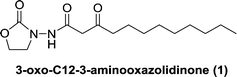
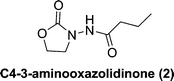
Open Access Article
Open Access Article3-Aminooxazolidinone AHL analogs as hydrolytically-stable quorum sensingagonists in Gram-negative bacteria†
Min
Guo
,
Yue
Zheng
,
Rusty
Starks
,
Clement
Opoku-Temeng
,
Xiaochu
Ma
and
Herman O.
Sintim
*
Department of Chemistry and Biochemistry, University of Maryland, Building 091, College Park, MD 20742, USA. E-mail: hsintim@umd.edu
First published on 20th April 2015
Abstract
Synthetic molecules that modulate quorum sensing, QS, in bacteria have great potential for use in synthetic biology applications as well as acting as anti-virulence and anti-biofilm agents. Acylhomoserine lactone (AHL)-based autoinducer analogs have been extensively developed as QS modulators but these suffer from both chemical and enzymatic degradations. Here, we reveal that 3-aminooxazolidinone acylhomoserine lactone analogs are hydrolytically stable and are as potent in activating LuxR-type receptors. Docking analysis revealed that 3-oxo-C12-3-aminooxazolidinone docked in LasR of P. aeruginosa, making similar interactions with the protein's active-site residues to the native ligand, 3-oxo-C12 HSL. Experimentally, 3-oxo-C12-3-aminooxazolidinone was equally as potent as the natural ligand in inducing bioluminescence in E. coli carrying a bioluminescent gene that was under the control of LasR. In C. violaceum CV026, the 3-aminooxazolidinone analogs could also modulate pigment (violacein) formation, albeit this time not as potent as the natural AHL ligands.
Introduction
The old view that bacteria live in solitary mode has now been replaced with a community-based bacterial lifestyle, whereby most bacteria live on surfaces as part of polymicrobial biofilms1 and communicate with neighbors using diffusible molecules. Bacteria also communicate via contact, using surface associated receptors2 or connecting nanotubes.3 Even in the planktonic state, bacteria can still communicate with neighboring self and non-self cells and react to population density via response to small moleculeautoinducers secreted by other bacteria.4 The cell-to-cell communication between bacteria, called quorum sensing (QS), regulates diverse phenotypes, including biofilm formation, competence, bioluminescence, virulence factors production and antibiotic synthesis.5 Additionally, both plant and animal hosts respond to bacterial signaling molecules and some QS molecules have been shown to promote apoptosis or programmed cell death in diverse eukaryotic cell types.6–9In the past decade, many small molecules that modulate quorum sensing have been developed.10–13 These QS modulators have been either agonists or antagonists and have the potential for use in diverse applications, ranging from inhibition of bacterial toxin production and biofilm formation (QS antagonists),14–18 manipulation of bacterial behavior and synthetic biology applications (both agonists and antagonists)19–21 to the inhibition of cancer (by 3-oxo-C12 HSL of Pseudomonas aeruginosa).22 Thus far, acylhomoserine lactone (AHL)-based QS modulators have been the most rigorously pursued by many groups. The majority of these compounds have targeted LasR from P. aeruginosa.9–15
Most of the AHL analogs developed to date have kept the acylhomoserine lactone head group and modified the acyl chain. A few lactone head group modifications have also been reported but often, modification of the head group usually leads to a dramatic reduction of activity.23 Unfortunately γ-lactones are not chemically stable and can hydrolyze in mild acidic or basic environments.24 Additionally bacterial, plant or animal lactonases25–28 and acylases29–32 have been shown to readily inactivate AHLs so there is clearly a need for an AHL head group that is resistant to hydrolysis and at the same time maintains the high QS modulatory activity seen with homoserinelactones.
We docked several lactone mimics into the active site of P. aeruginosa LasR and found that oxazolidinone-based AHL analogs had similar conformation in the binding site of LasR to the native 3-oxo-C12-HSL. The docking results were somehow surprising to us because many reports have documented the importance of the chirality at the C3 position for AHLautoinducers in activating QS-mediated processes.13,33,34 In this report we show that 3-aminooxazolidinone that lacks a C3 chirality could still bind to some LuxR-type receptors and is as potent, in binding to LasR, as the native 3-oxo-C12 HSL. As an added advantage, the 3-aminooxazolidinone head group is more resistant to hydrolysis than the AHLs and is therefore a good replacement for the lactone head group in AHL-based QS modulators. 3-Aminooxazolidinone-based analogs (Fig. 1) can be made from inexpensive materials in a few steps and are drug-like (examples of oxazolidinonedrugs are linezolid35 and rivaroxaban36).
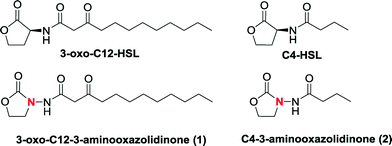 | ||
| Fig. 1 Structures of oxazolidinoneAHL analogs and natural AI-1. | ||
Results and discussion
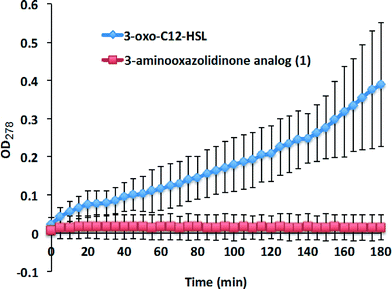
3-Oxo-HSLs are known to degrade under weakly basic conditions and eventually tautomerize to a tetramic acid derivative via a mechanism shown in Scheme 1.24 To demonstrate that 3-aminooxazolidinone-based analogs (see Scheme 2 for synthetic scheme) are superior to natural AHLs, in terms of chemical stability, we monitored the degradation of 3-oxo-C12-HSL and 3-oxo-C12-3-aminooxazolidinone (1) at pH = 8.0 by monitoring UV absorption at 278 nm, which is an absorption maxima for tetramic acid, as a function of time (Fig. 2). Whereas the UV absorption for the 3-oxo-C12-HSL incubation increased over time, that of the analog 1 remained stable over 3 h. HPLC analysis also revealed that analog 1 remained intact after 3 h of incubation in Tris buffer (pH = 8.0) (see the ESI, Fig. S1†). We therefore concluded that 3-aminooxazolidinone analog (1) is more stable than 3-oxo-C12-HSL to basic hydrolysis.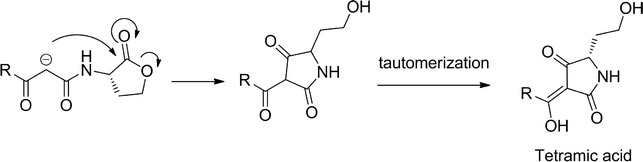 | ||
| Scheme 1 Degradation of AHL under basic conditions. | ||
 | ||
| Scheme 2 Syntheses of the oxazolidinone analogs. | ||
Recently, Raines revealed that the conformation of free AHLs is different when complexed to LasR.37 In the free state, the lone pairs of the amide carbonyl form a favorable interaction with the π* of the lactone carbonyl.37 This n to π* interaction (about 0.64 kcal mol−1) is disrupted upon binding to LasR. Interestingly the substitution of the C3 in 3-oxo-C12-HSL with N3 (aminooxazolidinone-based analogs) did not abrogate the n to π* interaction in the free state (see Fig. 3). Also, the C3 to N3 substitution did not drastically change the surface charge potentials of the head group moieties (compare compounds C2-HSL and C2-3-aminooxazolidinone in Fig. 3), implying that our analogs would be able to partake in charge–charge interactions in the 3-oxo-C12-HSL binding site.
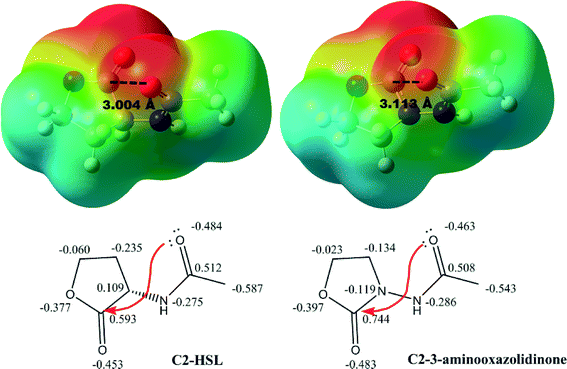 | ||
| Fig. 3 Surface charge potential on simplified models of AHL and oxazolidinone-based mimic. n → π* Interactions from lone pair (n) of the acyl carbonyl group oxygen to the empty π* on the carbon of carbonyl group in the lactone ring and the distances are highlighted. Computational level: B3LYP/6-311+G(d,2p).38 | ||
In most LuxR-type proteins reported to date, Trp60 is highly conserved.39–44 Both Suga and Blackwell have shown that this residue determines whether a ligand acts as an agonist or antagonist.45,46 Ligands that exhibit unfavorable interactions with Trp60 have antagonistic profiles. Recently Blackwell also revealed that the interactions between a ligand and Tyr56 and Ser129 in LasR are also important in determining whether a ligand acts as an antagonist or agonist since these residues bond to the carbonyl of the 3-oxo-C12-HSL ligand to position the lactone head group towards Tyr 60, which is a key residue.45,47 Docking experiments48–50 revealed that the docked poses of 3-oxo-C12-HSL and of the 3-aminooxazolidinone analog (1) are similar, with the exception of the orientation of the 3-oxo group (see Fig. 4). Importantly, the carbonyl head group of both the native ligand and the 3-aminooxazolidinone analog (1) are similarly oriented towards the key Trp60 residue, hinting that the 3-aminooxazolidinone analog (1) would also act as an agonist.
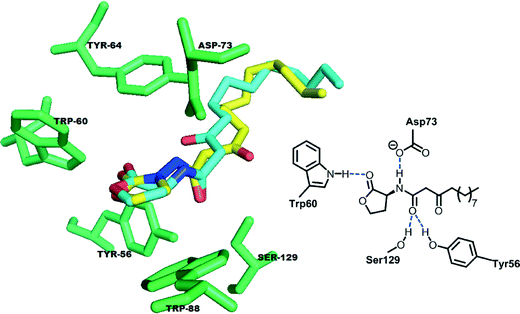 | ||
| Fig. 4 The binding domain (green) in the crystal structure of LasR (PDB code: 2UV0) with native 3-oxo-C12-HSL (cyan) and re-docked 3-aminooxazolidinone analog 1 (yellow). | ||
To test whether the 3-aminooxazolidinone analog (1) would function similarly to native 3-oxo-C12-HSL, as predicted by the docking experiment (see Fig. 4), we used bacterial reporter strain E. coli, pSB1075, (lasRI'::luxCDABE) to test for agonism. In the presence of native 3-oxo-C12-HSL, this bacterial strain produced bioluminescence as expected (see Fig. 5). Similarly, the 3-aminooxazolidinone analog (1) could also induce bioluminescence in E. coli (pSB1075) and the bioluminescence intensities induced by both the native 3-oxo-C12-HSL and the 3-oxo-C12-3-aminooxazolidinone (1) were remarkably similar (Fig. 5). The EC50 of 3-oxo-C12-HSL is 1.5 ± 0.7 nM, while the analog 1 gives an EC50 of 2.1 ± 0.3 nM (see Fig. S2†).
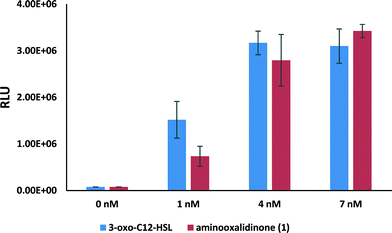 | ||
| Fig. 5 Bioluminescence induction in E. coli pSB1075 after an 8 hour incubation with native 3-oxo-C12-HSL and 3-aminooxazolidinone analog 1 at different concentrations. | ||
Next, we investigated if other LuxR-type proteins would also respond to oxazolidinone analogs. Chromobacterium violaceum CV026 is a biosensor strain that does not produce its own AI-1 but can respond to C4 to C8 AHL molecules, via binding to its LuxR-type QS system CviR, to produce violacein.51 However, long chain AHLs such as 3-oxo-C12-HSL can inhibit the C4–C8 AHL-induced production of violacein.51 Addition of 20 μM C4-HSL to agar incubated with CV026 led to the production of a dark violet pigment (Fig. 6a). C4-3-Aminooxazolidinone (2) was also able to induce the violacein production. Unlike LasR, CviR preferred the native C4 HSL to C4-3-aminooxazolidinone (2). 3-Oxo-C12-HSL can inhibit the C4-HSL-induced violacein production in CV026. In another set of experiment (see Fig. 6b), 3-oxo-C12-3-aminooxazolidinone (1) could inhibit C4-HSL-induced violacein production in CV026 but the concentration of 3-oxo-C12-3-aminooxazolidinone (1) needed to inhibit the activity of 20 μM C4-HSL was higher than the natural 3-oxo-C12-HSL. Whereas 2 μM 3-oxo-C12-HSL could completely inhibit 20 μM C4-HSL-induced violacein production, it required ~200 μM analog 1 to achieve a similar inhibition level (see Fig. 6b).
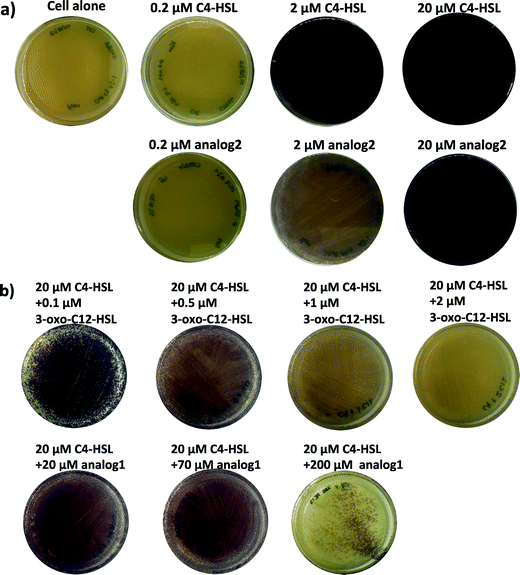 | ||
| Fig. 6 Chromobacterium violaceum CV026 agar plate assay. a) CV026 cultured with different concentrations of C4-HSL and C4-3-aminooxazolidinone (2). b) CV026 cultured with different concentrations of 3-oxo-C12-HSL and 3-oxo-C12-3-aminooxazolidinone (1) in the presence of 20 μM C4-HSL. | ||
Conclusion
In the past decade intensive efforts have been dedicated for the discovery of QS agonists and inhibitors. QS autoinducers have been shown to activate the immune system and hence these molecules and more stable analogs thereof have the potential for use in cancer immunotherapy.22 Hence the hydrolytically stable 3-oxo-C12-HSL analog 1 described in this manuscript could have anticancer properties and future studies along this line are planned. AI-1-based agonists also have the potential for use in synthetic biology applications whereby genetic circuits that are regulated by engineered LuxR-type proteins could be regulated by these molecules.52,53 In this regard, agonists described in this paper, which are more stable to chemical degradation than the natural autoinducer, could become useful in these applications. LasR receptors could accommodate the C3 to N3 substitution better than CviR protein. LasR is key to the production of various virulence factors during P. aeruginosa infection and future study will focus on making side chain variants of the oxazolidinone analogs and test for their activities against P. aeruginosa.Methods
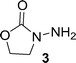
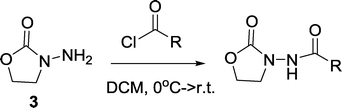
General procedures for preparation of oxazolidinone analogs:The starting material 3 is commercially available. It is however expensive but can be easily made in the gram scale as follows: a solution of NaOH (0.1 g, 2.5 mmol) in 0.5 ml of methanol was added to a mixture of 2-hydroxylethylhydrazine (2.3 g, 30 mmol) and dimethyl carbonate (4 ml, 48 mmol). The resulting mixture was heated and was stirred at 70 °C for 3 h. Then the reaction was cooled down to room temperature and the unreacted dimethyl carbonate was removed in vacuo. The residue was purified by silica column chromatography (methanol : dichloromethane = 1 : 30, v/v), affording 3 as a white solid (2.01 g, 65%).
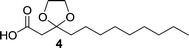
Compound 4 was synthesized according to a procedure in the literature.54
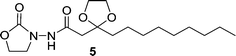
Oxalyl chloride (40 μL, 2.3 equiv.) was added to a solution of 4 (50 mg, 0.19 mmol) in dry dichloromethane at room temperature. The mixture was stirred for 5 h. Then the reaction was concentrated to remove the solvent and excess oxalyl chloride. The residue was re-subjected to dry dichloromethane and the resulting solution was added slowly to a solution of 3 (39 mg, 2 equiv.) in dry dichloromethane at 0 °C. The mixture was allowed to slowly warm up to room temperature and was stirred overnight. Then the reaction was concentrated under vacuum, and the residue was purified by silica column chromatography (methanol : dichloromethane = 1 : 40, v/v), affording 5 as a white solid (59 mg, 90% yield). 1H NMR (CDCl3, 400 MHz) δ 8.39 (s, 1H), 4.41 (t, J = 7.8 Hz, 2H), 4.11–4.02 (m, 2H), 4.02–3.93 (m, 2H), 3.81 (t, J = 7.8 Hz, 2H), 2.65 (s, 2H), 1.78–1.67 (m, 2H), 1.44–1.32 (m, 2H), 1.32–1.18 (m, 12H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ 168.8, 157.8, 109.8, 65.6, 62.3, 46.3, 43.5, 38.0, 32.3, 30.1, 29.9, 29.7, 24.0, 23.1, 14.5; HRMS (ESI-TOF) m/z calcd. for C17H31N2O5 [M + 1]+ 343.2233, found 343.2199.
Compound 5 (55 mg, 0.16 mmol) was dissolved in trifluoroacetic acid (0.64 ml) and water (0.16 ml). The mixture was stirred at room temperature overnight. Then the reaction was quenched by saturated NaHCO3(aq) until the solution turned neutral. Dichloromethane was used to extract the product three times and the organic phase was dried using anhydrous MgSO4. The product was purified by silica column chromatography (methanol : dichloromethane = 1 : 40, v/v) and afforded 3-oxo-C12-3-aminooxazolidinone (1) as a white solid (34 mg, 71% yield). 1H NMR (CDCl3, 400 MHz) δ 9.08 (s, 1H), 4.44 (t, J = 7.8 Hz, 2H), 3.84 (t, J = 7.8 Hz, 2H), 3.52 (s, 2H), 2.56 (t, J = 7.4 Hz, 2H), 1.64–1.52 (m, 2H), 1.35–1.18 (m, 12H), 0.88 (t, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ 205.8, 165.9, 158.0, 62.5, 48.0, 46.4, 44.1, 32.2, 29.8, 29.6, 29.4, 23.1, 14.5; HRMS (ESI-TOF) m/z calcd. for C15H27N2O4 [M + 1]+ 299.1971, found 299.1967.
Butyryl chloride (50 μL, 0.5 mmol, 1 equiv.) was added to a solution of 3 (102 mg, 1 mmol, 2 equiv.) in anhydrous dichloromethane at 0 °C. The mixture was allowed to warm up to room temperature slowly and was stirred for 3 h. Then the reaction was concentrated under vacuum, and the residue was purified by silica column chromatography (methanol : dichloromethane = 1 : 30, v/v) and afforded 2 as a 34 mg pale yellow oil (40% yield).1H NMR (CDCl3, 400 MHz) δ 8.49 (brs, 1H), 4.45 (t, J = 8.1 Hz, 2H), 3.84 (t, J = 8.1 Hz, 2H), 2.23 (t, J = 7.4 Hz, 2H), 1.78–1.61 (m, 2H), 0.98 (t, J = 7.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ 172.9, 158.5, 62.5, 46.4, 36.0, 19.0, 14.0; HRMS (ESI-TOF) m/z calcd. for C7H13N2O3 [M + 1]+ 173.0926, found 173.0903.
Docking calculations
Docking calculations were performed using Autodock Vina 1.1.1.48 A large grid box, which is enough to encompass the ligand in the binding pocket, was chosen. The exhaustiveness value was set to 32 in the Autodock calculations and the rest of the parameters were used as default. The ligand PDB files were prepared with ChemDraw. Autodock Tools 1.5.4 was used to convert the PDB files into PDBPT files for the Autodock Vina calculations. The top-ranked conformation poses were selected for analysis. Results were visualized using PyMOL viewer version 1.3.49Stability studies
The stability of 3-oxo-C12-HSL and 3-oxo-C12-3-aminooxazolidinone (1) to basic pH was determined viaHPLC monitoring.24,55 Briefly, the decomposition of AI-1 or the analog (1 mM) in 180 mM Tris-HCl, pH 8.0 at 25 °C was monitored by following absorbance changes at 278 nm, using a Jasco V-630 spectrophotometer for 3 hours.The stability of 3-oxo-C12-3-aminooxazolidinone (1) to basic pH was determined viaTLC as well (Fig. S1†). 10 mM analog 1 in methanol was mixed with an equivolume of 250 mM Tris-HCl buffer (pH = 8.0) and left at 25 °C for 3 hours. The mixture was spotted on TLC plate and developed using eluent (methanol : dichloromethane = 1 : 40, v/v). After developing, the TLC plate was air dried and stained using KMnO4solution.
Bioluminescence assay
E. coli JM109 (pSB1075) (containing lasRI'::luxCDABE, a bioluminescent reporter gene) was cultured at 37 °C overnight and diluted 10 times with fresh LB medium. After culture at 37 °C for 7 hours, OD600 was measured and diluted to OD600 = 0.01. A cell culture was grown in 37 °C for another hour and diluted to OD600 = 0.005. 3-Oxo-C12-HSL and analogs were added to the cell cultures and incubated at 37 °C with shaking for 8 hours. Bioluminescence was measured using a Nichols Institute Diagnostics luminometer.C. violaceum CV026 AHL reporter assay
Agar plate assay:56 different concentrations of AI-1 and analogs were added into LB agar. C. violaceum CV026 culture was diluted to OD600 of 0.1 and spread onto the agar plates. The plates were incubated at 37 °C overnight and 25 °C for another day.Acknowledgements
We are grateful to the NSF (CBET 1264509) and the Camille Dreyfus Foundation (Teacher–Scholar Fellowship to HOS) for funding. YZ is supported by the Mehta Graduate Research Award and the Kraybill Biochemistry Fellowship. We also thank Professors Shamala Devi Sekaran and Max Teplitski for their generous gifts of bacterial strains Chromobacterium violaceum CV026 and E. coli JM109 (pSB1075), respectively.References
- L. Hall-Stoodley, J. W. Costerton and P. Stoodley, Nat. Rev. Microbiol., 2004, 2, 95–108 CrossRef CAS PubMed.
- A. H. Rickard, R. J. Palmer, Jr., D. S. Blehert, S. R. Campagna, M. F. Semmelhack, P. G. Egland, B. L. Bassler and P. E. Kolenbrander, Mol. Microbiol., 2006, 60, 1446–1456 CrossRef CAS PubMed.
- G. P. Dubey and S. Ben-Yehuda, Cell, 2011, 144, 590–600 CrossRef CAS PubMed.
- K. H. Nealson, T. Platt and J. W. Hastings, J. Bacteriol., 1970, 104, 313–322 CAS.
- C. M. Waters and B. L. Bassler, Annu. Rev. Cell Dev. Biol., 2005, 21, 319–346 CrossRef CAS PubMed.
- L. R. Usher, R. A. Lawson, I. Geary, C. J. Taylor, C. D. Bingle, G. W. Taylor and M. K. Whyte, J. Immunol., 2002, 168, 1861–1868 CrossRef CAS.
- M. R. Kaufman, J. Jia, L. Zeng, U. Ha, M. Chow and S. Jin, Microbiology, 2000, 146(Pt 10), 2531–2541 CrossRef CAS PubMed.
- C. A. Jacobi, F. Schiffner, M. Henkel, M. Waibel, B. Stork, M. Daubrawa, L. Eberl, M. Gregor and S. Wesselborg, Int. J. Med. Microbiol., 2009, 299, 509–519 CrossRef CAS PubMed.
- H. Li, L. Wang, L. Ye, Y. Mao, X. Xie, C. Xia, J. Chen, Z. Lu and J. Song, Med. Microbiol. Immunol., 2009, 198, 113–121 CrossRef CAS PubMed.
- H. O. Sintim, J. A. I. Smith, J. Wang, S. Nakayama and L. Yan, Future Med. Chem., 2010, 2, 1005–1035 CrossRef CAS PubMed.
- W. R. Galloway, J. T. Hodgkinson, S. D. Bowden, M. Welch and D. R. Spring, Chem. Rev., 2011, 111, 28–67 CrossRef CAS PubMed.
- C. A. Lowery, T. J. Dickerson and K. D. Janda, Chem. Soc. Rev., 2008, 37, 1337–1346 RSC.
- G. D. Geske, J. C. O'Neill and H. E. Blackwell, Chem. Soc. Rev., 2008, 37, 1432–1447 RSC.
- M. Givskov, R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg and S. Kjelleberg, J. Bacteriol., 1996, 178, 6618–6622 CAS.
- M. Hentzer, H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffersen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Hoiby and M. Givskov, EMBO J., 2003, 22, 3803–3815 CrossRef CAS PubMed.
- T. Hjelmgaard, T. Persson, T. B. Rasmussen, M. Givskov and J. Nielsen, Bioorg. Med. Chem., 2003, 11, 3261–3271 CrossRef CAS PubMed.
- J. C. Kwan, T. Meickle, D. Ladwa, M. Teplitski, V. Paul and H. Luesch, Mol. BioSyst., 2011, 7, 1205–1216 RSC.
- (a) G. D. Geske, J. C. O'Neill, D. M. Miller, M. E. Mattmann and H. E. Blackwell, J. Am. Chem. Soc., 2007, 129, 13613–13625 CrossRef CAS PubMed; (b) C. T. O'Loughlin, L. C. Miller, A. Siryaporn, K. Drescher, M. F. Semmelhack and B. L. Bassler, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17981–17986 CrossRef PubMed.
- B. L. Adams, K. K. Carter, M. Guo, H. C. Wu, C. Y. Tsao, H. O. Sintim, J. J. Valdes and W. E. Bentley, ACS Synth. Biol., 2013, 3, 210–219 CrossRef PubMed.
- S. H. Hong, M. Hegde, J. Kim, X. Wang, A. Jayaraman and T. K. Wood, Nat. Commun., 2012, 3, 613 CrossRef PubMed.
- J. Shong and C. H. Collins, ACS Synth. Biol., 2013, 2, 568–575 CrossRef CAS PubMed.
- V. Kravchenko, A. L. Garner, J. Mathison, A. Seit-Nebi, J. Yu, I. P. Gileva, R. Ulevitch and K. D. Janda, ACS Chem. Biol., 2013, 8, 1117–1120 CrossRef CAS PubMed.
- A. L. Schaefer, B. L. Hanzelka, A. Eberhard and E. P. Greenberg, J. Bacteriol., 1996, 178, 2897–2901 CAS.
- G. F. Kaufmann, R. Sartorio, S. H. Lee, C. J. Rogers, M. M. Meijler, J. A. Moss, B. Clapham, A. P. Brogan, T. J. Dickerson and K. D. Janda, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 309–314 CrossRef CAS PubMed.
- Y. H. Dong, A. R. Gusti, Q. Zhang, J. L. Xu and L. H. Zhang, Appl. Environ. Microbiol., 2002, 68, 1754–1759 CrossRef CAS PubMed.
- D. I. Draganov, J. F. Teiber, A. Speelman, Y. Osawa, R. Sunahara and B. N. La Du, J. Lipid Res., 2005, 46, 1239–1247 CrossRef CAS PubMed.
- Y. H. Dong and L. H. Zhang, J. Microbiol., 2005, 43, 101–109 CAS.
- M. Teplitski, U. Mathesius and K. P. Rumbaugh, Chem. Rev., 2011, 111, 100–116 CrossRef CAS PubMed.
- J. R. Leadbetter and E. P. Greenberg, J. Bacteriol., 2000, 182, 6921–6926 CrossRef CAS PubMed.
- T. Morohoshi, S. Nakazawa, A. Ebata, N. Kato and T. Ikeda, Biosci., Biotechnol., Biochem., 2008, 72, 1887–1893 CrossRef CAS PubMed.
- J. J. Huang, J. I. Han, L. H. Zhang and J. R. Leadbetter, Appl. Environ. Microbiol., 2003, 69, 5941–5949 CrossRef CAS PubMed.
- M. Romero, S. P. Diggle, S. Heeb, M. Camara and A. Otero, FEMS Microbiol. Lett., 2008, 280, 73–80 CrossRef CAS PubMed.
- T. Ikeda, K. Kajiyama, T. Kita, N. Takiguchi, A. Kuroda, J. Kato and H. Ohtake, Chem. Lett., 2001, 30, 314–315 CrossRef.
- G. D. Geske, J. C. O'Neill, D. M. Miller, R. J. Wezeman, M. E. Mattmann, Q. Lin and H. E. Blackwell, ChemBioChem, 2008, 9, 389–400 CrossRef CAS PubMed.
- S. J. Brickner, Curr. Pharm. Des., 1996, 2, 175–194 CAS.
- S. Roehrig, A. Straub, J. Pohlmann, T. Lampe, J. Pernerstorfer, K. H. Schlemmer, P. Reinemer and E. Perzborn, J. Med. Chem., 2005, 48, 5900–5908 CrossRef CAS PubMed.
- R. W. Newberry and R. T. Raines, ACS Chem. Biol., 2014, 9, 880–883 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford, CT, USA, 2009 Search PubMed.
- R. G. Zhang, K. M. Pappas, J. L. Brace, P. C. Miller, T. Oulmassov, J. M. Molyneaux, J. C. Anderson, J. K. Bashkin, S. C. Winans and A. Joachimiak, Nature, 2002, 417, 971–974 CrossRef CAS PubMed.
- M. J. Lintz, K. Oinuma, C. L. Wysoczynski, E. P. Greenberg and M. E. Churchill, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 15763–15768 CrossRef CAS PubMed.
- M. J. Bottomley, E. Muraglia, R. Bazzo and A. Carfi, J. Biol. Chem., 2007, 282, 13592–13600 CrossRef CAS PubMed.
- G. Chen, L. R. Swem, D. L. Swem, D. L. Stauff, C. T. O'Loughlin, P. D. Jeffrey, B. L. Bassler and F. M. Hughson, Mol. Cell, 2011, 42, 199–209 CrossRef CAS PubMed.
- Y. Zou and S. K. Nair, Chem. Biol., 2009, 16, 961–970 CrossRef CAS PubMed.
- A. Vannini, C. Volpari, C. Gargioli, E. Muraglia, R. Cortese, R. De Francesco, P. Neddermann and S. D. Marco, EMBO J., 2002, 21, 4393–4401 CrossRef CAS PubMed.
- J. P. Gerdt, C. E. McInnis, T. L. Schell, F. M. Rossi and H. E. Blackwell, Chem. Biol., 2014, 1361–1369 CrossRef CAS PubMed.
- G. J. Jog, J. Igarashi and H. Suga, Chem. Biol., 2006, 13, 123–128 CrossRef CAS PubMed.
- Y. Zheng and H. O. Sintim, Chem. Biol., 2014, 21, 1261–1263 CrossRef CAS PubMed.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CAS.
- PyMOL is readily available and can be uploaded via the Internet.
- M. Ahumedo, J. C. Drosos and R. Vivas-Reyes, Mol. BioSyst., 2014, 10, 1162–1171 RSC.
- K. H. McClean, M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart and P. Williams, Microbiology, 1997, 143(Pt 12), 3703–3711 CrossRef CAS PubMed.
- C. H. Collins, J. R. Leadbetter and F. H. Arnold, Nat. Biotechnol., 2006, 24, 708–712 CrossRef CAS PubMed.
- B. Koch, T. Liljefors, T. Persson, J. Nielsen, S. Kjelleberg and M. Givskov, Microbiology, 2005, 151, 3589–3602 CrossRef CAS PubMed.
- J. T. Hodgkinson, W. R. J. D. Galloway, M. Casoli, H. Keane, X. Su, G. P. C. Salmond, M. Welch and D. R. Spring, Tetrahedron Lett., 2011, 52, 3291–3294 CrossRef CAS.
- K. Kamaraju, J. Smith, J. Wang, V. Roy, H. O. Sintim, W. E. Bentley and S. Sukharev, Biochemistry, 2011, 50, 6983–6993 CrossRef CAS PubMed.
- D. Anbazhagan, M. Mansor, G. O. Yan, M. Y. Md Yusof, H. Hassan and S. D. Sekaran, PLoS One, 2012, 7, e36696 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5md00015g |
| This journal is © The Royal Society of Chemistry 2015 |