 31, followed by fluorination with DAST to obtain 32 and 33. Finally, compound 32 or 33 reacted with intermediate 16 to provide 3a and 3b, respectively. Compound 32 reacted with 25 to obtain 3c.
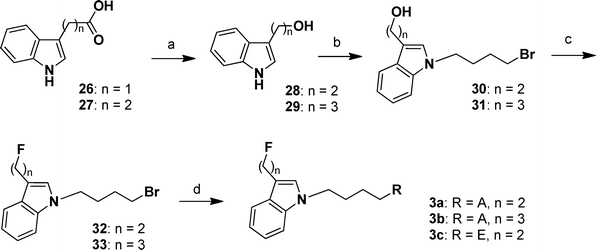 |
| | Scheme 3 Synthetic routes of compounds 3a–3c. Reagents and conditions: (a) Ar atmosphere, 0 °C, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSLiAlH4, anhydrous COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTHF, 4 h, 86% for 28, 79% for 29; (b) Ar atmosphere, 110 °C, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS1,4-dibromobutane, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNaH, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF, overnight, 19% for 30, 34% for 31; (c) Ar atmosphere, −78 °C, DAST, anhydrous COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2, 2 h, 58% for 32, 87% for 33; (d) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3, NaI, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN, 80 °C, 4 h, for 3a, 32, 16, 43%; for 3b, 33, 16, 36%; for 3c, 32, 25, 41%. | |
The synthetic routes of compounds 4 and 5 are depicted in Scheme 4. Synthesis of compound 4 was similar to that of compound 1c. Ullmann reaction between compound 19 and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS1,4-diiodobenzene22 instead of the 4-fluorophenyl residue provided 34 which was subsequently treated with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSPBr3 to yield 35. Finally, reaction between 35 and intermediate 18 provided target compound 4. N-Alkylation of intermediate 16 with 5-bromo-1-(4-fluorophenyl)pentan-1-one (36) gave compound 5 with yield of 62%.
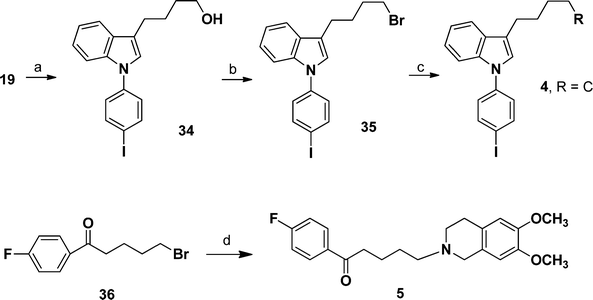 |
| | Scheme 4 Synthetic routes of compounds 4 and 5. Reagents and conditions: (a) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS1,4-diiodobenzene, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTScopper powder, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF, 120 °C, 5 h, 23%; (b) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSPBr3, anhydrous COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2, 0 °C, 2 h, 58%; (c) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3, NaI, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN, 18, 80 °C, 4 h, 49%. (d) COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3, NaI, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN, 16, 80 °C, 4 h, 62%. | |
2.2
In vitro radioligand competition studies and structure–affinity relationship analyses
The affinities of the indole-based analogues for the σ1 and σ2 receptors were determined by radioligand competition binding assays as reported previously.23(+)-[3H]Pentazocine and [3H]1,3-di-o-tolyl-guanidine (in the presence of 10 μM dextrallorphan) were used as radioligands for the σ1 and σ2 receptors, respectively. The results are listed in Table 1.
| Compound |
K
i(σ1) (nM) |
K
i(σ2) (nM) |
K
i(σ1)/Ki(σ2) |
|
Values are means ± standard deviation (SD) of three experiments performed in triplicate.
From ref. 25.
IC50 value, from ref. 24.
From ref. 27.
|
|
1a
|
530.8 ± 181.1 |
49.2 ± 11.7 |
10.8 |
|
1a
b
|
1390 ± 20 |
5.34 ± 1.22 |
260 |
|
1b
|
255.6 ± 14.8 |
53.8 ± 1.4 |
4.8 |
|
1c
|
2.58 ± 0.82 |
3.03 ± 0.75 |
0.9 |
|
1d
|
614 ± 137 |
68.0 ± 0.04 |
9.0 |
|
1e
|
1110 ± 252 |
458 ± 51 |
2.4 |
|
1f
|
2158 ± 404 |
1879 ± 11 |
1.1 |
|
1g
|
257 ± 62.8 |
48.4 ± 2.65 |
5.3 |
|
2
|
246 ± 59.4 |
44.0 ± 28.1 |
5.6 |
|
3a
|
493.5 ± 84.1 |
27.5 ± 0.7 |
17.9 |
|
3b
|
262.5 ± 62.9 |
28.5 ± 4.9 |
9.2 |
|
3c
|
11.0 ± 0.5 |
29.8 ± 1.6 |
0.4 |
|
4
|
386 ± 94 |
18.5 ± 5.7 |
20.9 |
|
5
|
16.6 ± 1.1 |
12.4 ± 0.6 |
1.3 |
| Siramesine |
4.69 ± 2.36 |
3.08 ± 0.68 |
1.5 |
| Siramesineb |
10.5 ± 2.6 |
12.6 ± 0.1 |
0.8 |
| Siramesinec |
17 |
0.12 |
140 |
| ISO-1 |
102.3 ± 15.1 |
28.2 ± 0.9 |
3.6 |
| ISO-1d |
330 ± 25 |
6.95 ± 1.63 |
47.5 |
It was reported that siramesine possessed a subnanomolar affinity (IC50(σ2) = 0.12 nM) and high subtype selectivity (IC50(σ1) = 17 nM, IC50(σ1)/IC50(σ2) = 140) for σ2 receptors.24 More recently, Niso et al. revealed its high affinity (Ki(σ2) = 12.6 nM) and low subtype selectivity (Ki(σ1)/Ki(σ2) = 0.83).25 For comparison, we also synthesized this compound and our sample showed nanomolar affinity (Ki(σ2) = 3.08 nM) and low subtype selectivity (Ki(σ1)/Ki(σ2) = 1.52), which is in good agreement with that reported by Niso et al.
Keeping the 4-fluorophenyl ring at the indole N-atom and the butyl chain constant, replacement of 3H-spiro(2-benzofuran-1,4′-piperidinyl) moiety with σ1 preferred group C retained the low nanomolar affinity and non-selectivity for σ2 receptors (1cvs. siramesine). On the other hand, replacement with σ2 preferred group A, B, or D decreased the affinity for σ2 receptors but increased the subtype selectivity. Compounds 1a, 1b, 1d and 1g showed moderate affinity (Ki(σ2) = 48.4–68 nM) and increased selectivity (Ki(σ1)/Ki(σ2) = 4.8–10.7) compared to siramesine. Compound 1d with substitution at the para-position of the phenyl ring in group D showed comparable affinity to compound 1g with substitution at the meta-position but had a somewhat higher subtype selectivity. Substitution at the para-position with an increased length of the fluorooligoethoxylated chain (n = 2, 3) dramatically decreased the affinities for σ1 and σ2 receptors (1e and 1f). In the literature, compound 1a was reported to possess nanomolar affinity (Ki(σ2) = 5.34 nM) and high subtype selectivity (Ki(σ1)/Ki(σ2) = 260) for σ2 receptors.25 However, our sample displayed only moderate affinity (Ki(σ2) = 49.2 nM) and selectivity (Ki(σ1)/Ki(σ2) = 10.8). The above discrepancy may result from the different experimental conditions employed by different groups. It is interesting to note that compound 1b with the 5,6-dimethoxyisoindoline moiety displayed comparable affinity and selectivity to compound 1a. In the literature, 5-bromo-N-[4-(5,6-dimethoxyisoindolin-2-yl)butyl]-2,3-dimethoxybenzamide (Ki(σ2) = 0.82 nM) was found to possess ten-fold higher affinity for σ2 receptors compared to 5-bromo-N-{4-[6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl]butyl}-2,3-dimethoxybenzamide (Ki(σ2) = 8.2 ± 1.4 nM).26 These data indicate that the 5,6-dimethoxyisoindoline moiety is a promising σ2 preferred group with less lipophilicity.
To evaluate a suitable approach for future fluorine-18 radiotracer development for imaging of σ2 receptors by positron emission tomography, a fluoroalkyl group was introduced. Replacement of the 4-fluorophenyl ring at the indole N-atom with a 2-fluoroethyl residue decreased the affinity for σ2 receptors but slightly increased the subtype selectivity (2vs. siramesine). Compounds 3a and 3b with the σ2 preferred group A displayed comparable affinity for σ2 receptors to ISO-1.27 However, compound 3b with a 3-fluoropropyl group showed decreased selectivity in comparison to compound 3a with a 2-fluoroethyl group. Replacement of σ2 preferred group A with 3H-spiro(2-benzofuran-1,4′-piperidinyl) moiety increased the σ1 affinity significantly and thus decreased the selectivity (3cvs.3a). Moreover, replacement of 1-(4-fluorophenyl) moiety with 1-(4-iodophenyl) group slightly decreased the affinity for σ2 receptors but increased the selectivity (4vs.1c). Replacement of the whole indole moiety with 1-(4-fluorophenyl)carbonyl group dramatically increased the affinity for σ1 receptors (5vs.1a, 5vs.3a, 5vs.3b), indicating the high importance of the indole moiety to retain the selectivity for σ2 receptors.
2.3 Antiproliferative activity
Recently, a series of compounds with the indole moiety were reported to display antiproliferative activity in MCF7 and MCF7/adr cells.25 In order to find new scaffolds and new σ2 receptor ligands as potent antitumor agents, antiproliferative activity of compounds 1a and 1b was evaluated in MCF7 (breast cancer), DU145 (androgen-independent human prostate cancer) and C6 (rat glioma) cells using the MTT assay. Antiproliferative activity of siramesine was also determined in these cells as comparison. The effects of these compounds on cellular viability were analyzed using different concentrations between 100 nM and 100 μM. The results expressed as EC50 values are shown in Table 2. All EC50 values were found to be in the micromolar range. Compound 1a and siramesine showed notable antiproliferative effects in MCF7 cells with EC50 values of 20.9 and 23.6 μM, respectively, which are consistent with that reported in the literature (with EC50 values of 17.8 and 12.3 μM, respectively).25 It is interesting to note that the new compound 1b exhibited the highest activity in MCF7 cells. Moreover, compound 1b displayed notable and comparable antiproliferative effects to compound 1a and siramesine in DU145 cells. However, all of the three compounds displayed a higher EC50 value in C6 cells than those in the human DU145 and MCF7 tumor cells. Besides compound 1a and siramesine, the indole-based compound 1b with the 5,6-dimethoxyisoindoline moiety seems to be promising as an anti-tumor agent and warrants further evaluation.
Table 2 EC50 values of compounds 1a and 1b in different tumor cellsa
|
Cell lines |
EC50 (μM) |
|
1a
|
1b
|
Siramesine |
|
Values are means ± standard deviation (SD) of two to three experiments performed in triplicate.
From ref. 25.
|
| MCF7 |
20.9 ± 6.3 |
17.0 ± 6.5 |
23.6 ± 7.8 |
| MCF7b |
17.8 ± 0.4 |
— |
12.3 ± 0.6 |
| DU145 |
28.8 ± 3.9 |
26.9 ± 6.9 |
13.9 ± 0.7 |
| C6 |
76.5 ± 4.6 |
44.1 ± 9.9 |
43.1 ± 6.2 |
To further examine the antitumor activity of compounds 1a and 1b, their effects on the cell cycle progression were analyzed by flow cytometry in DU145 cells. Cell cycle phase distribution in control DU145 cells and cells treated with different concentrations of 1a, 1b and siramesine at 24 h time point is presented in Fig. 3. The percentages of G1, S and G2 phases of the untreated DU145 cells (control) are 58.2%, 38.6% and 3.25%, respectively. Treatment with compound 1a or 1b or siramesine increased the percentage of G1 cells in a dose-dependent manner. After treatment with 40 μM 1a or 30 μM 1b, the percentages of G1 cells increased to 84.1% and 80.5%, respectively. At the same time, the percentages of S cells decreased to 15.9% and 19.3%, respectively. The percentage of G1 phase cells was maintained at 75.7–77.2% after treatment with 15 to 25 μM siramesine. These data suggest that compounds 1a and 1b and siramesine could induce cell cycle delay and arrest the cell cycle progression predominantly at the G1 phase in DU145 cells. It was reported that σ2 ligands can induce the tumor cell death by multiple signaling pathways.15 10 μM siramesine decreased the expression levels of cyclin D1, which are responsible for progression through the G1 phase in MDA-MB-435 cells in a time-dependent manner. Thus, siramesine may block G1-phase progression by decreasing cyclin D1 expression. In addition, siramesine also mainly decreased cyclin B1 and pRb in MDA-MB-435 cells. The investigation of the detailed mechanism in which compounds 1a and 1b impair the G1 phase of the cell cycle progression in DU145 cells is in progress.
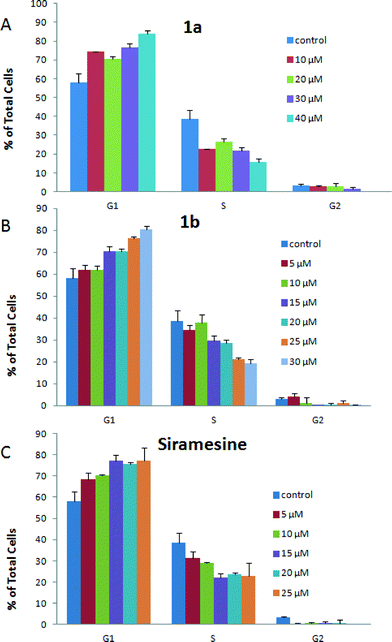 |
| | Fig. 3
Cell cycle phase distribution in control DU145 cells and cells treated with different concentrations of 1a (A), 1b (B) and siramesine (C) at 24 h time point. | |
3. Conclusion
We have developed a series of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSindole-based σ2 receptor ligands derived from siramesine. Structure–affinity relationship analyses indicated the high importance of the indole moiety and σ2 preferred group to improve the selectivity for σ2 receptors. In the MTT experiments, compound 1b displayed notable and comparable antiproliferative effects to compound 1a and siramesine in DU145 cells and exhibited the highest activity in MCF7 cells. Cell cycle analysis by flow cytometry demonstrated that compounds 1a, 1b and siramesine impaired the cell cycle progression predominantly at the G1 phase in DU145 cells. The indole-based compound 1b with the 5,6-dimethoxyisoindoline moiety shows potential as an antitumor agent and warrants further evaluation.
4. Experiments
4.1. Chemistry
All the chemicals or reagents were purchased from chemical suppliers and used without further purification unless otherwise noted. NMR spectra were recorded on a Varian Inova-400 spectrometer or on a Bruker Avance III NMR spectrometer at 400 (1H), 376 (19F), and 100 MHz (13C), respectively. The chemical shifts of the spectra were reported in parts per million (ppm) with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTStetramethylsilane (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTMS) as an internal standard, and coupling constants are reported in Hertz (Hz). MSspectra were obtained using a Xevo TQ-S spectrometer (Waters) with electrospray (ESI) as the ionization method. High-resolution mass spectrometry (HRMS) was performed on a LCT Premier XE ESI-TOF mass spectrometry instrument (Waters, USA). Chromatographic separations were carried out using Merck Silica Gel 60 (63–200 μm). TLC detections were carried out using Merck Silica Gel 60 F254 sheets and TLCs were developed by visualization under UV light (λ = 254 nm). Microanalyses were carried out using a Hekatech CHNS elemental analyser EuroEA 3000 or an Elementar 240C device (PerkinElmer). The HPLC analyses were performed using an AGILENT 1100 HPLC (Agilent Technologies, USA) equipped with a DAD detector. Analyses were carried out using a Nucleodur C18 ISIS column (250 × 4 mm, 5 μm, Macherey-Nagel, Germany) with an eluent of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSacetonitrile/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSH2O (0.1% COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTFA) (30 : 70) at a flow rate of 0.5 mL min−1. Cell cycle analysis was performed using a BD FACSCalibur flow cytometer (BD Biosciences, California, USA), and DNA distributions were analyzed using Modfit LT MacIntel (Verity Software House, Topsham, ME, USA).
4.1.1.
tert-Butyl 4-{4-[2-(2-fluoroethoxy)ethoxy]phenyl}piperazine-1-carboxylate (Boc-13).
Compounds 7 (168 mg, 0.60 mmol) and 10 (190 mg, 0.72 mmol) were dissolved in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN (25 mL), followed by addition of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (124 mg, 0.90 mmol) and NaI (27 mg, 0.18 mmol). The mixture was heated under reflux and stirred overnight. After cooling and filtration, the solvent was removed under reduced pressure. The residue was purified by silica gelchromatography (PE (petroleumether) : EE (ethyl acetate) = 1 : 1) to afford Boc-13 (203 mg, 92%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 6.92–6.80 (m, 4H), 4.59 (dt, J = 47.7, 4.2 Hz, 2H), 4.10 (t, J = 5.8 Hz, 2H), 3.86 (t, J = 5.0 Hz, 2H), 3.82 (dt, J = 29.8, 4.2 Hz, 2H), 3.57 (t, J = 5.0 Hz, 4H), 3.00 (t, J = 4.8 Hz, 4H), 1.48 (s, 9H).
4.1.2.
tert-Butyl 4-(4-{2-[2-(2-fluoroethoxy)ethoxy]ethoxy}phenyl)piperazine-1-carboxylate (Boc-14).
The procedure described for the synthesis of Boc-13 was applied to compounds 7 (152 mg, 0.55 mmol) and 11 (188 mg, 0.61 mmol) to afford Boc-14 (171 mg, 75%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 6.91–6.80 (m, 4H), 4.57 (dt, J = 47.7, 4.2 Hz, 2H), 4.09 (t, J = 4.8 Hz, 2H), 3.84 (t, J = 4.8Hz, 2H), 3.81–3.70 (m, 6H), 3.57 (t, J = 5.0 Hz, 4H), 3.00 (t, J = 4.8Hz, 4H), 1.48 (s, 9H).
4.1.3.
tert-Butyl 4-[3-(2-fluoroethoxy)phenyl]piperazine-1-carboxylate (Boc-15).
The procedure described for the synthesis of Boc-13 was applied to compounds 8 (414 mg, 1.49 mmol) and 9 (188 mg, 2.90 mmol) to afford Boc-15 (311 mg, 64%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.18 (t, J = 8.2 Hz, 1H), 6.57 (d, J = 8.2 Hz, 1H), 6.51 (s, 1H), 6.44 (d, J = 8.1 Hz, 1H), 4.74 (dt, J = 47.4, 4.1 Hz, 2H), 4.20 (dt, J = 27.9, 4.1 Hz, 2H), 3.57 (t, J = 4.7 Hz, 4H), 3.14 (t, J = 4.4 Hz, 4H), 1.48 (s, 9H).
4.2. General procedure for the syntheses of 1a–1g
3-(4-Bromobutyl)-1-(4-fluorophenyl)-1H-indole (6) and the respective amine (12–18) were dissolved in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN, followed by addition of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3. The mixture was heated under reflux and stirred overnight. After cooling and filtration, the solvent was removed under reduced pressure. The residue was purified by silica gelchromatography (PE : EE = 1 : 1) to afford 1a–1g.
4.2.1.
2-{4-[1-(4-Fluorophenyl)-1H-indol-3-yl]butyl}-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (1a).
The synthesis of 1a was similar to that reported in the literature.25 3-(4-Bromobutyl)-1-(4-fluorophenyl)-1H-indole (18) (321 mg, 0.92 mmol), 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (5) (176 mg, 0.77 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (233 mg, 1.69 mmol) dissolved in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN (25 mL) afforded 1a (185 mg, 44%) as light-yellow oil. 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.88–7.66 (m, 1H), 7.50–7.40 (m, 3H), 7.28–7.13 (m, 4H), 7.10 (s, 1H), 6.60 (s, 1H), 6.52 (s, 1H), 3.85 (s, 3H), 3.84 (s, 3H), 3.57 (s, 2H), 2.90–2.80 (m, 4H), 2.72 (t, J = 5.9 Hz, 2H), 2.62–2.54 (m, 2H), 1.89–1.82 (m, 2H), 1.82–1.70 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 160.7, 147.5, 147.2, 136.3, 136.0, 128.9, 126.1, 125.8, 125.7, 125.1, 122.4 (2C), 119.7, 119.3, 117.7, 116.3 (2C), 111.3, 110.1, 109.5, 58.1, 55.9 (2C), 55.7, 51.0, 28.5, 27.9, 27.1, 24.9; 19F NMR (376 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ –116.0; MS (ESI+): m/z = calcd. for C29H31FN2O2 [M + H]+ 459.2, found 459.0; HRMS (EI): m/z calcd. for C29H31FN2O2 [M + H]+ 459.2448, found 459.2441. Anal. calcd. for C29H31FN2O2·3/4H2O (472.08): C 73.78, H 6.94, N 5.93; found: C 73.52, H 6.83, N 5.74.
4.2.2.
3-[4-(5,6-Dimethoxyisoindolin-2-yl)butyl]-1-(4-fluorophenyl)-1H-indole (1b).
Compounds 6 (233 mg, 0.67 mmol) and 17 (119 mg, 0.67 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (144 mg, 1.04 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN (25 mL) afforded 1b as brown solid (156 mg, 52%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.65–7.69 (m, 1H), 7.49–7.41 (m, 3H), 7.25–7.15 (m, 4H), 7.11 (s, 1H), 6.74 (s, 2H), 3.91 (s, 4H), 3.86 (s, 6H), 2.87 (t, J = 7.4 Hz, 2H), 2.79 (t, J = 7.4 Hz, 2H), 1.92–1.83 (m, 2H), 1.78–1.67 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 160.7, 148.4 (2C), 136.3, 136.0, 131.6 (2C), 128.9, 125.8, 125.1, 122.4 (2C), 119.7, 119.3, 117.8, 116.3 (2C), 110.1, 106.8 (2C), 59.2 (2C), 56.1 (2C), 28.9, 27.8 (2C), 24.9; 19F NMR (376 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ –116.0; MS (ESI+): m/z = calcd. for C28H29FN2O2 [M + H]+ 445.2, found 445.2; HRMS (EI): m/z calcd. for C28H29FN2O2 [M + H]+ 445.2291, found 445.2296. Anal. calcd. for C28H29FN2O2 (444.54): C 75.65, H 6.58, N 6.30; found: C 75.14, H 6.62, N 6.30.
4.2.3. 1-{4-[1-(4-Fluorophenyl)-1H-indol-3-yl]butyl}-4-phenylpiperdine-4-carbonitrile (1c).
Compounds 6 (121 mg, 0.35 mmol) and 18 (89 mg, 0.48 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (483 mg, 3.5 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN (25 mL) afforded 1c as light-yellow oil (66 mg, 42%). 1H NMR (400 MHz, MeOD) δ 7.52 (d, J = 7.7 Hz, 1H), 7.44–7.36 (m, 4H), 7.36–7.27 (m 3H), 7.25–7.20 (m, 1H), 7.19–7.10 (m, 3H), 7.09–7.04 (m, 1H), 7.03–6.98 (m, 1H), 2.94 (d, J = 12.1 Hz, 2H), 2.75 (t, J = 7.3 Hz, 2H), 2.39 (t, J = 7.7 Hz, 2H), 2.36–2.26 (m, 2H), 1.98 (t, J = 7.7 Hz, 4H), 1.75–1.65 (m, 2H), 1.61–1.51 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 137.0, 136.4, 135.8, 129.5, 129.1, 128.5 (2C), 126.0, 125.9, 125.6 (2C), 122.7, 120.4 (2C), 120.1, 119.1. 116.6, 116.3 (2C), 110.4, 57.7, 50.4 (2C), 41.9, 33.5 (2C), 27.3, 24.5, 23.6; MS (ESI+): m/z = calcd. for C30H30FN3 [M + H]+ 452.3, found 452.7; HRMS (EI): m/z calcd. for C30H30FN3 [M + H]+ 452.2502, found 452.2505. Anal. calcd. for C30H30FN3·HCl·1/4H2O: C 73.16, N 8.53, H 6.45; found: C 73.19, N 8.48, H 6.69.
4.2.4.
3-(4-{4-[4-(2-Fluoroethoxy)phenyl]piperazin-1-yl}butyl)-1-(4-fluorophenyl)-1H-indole (1d).
Compounds 6 (180 mg, 0.52 mmol) and 12 (132 mg, 0.59 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (680 mg, 4.92 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN (25 mL) afforded 1d as white solid (210 mg, 83%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.65 (d, J = 7.6 Hz, 1H), 7.48–7.40 (m, 3H), 7.24–7.12 (m, 4H), 7.08 (s, 1H), 6.93–6.83 (m, 4H), 4.71 (dt, J = 47.4, 4.1 Hz, 2H), 4.16 (dt, J = 27.9, 4.2 Hz, 2H), 3.10 (t, J = 4.8 Hz, 4H), 2.84 (t, J = 7.4 Hz, 2H), 2.61 (t, J = 4.7 Hz, 4H), 2.46 (t, J = 7.6 Hz, 2H), 1.86–1.75 (m, 2H), 1.72–1.61 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 160.8, 152.5, 146.3, 136.4, 136.1, 128.9, 125.9 (2C), 125.1, 122.5, 119.8, 119.4, 118.0 (2C), 117.8, 116.4 (2C), 115.5 (2C), 110.2, 82.1, 67.7, 58.6 (2C), 53.4, 50.4 (2C), 28.0, 26.9, 25.0; MS (ESI+): m/z = calcd. for C30H33F2N3O [M + H]+ 490.3, found 490.6. Anal. calcd. for C30H33F2N3O (489.60): C 73.60, H 6.79, N 8.58; found: C 73.99, H 7.12, N 8.26.
4.2.5
3-[4-(4-{4-[2-(2-Fluoroethoxy)ethoxy]phenyl}piperazin-1-yl)butyl]-1-(4-fluorophenyl)-1H-indole (1e).
Compounds 6 (488 mg, 1.41 mmol) and 13 (138 mg, 0.51 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (84 mg, 0.61 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN (25 mL) afforded 1e as light-yellow oil (43 mg, 16%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.65 (t, J = 7.6 Hz, 1H), 7.50–7.40 (m, 3H), 7.24–7.13 (m, 4H), 7.08 (s, 1H), 6.91–6.82 (m, 4H), 4.58 (dt, J = 47.7, 4.1 Hz, 2H), 4.09 (t, J = 4.8 Hz, 2H), 3.89–3.75 (m, 4H), 3.10 (t, J = 4.7 Hz, 4H), 2.84 (t, J = 7.4 Hz, 2H), 2.62 (t, J = 4.5 Hz, 4H), 2.46 (t, J = 7.6 Hz, 2H), 1.85–1.74 (m, 2H), 1.72–1.62 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 160.8, 152.8, 146.0, 136.3, 136.0, 128.9, 125.8, 125.8 (2C), 125.1, 122.5, 119.6 (2C), 118.0 (2C), 117.8, 116.4, 115.4 (2C), 110.2, 83.2, 70.6, 70.1, 68.0, 58.6 (2C), 53.4, 50.5 (2C), 28.0, 26.8, 25.0. HRMS (EI): m/z calcd. for C32H37F2N3O2 [M + H]+ 534.2932, found 534.2916.
4.2.6.
3-{4-[4-(4-{2-[2-(2-Fluoroethoxy)ethoxy]ethoxy}phenyl)piperazin-1-yl]butyl}-1-(4-fluorophenyl)-1H-indole (1f).
Compounds 6 (142 mg, 0.41 mmol) and 14 (129 mg, 0.41 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (70 mg, 0.51 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN (25 mL) afforded 1f (68 mg, 29%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.65 (d, J = 7.6 Hz, 1H), 7.48–7.40 (m, 3H), 7.24–7.14 (m, 4H), 7.08 (s, 1H), 6.91–6.82 (m, 4H), 4.56 (dt, J = 47.7, 4.1 Hz, 2H), 4.08 (t, J = 4.8 Hz, 2H), 3.83 (t, J = 4.8 Hz, 2H), 3.80–3.68 (m, 6H), 3.10 (t, J = 4.7 Hz, 4H), 2.84 (t, J = 7.4 Hz, 2H), 2.61 (t, J = 4.8 Hz, 4H), 2.46 (t, J = 7.6 Hz, 2H), 1.85–1.75 (m, 2H), 1.74–1.63 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 160.7, 152.9, 145.9, 136.3, 136.0, 128.9, 125.8, 125.7, 125.1, 122.4, 119.5 (2C), 118.0 (2C), 117.7, 116.3 (2C), 115.3 (2C), 110.1, 83.1, 70.8, 70.5, 70.3, 69.9, 67.8, 58.6, 53.4 (2C), 50.5 (2C), 27.9, 26.8, 24.9; MS (ESI+): m/z = calcd. for C34H41F2N3O3 [M + H]+ 577.3, found 577.4. Anal. calcd. for C34H41F2N3O3·2HCl·H2O (668.64): C 61.07, H 6.78, N 6.28; found: C 61.47, H 6.80, N 6.38.
4.2.7.
3-(4-{4-[3-(2-Fluoroethoxy)phenyl]piperazin-1-yl}butyl)-1-(4-fluorophenyl)-1H-indole (1g).
Compounds 6 (259 mg, 0.75 mmol) and 15 (168 mg, 0.75 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (132 mg, 0.97 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN (25 mL) afforded 1g as light-yellow oil (130 mg, 35%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.68–7.61 (m, 1H), 7.47–7.39 (m, 3H), 7.22–7.11 (m, 5H), 7.07 (s, 1H), 6.59–6.53 (m, 1H), 6.49 (t, J = 2.3 Hz, 1H), 6.44–6.36 (m, 1H), 4.71 (dt, J = 47.1, 4.2 Hz, 2H), 4.17 (dt, J = 27.8, 4.2 Hz, 2H), 3.18 (t, J = 5.2 Hz, 4H), 2.83 (t, J = 7.4 Hz, 2H), 2.57 (t, J = 5.0 Hz, 4H), 2.43 (t, J = 7.6 Hz, 2H), 1.87–1.71 (m, 2H), 1.73–1.60 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 160.7, 159.4, 152.7, 136.3, 136.0, 129.7, 128.8, 125.8 (2C), 125.1, 122.4, 119.8, 119.3, 117.7, 116.3 (2C), 110.1, 109.3, 104.6, 103.2, 82.0, 67.0, 58.6, 53.2 (2C), 48.9 (2C), 27.9, 26.8, 24.9; 19F NMR (376 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ –120.7, −228.6; MS (ESI+): m/z = calcd. for C30H33F2N3O [M + H]+ 490.3, found 490.5; HRMS (EI): m/z calcd. for C30H33F2N3O [M + H]+ 490.2670, found 490.2673. Anal. calcd. for C30H33F2N3O·H2O (507.61): C 70.98, H 6.95, N 8.28; found: C 71.18, H 6.87, N 8.01.
4.2.8.
3-{4-[(tert-Butyldimethylsilyl)oxy]butyl}-1H-indole (20).
To a solution of 19 (2.10 g, 11.1 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2 (40 mL), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTBDMSCl (2.06 g, 13.7 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSimidazole (1.43 g, 21.0 mmol) were added. The mixture was stirred at room temperature for 2 h. After filtration, the solvent was removed under reduced pressure. The residue was purified by silica gelchromatography (PE : EE = 1 : 5) to afford 20 (2.80 g, 84%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.87 (s, 1H), 7.58 (d, J = 7.9 Hz, 1H), 7.32 (d, J = 8.1 Hz, 1H), 7.20–7.13 (m, 1H), 7.12–7.04 (m, 1H), 6.95 (s, 1H), 3.63 (t, J = 6.5 Hz, 2H), 2.75 (t, J = 7.5 Hz, 2H), 1.80–1.68 (m, 2H), 1.65–1.58 (m, 2H), 0.87 (s, 9H).
4.2.9. 2-(3-{4-[(tert-Butyldimethylsilyl)oxy]butyl}-1H-indol-1-yl)ethanol (21).
To a solution of compound 20 (1.88 g, 6.19 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS2-bromoethanol (1.23 g, 9.92 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNaH (240 mg, 10.0 mmol) were added. The mixture was stirred at 110 °C overnight. After cooling, the crude product was extracted with ethyl acetate, dried with anhydrous COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMgSO4, and purified by silica gelcolumn chromatography (PE : EE = 5 : 1) to afford 21 (805 mg, 37%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.57 (d, J = 7.9 Hz, 1H), 7.30 (d, J = 8.2 Hz, 1H), 7.20–7.15 (m, 1H), 7.10–7.05 (m, 1H), 6.91 (s, 1H), 4.21 (t, J = 5.3 Hz, 2H), 3.92 (t, J = 5.3 Hz, 2H), 3.62 (t, J = 6.4 Hz, 2H), 2.73 (t, J = 7.5 Hz, 2H), 1.79–1.65 (m, 2H), 1.65–1.53 (m, 2H), 0.87 (s, 9H).
4.2.10. 2-(3-{4-[(tert-Butyldimethylsilyl)oxy]butyl}-1H-indol-1-yl)ethyl 4-methylbenzenesulfonate (22).
Compound 21 (805 mg, 2.32 mmol), TsCl (661 mg, 3.48 mmol), DIPEA (450 mg, 3.48 mmol), and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMAP (425 mg, 3.48 mmol) were dissolved in 30 mL of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTHF. The mixture was stirred at room temperature for 2 h. After the solvent was removed under reduced pressure, the crude product was extracted with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2. The organic layer was dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMgSO4, filtered, and concentrated under vacuum. The residue was purified by silica gelchromatography (PE : EE = 10 : 1) to afford 22 (208 mg, 18%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.63–7.56 (m, 1H), 7.49 (d, J = 8.3 Hz, 2H), 7.17–7.04 (m, 5H), 6.80 (s, 1H), 4.36–4.24 (m, 4H), 3.72 (t, J = 6.3 Hz, 2H), 2.75 (t, J = 7.5 Hz, 2H), 2.36 (s, 3H), 1.86–1.73 (m, 2H), 1.73–1.64 (m, 2H), 0.97 (s, 9H).
4.2.11.
4-[1-(2-Fluoroethyl)-1H-indol-3-yl]butan-1-ol (23).
TBAF (345 mg, 1.32mmol) and 22 (265 mg, 0.53 mmol) were added into COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTHF (20 mL). The mixture was stirred at room temperature overnight. After the solvent was removed under reduced pressure, the crude product was extracted with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2. The organic layer was dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMgSO4, filtered, and concentrated under vacuum. The residue was purified by silica gelchromatography (PE : EE = 3 : 1) to afford 23 (69 mg, 56%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.63 (d, J = 7.9 Hz, 1H), 7.30 (d, J = 8.1 Hz, 1H), 7.43–7.21 (m, 2H), 7.17–7.10 (m, 1H), 6.95 (s, 1H), 4.69 (dt, J = 47.0, 5.0 Hz, 2H), 4.35 (dt, J = 25.7, 4.9 Hz, 2H), 3.68 (t, J = 6.5 Hz, 2H), 2.80 (t, J = 7.4 Hz, 2H), 1.87–1.75 (m, 2H), 1.75–1.63 (m, 2H).
4.2.12. 4-[1-(2-Fluoroethyl)-1H-indol-3-yl]butyl 4-methylbenzenesulfonate (24).
To a solution of 23 (120 mg, 0.51 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTHF (20 mL), TsCl (116 mg, 0.61 mmol), DIPEA (129 mg, 1.00 mmol), and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMAP (122 mg, 1.00 mmol) were added. The mixture was stirred at room temperature for 2 h. After the solvent was removed under reduced pressure, the crude product was extracted with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2. The organic layer was dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMgSO4, filtered, and concentrated under vacuum. The residue was purified by silica gelchromatography (PE : EE = 5 : 1) to afford 24 (88 mg, 44%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.76 (d, J = 8.3 Hz, 2H), 7.50 (d, J = 7.9 Hz, 1H), 7.32–7.16 (m, 4H), 7.11–7.05 (m, 1H), 6.86 (s, 1H), 4.66 (dt, J = 47.0, 5.0 Hz, 2H), 4.32 (dt, J = 25.8, 5.0 Hz, 2H), 4.07–3.99 (m, 2H), 2.68 (t, J = 6.7 Hz, 2H), 2.40 (s, 3H), 1.75–1.68 (m, 4H).
4.2.13.
1′-{4-[1-(2-Fluoroethyl)-1H-indol-3-yl]butyl}-3H-spiro[isobenzofuran-1,4′-piperidine] (2).
To a solution of 24 (88 mg, 0.23 mmol) in 20 mL of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN, 3H-spiro[isobenzofuran-1,4′-piperidine] (25) (32 mg, 0.17 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (32 mg, 0.23 mmol) were added. The mixture was stirred at 80 °C for 4 h. After the solvent was removed under reduced pressure, the residue was purified by silica gelchromatography (PE : EE = 1 : 1) to afford 2 (26 mg, 38%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.62 (d, J = 7.8 Hz, 1H), 7.31–7.25 (m, 3H), 7.24–7.20 (m, 2H), 7.18–7.09 (m, 2H), 6.96 (s, 1H), 5.08 (s, 2H), 4.70 (dt, J = 47.0, 5.0 Hz, 2H), 4.36 (dt, J = 25.5, 5.0 Hz, 2H), 2.90 (d, J = 11.2 Hz, 2H), 2.80 (t, J = 7.3 Hz, 2H), 2.49 (t, J = 10.0 Hz, 2H), 2.41 (t, J = 11.1 Hz, 2H), 2.07–1.97 (m, 2H), 1.84–1.63 (m, 6H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 145.9, 139.1, 136.6, 128.5, 127.8, 127.6, 125.4, 121.9, 121.2, 121.1, 119.5, 119.1, 116.3, 109.1, 84.2, 81.8, 71.0, 59.1, 50.5 (2C), 46.5, 36.8 (2C), 28.5, 27.2, 25.2; 19F NMR (376 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ –219.8; MS (ESI+): m/z = calcd. for C26H31FN2O [M + H]+ 407.2, found 407.2; HRMS (EI): m/z calcd. for C26H31FN2O [M + H]+ 407.2499, found 407.2492; purity (HPLC): 95%.
4.2.14.
2-(1H-Indol-3-yl)ethanol (28).
Under ice bath and argon atmosphere, a solution of 26 (1.00 g, 5.71 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTHF (60 mL) was added to a solution of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSLiAlH4 (642 mg, 17.1 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSTHF (40 mL). The mixture was stirred for 4 h at room temperature, followed by addition of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSethanol until no H2 was formed. Then 4 M COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTShydrochloric acid was added to adjust the pH to 5. After filtration, the solution was concentrated under reduced pressure. The crude product was extracted with ethyl acetate, dried with anhydrous COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMgSO4, and purified by silica gelcolumn chromatography (PE : EE = 5 : 1) to afford alcohol28 (795 mg, 86%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 8.02 (s, 1H), 7.60 (d, J = 7.8 Hz, 1H), 7.35 (d, J = 8.1 Hz, 1H), 7.21–7.16 (m, 1H), 7.13–7.09 (m, 1H), 7.06 (d, J = 2.1 Hz, 1H), 3.89 (t, J = 6.3 Hz, 2H), 3.02 (t, J = 6.3 Hz, 2H).
4.2.15.
3-(1H-Indol-3-yl)propan-1-ol (29).
The procedure described for the synthesis of 28 was applied to 27 (3.00 g, 15.8 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSLiAlH4 (2.93 g, 77.2 mmol) to afford alcohol29 (2.18 g, 79%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.93 (s, 1H), 7.65–7.55 (m, 1H), 7.37–7.30 (m, 1H), 7.23 (s, 1H), 7.19–7.14 (m, 1H), 7.12–7.07 (m, J = 8.0, 1H), 6.97 (s, 1H), 3.71 (t, J = 6.4 Hz, 2H), 2.90–2.78 (m, 2H), 2.04–1.93 (m, 2H).
4.2.16.
2-[1-(4-Bromobutyl)-1H-indol-3-yl]ethanol (30).
Under ice bath and argon atmosphere, alcohol28 (220 mg, 1.37 mmol), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS1,4-dibromobutane (875 mg, 4.11 mmol), and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNaH (98 mg, 4.11 mmol) were added into COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF (30 mL). The mixture was stirred at 110 °C overnight. After cooling and filtration, the crude product was extracted with ethyl acetate, dried with anhydrous COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMgSO4, and purified by silica gelcolumn chromatography (PE : EE = 3 : 1) to afford 30 (78 mg, 19%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.59 (d, J = 7.9 Hz, 1H), 7.30 (d, J = 8.2 Hz, 1H), 7.24–7.18 (m, 1H), 7.14–7.04 (m, 1H), 6.96 (s, 1H), 4.10 (t, J = 6.9 Hz, 2H), 3.87 (t, J = 6.4 Hz, 2H), 3.35 (t, J = 6.5 Hz, 2H), 3.00 (t, J = 6.4 Hz, 2H), 2.05–1.94 (m, 2H), 1.89–1.78 (m, 2H).
4.2.17.
3-[1-(4-Bromobutyl)-1H-indol-3-yl]propan-1-ol (31).
The procedure described for the synthesis of 30 was applied to alcohol29 (1.20 g, 6.86 mmol), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS1,4-dibromobutane (4.38 g, 20.6 mmol), and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNaH (329 mg, 13.7 mmol) to afford 31 (728 mg, 34%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.60 (d, J = 7.9 Hz, 1H), 7.29 (d, J = 8.2 Hz, 1H), 7.25–7.14 (m, 1H), 7.12–7.07 (m, 1H), 6.87 (s, 1H), 4.08 (t, J = 6.8 Hz, 2H), 3.70 (t, J = 6.4 Hz, 2H), 3.35 (t, J = 6.5 Hz, 2H), 2.84 (t, J = 7.5 Hz, 2H), 2.03–1.90 (m, 4H), 1.86–1.77 (m, 2H).
4.2.18.
1-(4-Bromobutyl)-3-(2-fluoroethyl)-1H-indole (32).
Under argon atmosphere (−78 °C), a solution of DAST (352 mg, 2.18 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2 (20 mL) was added into a solution of 30 (538 mg, 1.82 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2 (20 mL). The mixture was stirred for 2 h, followed by addition of saturated sodium hyposulfite to quench the reaction. The crude product was extracted with ethyl acetate, dried with anhydrous COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMgSO4, and purified by silica gelcolumn chromatography (PE :EE = 10 : 1) to afford 32 (315 mg, 58%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.58 (d, J = 7.9 Hz, 1H), 7.30 (d, J = 8.2 Hz, 1H), 7.23–7.18 (m, 1H), 7.14–7.08 (m, 1H), 6.96 (s, 1H), 4.67 (dt, J = 47.2, 6.7 Hz, 2H), 4.11 (t, J = 6.9 Hz, 2H), 3.36 (t, J = 6.5 Hz, 2H), 3.15 (dt, J = 22.2, 6.5 Hz, 2H), 2.03–1.95 (m, 2H), 1.90–1.79 (m, 2H).
4.2.19.
1-(4-Bromobutyl)-3-(3-fluoropropyl)-1H-indole (33).
The procedure described for the synthesis of 32 was applied to DAST (205 mg, 1.28 mmol) and 31 (360 mg, 1.16 mmol) to afford 33 (315 mg, 87%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.63 (d, J = 7.9 Hz, 1H), 7.33 (d, J = 8.2 Hz, 1H), 7.28–7.22 (m, 1H), 7.17–7.11 (m, 1H), 6.92 (s, 1H), 4.53 (dt, J = 47.3, 5.9 Hz, 2H), 4.12 (t, J = 6.8 Hz, 2H), 3.38 (t, J = 6.5 Hz, 2H), 2.92 (t, J = 7.5 Hz, 2H), 2.19–2.05 (m, 2H), 2.04–1.96 (m, 2H), 1.90–1.81 (m, 2H).
4.2.20. 2-{4-[3-(2-Fluoroethyl)-1H-indol-1-yl]butyl}-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (3a).
The procedure described for the synthesis of 3a was applied to 32 (78 mg, 0.26 mmol), 16 (76 mg, 0.39 mmol), and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (54 mg, 0.39 mmol) to afford 3a (46 mg, 43%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.57 (d, J = 7.9 Hz, 1H), 7.31 (d, J = 8.2 Hz, 1H), 7.21–7.16 (m, 1H), 7.12–7.07 (m, 1H), 6.98 (s, 1H), 6.57 (s, 1H), 6.48 (s, 1H), 4.66 (dt, J = 47.2, 6.7 Hz, 2H), 4.11 (t, J = 7.0 Hz, 2H), 3.82 (s, 3H), 3.81 (s, 3H), 3.48 (s, 2H), 3.15 (dt, J = 22.1, 6.7 Hz, 2H), 2.78 (t, J = 5.7 Hz, 2H), 2.64 (t, J = 5.8 Hz, 2H), 2.48 (t, J = 5.8 Hz, 2H), 1.94–1.84 (m, 2H), 1.66–1.54 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 147.7, 147.4, 136.4, 128.1, 126.7, 126.4, 126.3, 121.7, 119.1, 119.0, 111.6, 109.8, 109.8, 109.7, 84.1, 57.9, 56.1, 56.0, 51.3, 46.3, 28.9, 28.4, 26.9, 26.6, 24.9; 19F NMR (376 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ –213.3. MS (ESI+): m/z = calcd. for C25H31FN2O2 [M + H]+ 411.2, found 411.1; HRMS (EI): m/z calcd. for C25H31FN2O2 [M + H]+ 411.2448, found 411.2443; purity (HPLC): 95%.
4.2.21. 2-{4-[3-(3-Fluoropropyl)-1H-indol-1-yl]butyl}-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (3b).
The procedure described for the synthesis of 3a was applied to 33 (113 mg, 0.36 mmol), 16 (73 mg, 0.38 mmol), and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (60 mg, 0.43 mmol) to afford 3b (55 mg, 36%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.60 (d, J = 7.8 Hz, 1H), 7.33 (d, J = 8.2 Hz, 1H), 7.20 (t, J = 7.6 Hz, 1H), 7.11 (t, J = 7.4 Hz, 1H), 6.93 (s, 1H), 6.60 (s, 1H), 6.51 (s, 1H), 4.51 (dt, J = 47.4, 5.9 Hz, 2H), 4.13 (t, J = 7.0 Hz, 2H), 3.85 (s, 3H), 3.84 (s, 3H), 3.51 (s, 2H), 2.90 (t, J = 7.4 Hz, 2H), 2.81 (t, J = 5.6 Hz, 2H), 2.67 (t, J = 5.8 Hz, 2H), 2.51 (t, J = 7.3 Hz, 2H), 2.18–2.02 (m, 2H), 1.97–1.88 (m, 2H), 1.67–1.57 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 147.7, 147.4, 136.6, 128.1, 126.8, 126.4, 125.6, 121.6, 119.2, 118.8, 113.9, 111.6, 109.7, 109.6, 83.7, 57.9, 56.1, 56.0, 51.3, 46.2, 31.3, 31.2, 28.9, 28.4, 24.9, 20.8; 19F NMR (376 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ –220.5; MS (ESI+): m/z = calcd. for C26H33FN2O2 [M + H]+ 425.3, found 425.0; HRMS (EI): m/z calcd. for C26H33FN2O2 [M + H]+ 425.2604, found 425.2601. Anal. calcd. for C26H33FN2O2 ·3/4H2O (438.06): C 71.29, N 6.39, H 7.94; found: C 71.24, N 6.57, H 7.63.
4.2.22. 1′-{4-[3-(2-Fluoroethyl)-1H-indol-1-yl]butyl}-3H-spiro[isobenzofuran-1,4′-piperidine] (3c).
To a solution of 32 (70 mg, 0.24 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN (20 mL), 25 (45 mg, 0.24 mmol) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (40 mg, 0.29 mmol) were added. The mixture was stirred at 80 °C for 4 h. After cooling and filtration, the solvent was removed under reduced pressure. The residue was purified by silica gelcolumn chromatography (PE : EE = 1 : 5) to afford 3c (40 mg, 41%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.58 (d, J = 7.9 Hz, 1H), 7.33 (d, J = 8.2 Hz, 1H), 7.29–7.16 (m, 4H), 7.15–7.07 (m, 2H), 6.99 (s, 1H), 5.06 (s, 2H), 4.66 (dt, J = 47.2, 6.7 Hz, 2H), 4.10 (t, J = 7.1 Hz, 2H), 3.16 (dt, J = 21.9, 6.7 Hz, 2H), 2.81 (d, J = 11.0 Hz, 2H), 2.47–2.29 (m, 4H), 2.03–1.81 (m, 4H), 1.75 (d, J = 12.5 Hz, 2H), 1.62–1.52 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 145.8, 139.1, 136.4, 128.1, 127.8, 127.6, 126.3, 121.8, 121.3, 121.0, 119.1, 109.8, 109.8, 109.7, 84.9, 83.3, 71.0, 58.6, 50.4 (2C), 46.4, 36.8 (2C), 28.6, 26.8, 24.8; 19F NMR (376 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ –213.2; MS (ESI+): m/z = calcd. for C26H31FN2O [M + H]+ 407.2, found 407.1; HRMS (EI): m/z calcd. for C26H31FN2O [M + H]+ 407.2499, found 407.2501; purity (HPLC): 96%.
4.2.23.
4-[1-(4-Iodophenyl)-1H-indol-3-yl]butan-1-ol (34).
Compound 19 (595 mg, 3.14 mmol), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS1,4-diiodobenzene (780 mg, 2.36 mmol), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (3.12 g, 23.6 mmol), a catalytic amount of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTScopper powder, and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTS18-crown-6 were added into 25 mL of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSDMF. The mixture was stirred at 120 °C for 5 h. After cooling and filtration, the crude product was extracted with ethyl acetate and washed with 1 M COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSHCl and saturated COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNaClsolution. The organic layer was dried over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMgSO4, and the solvent was removed under reduced pressure. The residue was purified by silica gelcolumn chromatography (PE : EE = 4 : 1) to afford 34 (208 mg, 23%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.76–7.72 (m, 2H), 7.57 (t, J = 7.6 Hz, 1H), 7.46 (t, J = 8.2 Hz, 1H), 7.18–7.08 (m, 4H), 7.03 (s, 1H), 3.64 (t, J = 6.5 Hz, 2H), 2.76 (t, J = 7.4 Hz, 2H), 1.80–1.73 (m, 2H), 1.67–1.60 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 139.6, 138.6 (2C), 135.8, 129.2, 125.7, 124.6, 122.7 (2C), 120.1, 119.4, 118.3, 110.3, 90.0, 62.9, 32.6, 26.1, 24.8; MS (ESI+): m/z = calcd. for C18H18INO [M + H]+ 392.0, found 392.2.
4.2.24.
3-(4-Bromobutyl)-1-(4-iodophenyl)-1H-indole (35).
Under argon atmosphere and ice bath, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSPBr3 (192 mg, 0.71 mmol) was added to a solution of 34 (550 mg, 1.41 mmol) in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2. The mixture was stirred at room temperature for 2 h, followed by addition of saturated COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSNaHCO3solution to quench the reaction. The crude product was extracted with ethyl acetate, dried with anhydrous COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMgSO4, and purified by silica gelcolumn chromatography (PE : EE = 4 : 1) to afford 35 (187 mg, 58%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.81 (t, J = 8.2 Hz, 2H), 7.64 (t, J = 7.0 Hz, 1H), 7.52 (t, J = 8.1 Hz, 1H), 7.26–7.16 (m, 4H), 7.10 (s, 1H), 3.47 (t, J = 6.5 Hz, 2H), 2.83 (t, J = 7.24 Hz, 2H), 2.03–1.98 (m, 2H), 1.96–1.91 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 139.6, 138.6 (2C), 135.8, 129.1, 125.7, 124.6, 122.7 (2C), 120.2, 119.4, 117.8, 110.4, 90.0, 33.8, 32.5, 28.4, 24.2; MS (ESI+): m/z = calcd. for C18H17BrIN [M + H]+ 454.0, found 453.9.
4.2.25.
1-{4-[1-(4-Iodophenyl)-1H-indol-3-yl]butyl}-4-phenylpiperidine-4-carbonitrile (4).
Compound 35 (163 mg, 0.36 mmol), 4-phenylpiperidine-4-carbonitrile (18) (147 mg, 0.72 mmol), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (496 g, 3.60 mmol), and KI (64 mg, 0.39 mmol) were added into COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CNsolution. The mixture was stirred at 80 °C for 4 h. After cooling and filtration, the solvent was removed under reduced pressure. The residue was purified by silica gelcolumn chromatography (PE : EE = 2 : 1) to afford 4 (99 mg, 49%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.74–7.71 (m, 2H), 7.57 (d, J = 3.8 Hz, 1H), 7.46–7.41 (m, 3H), 7.34 (t, J = 7.2 Hz, 2H), 7.30–7.23 (m, 2H), 7.19–7.17 (m, 2H) 7.12–7.08 (m, 1H), 7.03 (s, 1H), 2.98 (d, J = 11.96 Hz, 2H), 2.76 (t, J = 7.36 Hz, 2H), 2.47–2.38 (m, 4H), 2.04 (s, 4H), 1.74–1.68 (m, 2H), 1.61–1.58 (m, 2H); 13C NMR (100 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 139.2, 138.6 (2C), 137.6, 134.8, 128.2 (2C), 128.0, 127.1, 124.7 (2C), 124.6, 123.5, 121.7 (2C), 120.8, 119.1, 118.4, 117.3, 109.3, 88.9, 57.3, 49.8 (2C), 41.8, 35.4 (2C), 26.8, 25.7, 23.9; MS (ESI+): m/z = calcd. for C30H30IN3 [M + H]+ 560.1, found 560.1. Anal. calcd. for C30H30IN3·HCl·1/2H2O (604.95): C 59.56, N 6.95, H 5.33; found: C 59.76, N 6.82, H 5.17.
4.2.26.
2-{4-[1-(4-Fluorophenyl)-1H-indol-3-yl]butyl}-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (5).
Compound 36 (279 mg, 1.08 mmol), 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (153 mg, 0.67 mmol), and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSK2CO3 (220 mg, 1.59 mmol) were added into 25 mL of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH3CN. The mixture was stirred at 80 °C for 4 h. After the solvent was removed under reduced pressure, the residue was purified by silica gelcolumn chromatography (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCH2Cl2 : COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSMeOH = 20 : 1) to afford 5 (153 mg, 62%). 1H NMR (400 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 7.98–7.90 (m, 2H), 7.09–7.02 (m, 2H), 6.54 (s, 1H), 6.47 (s, 1H), 3.80 (s, 3H), 3.79 (s, 3H), 3.52 (s, 2H), 2.96 (t, J = 7.2 Hz, 2H), 2.77 (t, J = 5.8 Hz, 2H), 2.67 (t, J = 5.9 Hz, 2H), 2.52 (t, J =7.3 Hz, 2H), 1.82–1.71 (m, 2H), 1.71–1.61 (m, 2H); 13C NMR (101 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ 198.6, 165.5, 147.5, 147.2, 133.3, 130.6 (2C), 126.5, 126.1, 115.6 (2C), 111.3, 109.4, 57.8, 55.9, 55.9, 55.7, 50.9, 38.2, 28.6, 26.6, 22.3; 19F NMR (376 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound
Explore further on Open PHACTSCDCl3): δ –106.7; MS (ESI+): m/z = calcd. for C22H26FNO3 [M + H]+ 372.2, found 372.2; HRMS (EI): m/z calcd. for C22H26FNO3 [M + H]+ 372.1975, found 372.1972. Anal. calcd. for C22H26FNO3·3/4H2O (384.96): C 68.64, N 3.64, H 7.20; found: C 68.74, N 3.92, H 6.82.
4.3
In vitro radioligand competition studies
Competition assays of σ1 and σ2 receptors were performed as previous reported in the literature.28,29 The detailed procedures are provided in the ESI.†
The cancer cell lines MCF7 (human mammary carcinoma), DU145 (human prostate carcinoma) and C6 (rat glioma) were routinely cultured in Beijing Normal University. The MTT assay was used to determine the antiproliferative activity of compounds 1a and 1b and siramesine in these cell lines as described previously.30,31 The procedures are shown in the ESI.†
1a, 1b and siramesine were cultured in DU145 cell line for 24 h to examine cell cycle arrest as described previously.32 The detailed procedures are shown in the ESI.†
Acknowledgements
The excellent technical assistance of Aline Morgenegg, Catharina Heinig, Tina Spalholz and Peggy Wecke is greatly acknowledged. This work was supported by the National Natural Science Foundation of China (No. 21471019) and supported in part (C.N., J.P., T.K., and J.S.) by the Helmholtz Association within Helmholtz-Portfolio Topic “Technologie und Medizin – Multimodale Bildgebung zur Aufklärung des In-vivo-Verhaltens von polymeren Biomaterialien”. Fang Xie acknowledges the financial support from China Scholarship Council (CSC) for his study in HZDR.
Notes and references
- R. Quirion, W. D. Bowen, Y. Itzhak, J. L. Junien, J. M. Musacchio, R. B. Rothman, T. P. Su, S. W. Tam and D. P. Taylor, Trends Pharmacol. Sci., 1992, 13, 85–86 CrossRef CAS.
- M. Hanner, F. F. Moebius, A. Flandorfer, H. G. Knaus, J. Striessnig, E. Kempner and H. Glossmann, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 8072–8077 CrossRef CAS.
- E. Aydar, C. P. Palmer, V. A. Klyachko and M. B. Jackson, Neuron, 2002, 34, 399–410 CrossRef CAS.
- T. Hayashi and T. P. Su, Cell, 2007, 131, 596–610 CrossRef CAS PubMed.
- T. P. Su, T. Hayashi, T. Maurice, S. Buch and A. E. Ruoho, Trends Pharmacol. Sci., 2010, 31, 557–566 CrossRef CAS PubMed.
- J. Xu, C. Zeng, W. Chu, F. Pan, J. M. Rothfuss, F. Zhang, Z. Tu, D. Zhou, D. Zeng, S. Vangveravong, F. Johnston, D. Spitzer, K. C. Chang, R. S. Hotchkiss, W. G. Hawkins, K. T. Wheeler and R. H. Mach, Nat. Commun., 2011, 2, 380 CrossRef PubMed.
- B. J. Vilner, C. S. John and W. D. Bowen, Cancer Res., 1995, 55, 408–413 CAS.
- A. Van Waarde, A. A. Rybczynska, N. K. Ramakrishnan, K. Ishiwata, P. H. Elsinga and R. A. J. O. Dierckx, Curr. Pharm. Des., 2010, 16, 3519–3537 CrossRef CAS.
- I. Al-Nabulsi, R. H. Mach, L. M. Wang, C. A. Wallen, P. C. Keng, K. Sten, S. R. Childers and K. T. Wheeler, Br. J. Cancer, 1999, 81, 925–933 CrossRef CAS PubMed.
- K. T. Wheeler, L. M. Wang, C. A. Wallen, S. R. Childers, J. M. Cline, P. C. Keng and R. H. Mach, Br. J. Cancer, 2009, 82, 1223–1232 CrossRef PubMed.
- R. H. Mach, C. R. Smith, I. Al-Nabulsi, B. R. Whirrett, S. R. Childers and K. T. Wheeler, Cancer Res., 1997, 57, 156–161 CAS.
- M. S. Ostenfeld, N. Fehrenbacher, M. Høyer-Hansen, C. Thomsen, T. Farkas and M. Jäättelä, Cancer Res., 2005, 65, 8975–8983 CrossRef CAS PubMed.
- L. Groth-Pedersen, M. S. Ostenfeld, M. Høyer-Hansen, J. Nylandsted and M. Jäättelä, Cancer Res., 2007, 67, 2217–2225 CrossRef CAS PubMed.
- M. J. Parry, J.-M. I. Alakoskela, H. Khandelia, S. A. Kumar, M. Jäättelä, A. K. Mahalka and P. K. J. Kinnunen, J. Am. Chem. Soc., 2008, 130, 12953–12960 CrossRef CAS PubMed.
- C. Zeng, J. Rothfuss, J. Zhang, W. Chu, S. Vangveravong, Z. Tu, F. Pan, K. C. Chang, R. Hotchkiss and R. H. Mach, Br. J. Cancer, 2012, 106, 693–701 CrossRef CAS PubMed.
- R. H. Mach, C. Zeng and W. G. Hawkins, J. Med. Chem., 2013, 56, 7137–7160 CrossRef CAS PubMed.
- C. Abate, R. Perrone and F. Berardi, Curr. Pharm. Des., 2012, 18, 938–949 CrossRef CAS.
- M. H. Cesen, U. Repnik, V. Turk and B. Turk, Cell Death Dis., 2013, 4, e818 CrossRef PubMed.
- Y. Li, H. Jia, W. Deuther-Conrad, P. Brust, J. Steinbach and B. Liu, He Huaxue Yu Fangshe Huaxue, 2010, 32, 99–105 CAS.
- M. H. Herth, V. Kramer and F. Rösch, J. Labelled Compd. Radiopharm., 2009, 52, 201–207 CrossRef CAS PubMed.
- H. Kubota, M. Fujii, K. Ikeda, M. Takeuchi, T. Shibanuma and Y. Isomura, Chem. Pharm. Bull., 1998, 46, 351–354 CrossRef CAS.
- H. Shao, X. Chen, Z. Wang and P. Lu, J. Lumin., 2007, 127, 349–354 CrossRef CAS PubMed.
- C. Fan, H. Jia, W. Deuther-Conrad, P. Brust, J. Steinbach and B. Liu, Sci. China, Ser. B: Chem., 2006, 49, 169–176 CrossRef CAS.
- J. Perregaard, E. K. Moltzen, E. Meier and C. Sanchez, J. Med. Chem., 1995, 38, 1998–2008 CrossRef CAS.
- M. Niso, C. Abate, M. Contino, S. Ferorelli, A. Azzariti, R. Perrone, N. A. Colabufo and F. Berardi, ChemMedChem, 2013, 8, 2026–2035 CrossRef CAS PubMed.
- K.-H. Fan, J. R. Lever and S. Z. Lever, Bioorg. Med. Chem., 2011, 19, 1852–1859 CrossRef CAS PubMed.
- Z. Tu, J. Xu, L. A. Jones, S. Li, C. Dumstorff, S. Vangveravong, D. L. Chen, K. T. Wheeler, M. J. Welch and R. H. Mach, J. Med. Chem., 2007, 50, 3194–3204 CrossRef CAS PubMed.
- Y. Li, X. Wang, J. Zhang, W. Deuther-Conrad, F. Xie, X. Zhang, J. Liu, J. Qiao, M. Cui, J. Steinbach, P. Brust, B. Liu and H. Jia, J. Med. Chem., 2013, 56, 3478–3491 CrossRef CAS PubMed.
- X. Wang, Y. Li, W. Deuther-Conrad, F. Xie, X. Chen, M.-C. Cui, X.-J. Zhang, J.-M. Zhang, J. Steinbach, P. Brust, B.-L. Liu and H.-M. Jia, Bioorg. Med. Chem., 2013, 21, 215–222 CrossRef CAS PubMed.
- C. Mamat, B. Mosch, C. Neuber, M. Köckerling, R. Bergmann and J. Pietzsch, ChemMedChem, 2012, 7, 1991–2003 CrossRef CAS PubMed.
- B. Mosch, K. Mueller, J. Steinbach and J. Pietzsch, Int. J. Radiat. Biol., 2009, 85, 1002–1012 CrossRef CAS PubMed.
- S. Li, X. Wang, Y. He, M. Zhao, Y. Chen, J. Xu, M. Feng, J. Chang, H. Ning and C. Qi, Eur. J. Med. Chem., 2013, 67, 293–301 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5md00079c |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article






