Naphthoquinone-based chalcone hybrids and derivatives: synthesis and potent activity against cancer cell lines†
Guilherme A. M. Jardim a, Tiago T. Guimarães b, Maria do Carmo F. R. Pinto c, Bruno C. Cavalcanti d, Kaio M. de Farias d, Claudia Pessoa de, Claudia C. Gatto f, Divya K. Nair g, Irishi N. N. Namboothiri *g and Eufrânio N. da Silva Júnior *a
aInstitute of Exact Sciences, Department of Chemistry, Federal University of Minas Gerais, CEP 31270-901, Belo Horizonte-MG, Brazil. E-mail: eufranio@ufmg.br; Fax: +55 31 34095700; Tel: +55 31 34095720
bInstituto Nacional de Câncer, Hospital do Câncer – Unidade I – Seção de Medicina Nuclear, 20230-130, Rio de Janeiro, RJ, Brazil
cNúcleo de Pesquisas de Produtos Naturais, UFRJ, 21944-971, Rio de Janeiro, RJ, Brazil
dDepartamento de Fisiologia e Farmacologia, UFC, 60430-270, Fortaleza, CE, Brazil
eFiocruz – Ceará, 60180-900, Fortaleza, CE, Brazil
fInstitute of Chemistry, University of Brasilia, CEP 70904970, Brasilia-DF, Brazil
gDepartment of Chemistry, Indian Institute of Technology Bombay, Mumbai 400 076, India. E-mail: irishi@iitb.ac.in; Fax: +91-22-2576-7152; Tel: +91-22-2576-7196
First published on 24th September 2014
Abstract
Novel naphthoquinone-based chalcones were prepared from the reaction between 3-bromo-nor-β-lapachone and amino-chalcones. Lapachone derivatives are also described here. All the substances were evaluated against cancer and normal cell lines and several compounds demonstrated potent antitumor activity.
1. Introduction
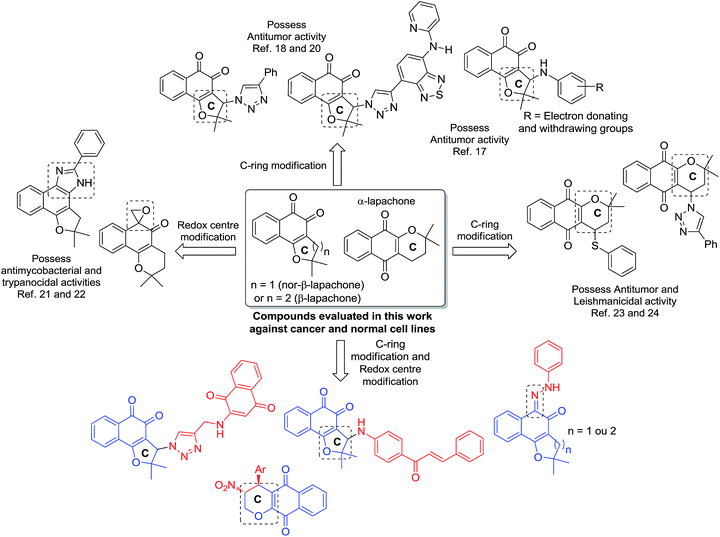
The population growth and aging associated with some risk factors have increased the incidence of new cases of cancer and related deaths in developed countries and in developing countries.1 In a four-year period, the estimated number increased from 12.7 million new cancer cases with 7.6 million cancer-related deaths in 2008, to 14.1 million new cancer cases and 8.2 million cancer-related deaths in 2012. The projection for the year 2030, estimates 27 million new cancer cases with 17 million cancer-related deaths.2,3Naphthoquinone containing natural products belong to an important class of naturally occurring secondary metabolites found in the bignoniaceae family.4 Among these, naphthoquinoidal compounds obtained from natural products, such as lapachol and lawsone (2-hydroxy-1,4-naphthoquinone), have received considerable attention because of their antitumor potential.5–8 1,4- and 1,2-naphthoquinones, and for instance, dehydro-α-lapachone, an important anti-vascular agent,9 and β-lapachone, a potent antitumoral compound, have also been used as prototypes for the development of new drugs.10 Generally, quinones are able to provoke apoptosis and act as topoisomerase inhibitors via DNA intercalation. Their toxicity can also be explained by inducing oxidative stress through reactive oxygen species (ROS) generation.11 Recently, described by Bolognesi and coworkers, quinones have attracted attention due to their activity being intrinsically related by a multitarget mechanism.12
In recent years, lapachones were employed as key substrates for the synthesis of complex diazaazulenones,13 spirolactones,14 oxazoles15 and other potentially bioactive heterocyclic compounds.15 Our research group has explored the potential of quinones against cancer, mainly, via structural modification of the nor-β-lapachone, particularly, the C-ring and redox centre modification16 since these moieties are deeply related with the generation of ROS (Scheme 1).
Earlier, we reported the synthesis of nor-β-lapachone-based 1,2,3-triazole and 3-arylamino derivatives with potent antitumor activity via C-ring modification of nor-β-lapachone and molecular hybridization19 with the junction of 1,2,3-triazole groups (Scheme 1).17,18 Recently, we described a derivative of nor-β-lapachone coupled benzothiadiazole (Scheme 1) with potent activity against twenty cancer cell lines and low cytotoxicity against three normal cells.20 These results were very promising and the mechanism of action of this substance in tumor cells is being currently investigated in our laboratories and it will be reported in due course.
Using redox centre modification, imidazoles and an oxirane derivative were obtained from nor-β-lapachone and β-lapachone (Scheme 1), with antimycobacterial21 and trypanocidal22 activities, indicating the importance of this approach. The C-ring modification in the α-lapachone was also accomplished and thio-derivatives with antitumor activities and 1,2,3-triazoles with leishmanicidal activity were recently reported.23,24
Finally, following our program to develop new bioactive molecules, particularly, new nor-β-lapachones with activity against cancer cell lines and low cytotoxicity against normal cells, the synthesis of quinone-based chalcones, is described herein for the first time. The strategies of molecular hybridization and C-ring modification were used because of chalcone's known antitumor activity.25 Redox centre modification in lapachones to obtain hydrazone derivatives and C-ring modification in α-lapachone in addition to nor-β-lapachone coupled with para-quinones were also accomplished. All the substances were evaluated against cancer and normal cell lines.
2. Results and discussion
Chemistry
To obtain the first class of compounds, nor-β-lapachone-based chalcones, initially amine-chalcones were prepared in good yields (Scheme 2). The syntheses of chalcones were easily accomplished by condensing 4′-aminoacetophenone with different aldehydes in the presence of sodium hydroxide in ethanol. Compounds 1–6 were prepared with electron-donating (methyl, phenyl, methoxy and isopropyl groups) and an electron-withdrawing group (nitro) in addition to the unsubstituted, 1. All the substances are known in the literature.26–28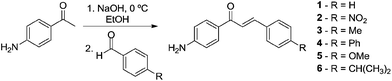 | ||
| Scheme 2 Synthesis of the chalcone intermediates 1–6. | ||
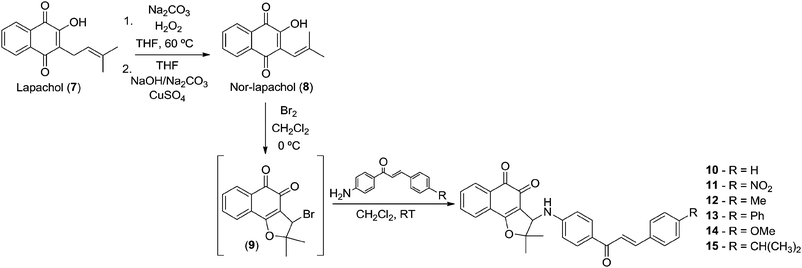
Using the Hooker oxidation methodology29 nor-lapachol 8 was prepared from lapachol 7 and used to obtain the classic intermediate 3-bromo-nor-β-lapachone 9 in quantitative yield. Compound 9 was reacted with the previously prepared amine-chalcones 1–6 to provide nor-β-lapachone-based chalcones 10–15 (Scheme 3).
New compounds 10–15 were well characterized by 1H and 13C NMR and high resolution mass spectrometry and all the data are in accordance with the proposed structures. It was observed in the 1H NMR spectrum of chalcone derivatives that the respective doublet signals with coupling constant, J = 15.6 Hz indicated the presence of the trans-olefin of the quinone coupled chalcone. Another important observation is a singlet at around δ 4.90–5.00 corresponding to the hydrogen attached to the C-3 in the chalcone derivatives. It is more shielded, compared to its counterpart in the bromo derivative 9, which is observed at δ 5.40. In addition, the 13C NMR spectra showed all the expected signals. The structure of quinone-based chalcone 14 was reconfirmed by X-ray crystallography (Fig. 1).
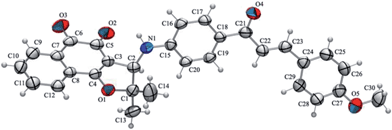 | ||
| Fig. 1 An ORTEP-3 representation (50% probability displacement ellipsoids) of the asymmetric unit of compound 14. | ||
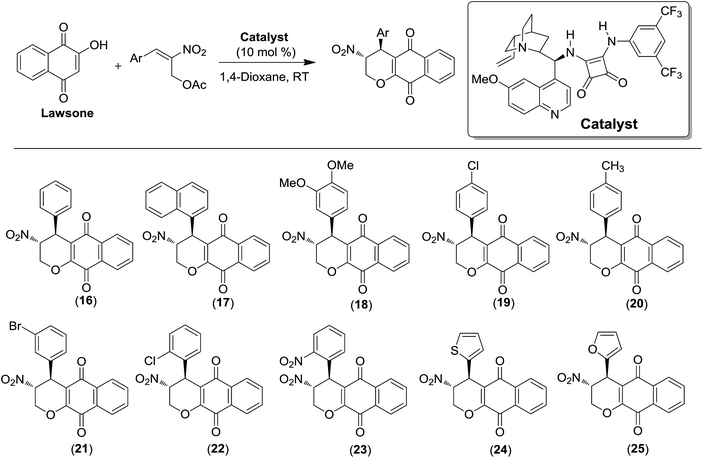
Recently, the second class of substances was reported by us (Scheme 4).30 Lawsone was reacted with Morita–Baylis–Hillman acetates of nitroalkenes in the presence of a quinine–squaramide organocatalyst to provide enantioenriched disubstituted α-lapachones. This methodology allowed us to access asymmetric lapachones that are C-ring modified in a single step. In general, the products were obtained in high yields with excellent diastereo- and enantioselectivities.30 The catalyst shown in Scheme 4 was the most effective.
The compounds 16–25 assayed against cancer cell lines here were previously described.30
In view of the ease of access to lapachone derivatives by selective chemical transformation of lapachol 7, we invested to study the corresponding hydrazone derivatives. In addition, the hydrazones of the β-lapachone 26, 3-iodo-β-lapachone 27, 3-bromo-β-lapachone 28, α-lapachone 32, nor-β-lapachone 34, nor-α-lapachone 36 and 3-hydroxy-nor-β-lapachone 38, were obtained in high yields. In fact, the hydrazones of naturally occurring quinones have been considered for a long time as simple derivatives to confirm the identity of quinones. To the best of our knowledge, the use of hydrazones from quinones of the lapachol's group was not considered for major antitumor studies until now.
Initially, β-lapachone 26, its derivatives 27 and 28 and α-lapachone 32, were prepared from lapachol 7, by reaction with sulfuric acid, iodine, bromine and CH3COOH/HCl, respectively, as previously reported.31,32 Nor-lapachol 8 was used to obtain 3-hydroxy-nor-β-lapachone 38, nor-β-lapachone 34 and nor-α-lapachone 36.33 The hydrazone derivatives were prepared according to the methodology previously published with minor modifications.34 The reaction of the appropriate quinones with phenylhydrazine hydrochloride in acetic acid yielded the respective derivatives 29–31, 33, 35, 37 and 39 in excellent yields (Scheme 5).
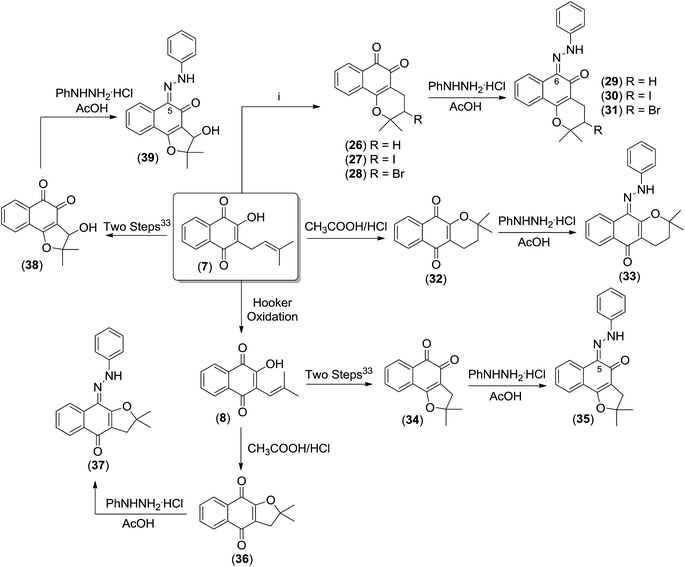 | ||
| Scheme 5 Synthesis of the hydrazone derivatives 29–31, 33, 35, 37 and 39. (i) To obtain compound 26: H2SO4, 27: I2, Py, CH2Cl2, 0 °C, 28: Br2, CH2Cl2. | ||
The spectroscopic data of hydrazone derivatives are in accordance with the proposed structures. The formation of these compounds followed the regioselective pattern previously observed and it is possible to conclude that the benzylic carbonyl group formed the hydrazone in all the cases.34 This is evident from 1H NMR data that the peri-hydrogen of the naphthalene ring appeared at δ 7.55 to 7.14 ppm, due to the anisotropic hydrazo group at carbon C-6. In addition, the 13C NMR spectra of 30, 35 and 39, for instance, showed similar chemical shifts for the carbonyls, at δ 179.1, 178.2 and 177.0 ppm, respectively, which is consistent with carbonyl at carbon C-6 (for 30) and C-5 (for 35 and 39). A common feature observed for compounds 30, 35 and 39 in the 1H NMR spectra using CDCl3 as solvent is the presence of sharp singlet signals in the downfield region (δ 15.5–16.5 ppm) attributed to one N–H, which could form an internal hydrogen bond with the carbonyl of the ortho-hydrazone moiety, a strong H-bond acceptor.
α-Lapachone 32 gave the regioselective para-hydrazone 33, which is confirmed by the presence in its 1H NMR spectrum of an isolated downfield doublet in the aromatic region, attributed to one naphthalene peri-hydrogen. Thus, as in the case of the above ortho-hydrazones, the anisotropic effect of the hydrazine moiety on the peri-hydrogen of naphthalene is also observed in the case of para-hydrazones. On the other hand, in comparison to the original methylene hydrogens of the pyran ring in 32, the 1H NMR of the corresponding ones of 33, shows only negligible effects, indicating that the hydrazo group has little influence over the pyran ring.
Recently, the trypanocidal activity of nor-β-lapachone-derived 1,2,3-triazole-conjugated aminoquinones was described.35 These compounds present ortho- and para-carbonyl moieties that are able to generate ROS and these can be considered as important structures for evaluation against cancer cell lines because the activity of quinoidal molecules is deeply related with ROS generation as previously discussed.11,12 Bearing in mind the importance of these substances, we considered the compounds 41–43, obtained from lapachol 7 (Scheme 6) for antitumor studies. Nor-lapachol 8 was cyclized to 3-bromo-nor-β-lapachone that was used to prepare compound 40. The azido derivative 40 was used to synthesize 41–43 and all spectroscopy data were in accordance with the literature.35
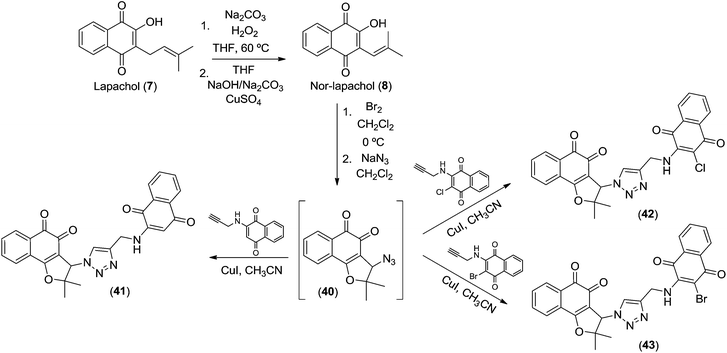 | ||
| Scheme 6 Lapachones 41–43 obtained from lapachol (7). | ||
3. Biology
All the substances described (Schemes 2–5), were evaluated in vitro using the MTT assay against four cancer cell lines: HL-60 (human promyelocytic leukemia), OVCAR-8 (human ovarian adenocarcinoma), SF295 (human glioblastoma) and HCT-116 (human colon carcinoma). Doxorubicin was used as the positive control (Table 1).36 The selectivity of the compounds toward a normal proliferating cell line was investigated using the MTT assay with human peripheral blood mononuclear cells (PBMC) after 72 h of drug exposure.| Compounds | HL-60 | HCT-116 | OVCAR-8 | SF295 | PBMC |
|---|---|---|---|---|---|
| a Results obtained by nonlinear regression for all assayed cell lines from three independent experiments (deviations in parenthesis). | |||||
| 1 | 6.40 (5.24–7.84) | 14.1 (12.86–15.59) | >22.4 | >22.4 | 20.2 (19.54–22.00) |
| 2 | 1.75 (0.89–3.46) | 10.96 (9.99–12.01) | >18.65 | >18.65 | 13.83 (13.54–14.54) |
| 3 | 0.46 (0.08–1.94) | 15.35 (12.73–18.47) | >21.08 | >21.08 | >21.08 |
| 4 | >16.71 | >16.71 | >16.71 | >16.71 | >16.71 |
| 5 | 10.50 (8.53–12.99) | >19.75 | >19.75 | >19.75 | >19.75 |
| 6 | 10.0 (12.52–17.53) | >18.85 | >18.85 | >18.85 | >18.85 |
| 10 | 0.04 (0.02–0.04) | 0.71 (0.62–0.82) | 1.53 (1.33–1.75) | 0.37 (0.26–0.53) | 2.20 (1.82–2.64) |
| 11 | 0.20 (0.16–0.26) | 2.22 (1.94–2.57) | 4.35 (3.78–4.81) | 2.89 (2.50–3.39) | 6.51 (5.80–6.88) |
| 12 | 0.10 (0.04–0.21) | 1.94 (1.77–2.15) | 2.13 (1.81–2.52) | 4.57 (4.14–5.05) | 7.90 (7.66–8.26) |
| 13 | 2.20 (1.84–2.60) | >9.5 | >9.5 | >9.5 | >9.5 |
| 14 | 0.10 (0.08–0.12) | 1.54 (0.91–1.89) | 0.64 (0.33–1.08) | 1.08 (0.56–1.46) | 1.33 (0.85–1.73) |
| 15 | 0.14 (0.10–0.18) | 1.56 (1.18–2.23) | 2.54 (1.99–2.91) | 1.62 (1.30–2.13) | 2.28 (1.77–2.64) |
| 16 | >14.92 | >14.92 | >14.92 | >14.92 | >14.92 |
| 17 | >12.98 | >12.98 | >12.98 | >12.98 | >12.98 |
| 18 | >12.65 | >12.65 | >12.65 | >12.65 | >12.65 |
| 19 | >13.54 | >13.54 | >13.54 | >13.54 | >13.54 |
| 20 | >14.32 | >14.32 | >14.32 | >14.32 | >14.32 |
| 21 | >12.10 | >12.10 | >12.10 | >12.10 | >12.10 |
| 22 | >13.54 | >13.54 | >13.54 | >13.54 | >13.54 |
| 23 | >13.15 | >13.15 | >13.15 | >13.15 | >13.15 |
| 24 | >14.66 | >14.66 | >14.66 | >14.66 | >14.66 |
| 25 | >15.38 | >15.38 | >15.38 | >15.38 | >15.38 |
| 29 | >15.05 | >15.05 | >15.05 | >15.05 | >15.05 |
| 30 | >10.91 | >10.91 | >10.91 | >10.91 | >10.91 |
| 31 | >12.19 | >12.19 | >12.19 | >12.19 | >12.19 |
| 33 | >15.05 | >15.05 | >15.05 | >15.05 | >15.05 |
| 35 | >15.71 | >15.71 | >15.71 | >15.71 | >15.71 |
| 37 | >15.71 | >15.71 | >15.71 | >15.71 | >15.71 |
| 39 | >14.96 | >14.96 | >14.96 | >14.96 | >14.96 |
| 41 | 0.45 (0.39–0.54) | 0.58 (0.52–0.66) | 1.33 (1.12–1.58) | 1.29 (1.14–1.43) | 0.27 (0.14–0.37) |
| 42 | 1.76 (1.43–2.17) | 1.90 (1.65–2.21) | 3.32 (2.82–4.06) | 1.80 (1.55–2.10) | 1.10 (0.83–1.18) |
| 43 | 1.75 (1.37–2.25) | 2.32 (2.25–2.72) | 4.36 (3.83–4.78) | 2.21 (1.52–2.79) | 1.00 (0.87–1.07) |
| Nor-β-lapachone | 1.75, 1.59–1.83 | 1.41, 1.28–1.54 | 1.25, 1.07–1.43 | 1.58, 1.31–1.88 | >21.90 |
| β-Lapachone | 1.65, 1.49–1.78 | 0.97, 0.81–1.04 | 1.16, 0.97–1.25 | 0.91, 0.74–1.11 | >20.60 |
| α-Lapachone | 8.18, 7.55–8.92 | 19.11, 18.75–19.88 | 14.79, 14.42–18.15 | 18.77, 18.15–19.03 | >20.66 |
| Doxorubicin | 0.03, 0.01–0.05 | 0.02, 0.01–0.03 | 0.09, 0.04–0.12 | 0.48, 0.37–0.52 | 0.37, 0.30–0.43 |
Our strategy was based on the modification of the prototypes β-lapachone 26, α-lapachone 32, nor-β-lapachone 34 and nor-α-lapachone 36. Recently, 1,2,3-triazole-, arylamino- and thio-substituted α-lapachones were synthesized and evaluated against eleven cancer cell lines.24,35,36 Most of them were considered moderately active and several compounds were considered highly active (IC50 < 2 μM). Following this previous work, herein, the antitumor activities of asymmetric α-lapachone C-ring modified 16–25 were evaluated.
The selected compounds have different patterns of substitution with an aryl ring substituted by electron withdrawing (Cl and NO2) and donating (OMe and CH3) groups in different positions, in addition to furan and thiophene rings. Unfortunately, in this study our strategy has failed and none of the compounds presented antitumor activity with IC50 values > 12.1 μM (Table 1). At present, several modifications of the asymmetric lapachones, for instance the insertion of 1,2,3-triazoles, are being currently investigated and will be reported in due course.
Recently, redox centre modification in quinones was successfully employed to obtain antitumor compounds.16 Imine derivatives obtained from β-lapachone 26 presented potent antitumor activity.37 In this context, we conducted the synthesis of hydrazone derivatives from β-lapachone 26, their iodine and bromine derivatives 27 and 28, α-lapachone 32, nor-β-lapachone 34, nor-α-lapachone 36 and 3-hydroxy-nor-β-lapachone 38, all the substances from the lapachol group. The derivatives 29–31, 33, 35, 37 and 39 were evaluated against four cancer cell lines. In the same manner as the asymmetric quinones, the hydrazones were not active against all the lineages evaluated (Table 1). In comparison of these structures with the imines, previously obtained from β-lapachone 26,37 the simple insertion of the NH-bond was enough to provoke the suppression of the activity. As suggested by Burton and coworkers,37 the change in the redox centre does not affect the overall activity and selectivity of the lapachone imine derivatives and it was described as an alternative in the search for new anticancer agents considering the high cytotoxicity of the β-lapachone 26 in normal cell lines. On the basis of this principle, we considered the redox centre modification of lapachone derivatives as an important strategy to obtain novel antitumor drugs despite the lack of activity of the compounds described herein.
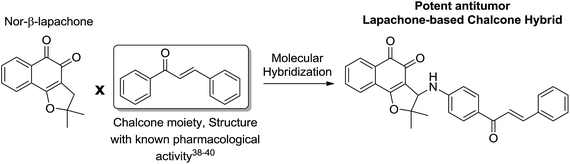
The third group of compounds was strategically planned by C-ring modification16 of nor-β-lapachone 34 and molecular hybridization19 with chalcone moieties (Scheme 7).
Chalcones are recognized by their biological activities, for instance, antitumor,38 leishmanicidal39 and antimalarial.40 Considering the potential of the chalcones, naphthoquinoidal compounds 10–15 were prepared and evaluated against cancer and normal cell lines (Table 1). The selectivity index was considered to establish the potential of these structures (Table 2).
| Compounds | PBMC vs. HL 60 | PBMC vs. HCT-116 | PBMC vs. OVCAR-8 | PBMC vs. SF295 |
|---|---|---|---|---|
| 1 | 3.1 | 1.4 | 0.9 | 0.9 |
| 2 | 7.9 | 1.2 | 0.7 | 0.7 |
| 3 | 45.8 | 1.3 | 1.0 | 1.0 |
| 10 | 55.0 | 3.0 | 1.4 | 5.9 |
| 11 | 32.5 | 2.9 | 1.4 | 2.2 |
| 12 | 79.0 | 4.0 | 3.7 | 1.7 |
| 13 | 4.3 | 1.0 | 1.0 | 1.0 |
| 14 | 13.3 | 0.8 | 2.0 | 1.2 |
| 15 | 16.2 | 1.4 | 0.8 | 1.4 |
| 41 | 0.6 | 0.4 | 0.2 | 0.2 |
| 42 | 0.6 | 0.5 | 0.3 | 0.6 |
| 43 | 0.5 | 0.4 | 0.2 | 0.4 |
| Doxorubicin | 12.0 | 18.5 | 4.1 | 0.7 |
For the insertion of chalcone in the naphthoquinoidal moiety, compounds 1–6 were prepared. The structures 1–3 were also evaluated and showed activity against HL-60, HCT-116 and OVCAR-8 with IC50 values in the range of 0.46 to 15.35 μM. Compound 4 was not active and the structures 5 and 6 presented activity only for HL-60 with IC50 values = 10.5 and 10.0 μM, respectively.
After evaluating nor-β-lapachone based chalcone 10–15, the data confirmed the success of our approach. As previously described,8 the compounds were classified according to their activity as highly active (IC50 < 2 μM), moderately active (2 μM < IC50 < 10 μM), or inactive (IC50 > 10 μM). The structures 10–15 were considered highly active against HL-60 and HCT-116 with IC50 values between 0.04 to 2.2 μM. Compounds 3, 10–12, 14 and 15 were considered very promising against HL-60 after assaying these structures against peripheral blood mononuclear cells (PBMC). The selectivity index (SI), which is an excellent parameter to determine the efficiency of the compounds in cancer cells with low cytotoxicity in normal cell lines is expressed by the ratio of cytotoxicities between normal cell (PBMC) and different cancer cell lines. For compounds 3, 10, 11, 12, 14 and 15 the values of SI (considering HL-60) were 45.8, 55.0, 32.5, 79, 13.3 and 16.2 (Table 2), respectively. For doxorubicin, the drug used clinically against several types of cancer, the SI value is 12.0. In comparison with the positive control, doxorubicin, nor-β-lapachone-based chalcone 12 is more active and this structure could be considered as an important prototype for further studies. To the best of our knowledge, 12 can be considered as one of the most active substances for HL-60 cancer cell line with a high selectivity index obtained from lapachol 7 already reported.
Another important characteristic that points to the success of the strategy herein employed was the increase of the activity of nor-β-lapachone 34 after the insertion of the chalcone moieties (IC50 values for 34 in the range of 1.25–1.75 μM), Table 1. The hybrid compounds were also more active than the non-hybridized chalcones 1–4.
Finally, compound 13 was not active against HCT-116, OVCAR-8 and SF295, but this molecule presented selective activity for HL-60 with IC50 value = 2.20 μM. In terms of selectivity index this structure was not promising, but further modifications in this prototype are under investigation in our research group to develop drugs with selectivity against a certain type of cancer. Compounds 14 and 15 were highly active against all the cancer cell lines evaluated, but these compounds were more cytotoxic than the other derivatives of this class.
In general terms, lapachone-based chalcone hybrids presented important activity against cancer cell lines with low cytotoxicity in normal cancer cell lines. This manuscript represents the first report on this class of compounds with promising activity, and based on our experience with lapachone derivatives, the compounds herein described deserve further subsequent studies.
The last class of compounds herein evaluated has been chosen because of the intrinsic ability of these structures to generate ROS. In fact, the compounds 41–43 were considered highly active (IC50 < 2 μM) against all the cancer lineages evaluated with some exceptions. The main problem observed for these compounds was the high cytotoxicity observed in normal cell lines (PBMC). For instance, compound 41 was highly active against HL-60 cell lines with IC50 = 0.45 μM, but it presented IC50 value = 0.27 μM for normal cells. In general, 41–43 are more cytotoxic for normal cell than cancer cell lines.
Lately, microencapsulation aimed controlled release is an important strategy to increase the efficiency of antitumor drugs.41 Recent studies using β-lapachone were described to try to diminish the cytotoxicity of this important antitumor quinone.42 Considering the promising results observed for 41–43, in four types of cancer cell lineage, studies aiming controlled delivery systems, could be an interesting possibility to solve the problem of cytotoxicity.
4. Conclusions
We evaluated thirty-two compounds against four cancer cell lines and peripheral blood mononuclear cells (PBMC). These compounds were obtained by C-ring and redox centre modifications based on the prototypes nor-β-lapachone, α-lapachone, nor-α-lapachone and β-lapachone analogues. Fourteen compounds were discovered as important substances with potent antitumor activity. In general, all the compounds were highly active (IC50 < 2 μM) and the substances were more active than doxorubicin, a clinically used drug against several types of cancer. Compound 12 presented good selectivity for HL-60 cell line with high selectivity index even better than doxorubicin the positive control. This compound presents a promising profile for further experimental investigations in this study.5. Experimental section
Melting points were obtained on a Thomas Hoover apparatus and are uncorrected. Analytical grade solvents were used. Column chromatography was performed on silica gel (Acros Organics 0.035–0.070 mm, pore diameter ca. 6 nm). 1H and 13C NMR were recorded at room temperature using a Bruker AVANCE DRX 200 and Varian Mercury 400 and 500 MHz spectrometers in the solvents indicated with TMS as an internal reference. Chemical shifts (δ) are given in ppm. Electron-impact mass spectra (70 eV) were obtained using a VG Autospec apparatus (Micromass, Manchester, UK). The main fragments were described as a relation between atomic mass units and the charge (m/z) and the relative abundance as a percentage of the base peak intensity. Lapachol (5) (2-hydroxy-3-(3′-methyl-2′-butenyl)-1,4-naphthoquinone) was extracted from the heartwood of Tabebuia sp. (Tecoma) and purified by a series of recrystallizations in an appropriate solvent. From this quinone, nor-lapachol (2-hydroxy-3-(2′-methyl-propenyl)-1,4-naphthoquinone) was obtained by the Hooker oxidation method.28General procedure to prepare the quinone-based chalcones
An excess of bromine (2 mL) was added to a cooled solution of nor-lapachol (228 mg, 1 mmol) in 25 mL of dichloromethane. The brominated intermediate 9 was obtained as an orange solid. After the removal of excess bromine, a solution of the respective chalcone (1 mmol) in 25 mL of dichloromethane was added and stirred overnight. The reaction mixture was concentrated under reduced pressure and the residue was purified by column chromatography in silica gel by eluting with an increasing polarity gradient mixture of hexane and ethyl acetate.General procedure to prepare the hydrazone-derivatives
Phenylhydrazine hydrochloride (288 mg, 2 mmol) was added all at once to a solution of respective quinone (1 mmol) in acetic acid (15 mL). The mixture was stirred at reflux and monitored by TLC until complete consumption of the quinone. The residue was poured into water and the precipitate was filtered and washed with plenty of water. After dried, the crude hydrazones were purified by column chromatography in silica gel by eluting with an increasing polarity gradient mixture of hexane and ethyl acetate.Crystallographic data for compound 14
X-ray data collection was accomplished on a Bruker CCD SMART APEX II single crystal diffractometer with Mo Kα radiation (0.71073 Å) at 296 K. The data were processed with SAINT43 and were corrected for absorption using SADABS.44 The structures were solved by direct methods using SHELXS-97 and subsequent Fourier-difference map analyses yielded the positions of the nonhydrogen atoms, the refinement was performed using SHELXL-97.45 Molecular graphics, ORTEP-3,46 software was used to prepare material for publication, WinGX-Routine.47†Crystal data for compound 14
C30H25NO5; M = 479.51; monoclinic, space group C2/c; a = 37.446(2) Å, b = 10.070(7) Å, c = 13.198(10) Å; α = γ = 90°, β = 101.015(5)°; V = 4885.3(6) Å3; Z = 8; Dc = 1.304 g cm−1; F(000) = 2016; red prism, size 0.39 × 0.22 × 0.11 mm; 4944 independent measured reflections, refinement based on F2 to give R1[F2 > 4σ(F2)] = 0.055; w2 = 0.106 for 17 276 observed reflections, and 393 parameters.Cytotoxicity against cancer and normal cell lines
Compounds (0.001–5 μg mL−1) were tested for cytotoxic activity against several human cancer cell lines obtained from the National Cancer Institute, NCI (Bethesda, MD). Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood from healthy, non-smoker donors who had not taken any medication at least 15 days prior to sampling by a standard method of density-gradient centrifugation on Histopaque-1077 (Sigma Aldrich Co. – St. Louis, MO/USA). All cancer cell lines and PBMC were maintained in RPMI 1640 medium. All culture media were supplemented with 20% (PBMC) or 10% (HL-60, HCT-116, OVCAR-8 and SF295) fetal bovine serum, 2 mM L-glutamine, 100 IU mL−1 penicillin, 100 μg mL−1 streptomycin at 37 °C with 5% CO2. PBMC cultures were also supplemented with 2% phytohaemagglutinin. In cytotoxicity experiments, cells were plated in 96-well plates (1 × 106 cells per well for leukemia HL-60 cells and 1 × 106 cells per well for solid tumors cell lines and PBMC). All the tested compounds were dissolved with DMSO. The final concentration of DMSO in the culture medium was kept constant (0.1%, v/v). Doxorubicin (0.001–1.10 μM) was used as the positive control, and negative control groups received the same amount of vehicle (DMSO). The cell viability was determined by reduction of the yellow dye 3-(4,5-dimethyl-2-thiazol)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to a blue formazan product as described by Mosmann.48 At the end of the incubation time (72 h), the plates were centrifuged and the medium was replaced by fresh medium (200 μL) containing 0.5 mg mL−1 MTT. Three hours later, the MTT formazan product was dissolved in DMSO (150 μL) and the absorbance was measured using a multiplate reader (Spectra Count, Packard, Ontario, Canada). Drug effect was quantified as the percentage of control absorbance of the reduced dye at 550 nm. All cell treatments were carried out with three replicates.Acknowledgements
This work was partially supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Edital Jovens pesquisadores 550579/2012-5, CAPES, FAPEMIG, and RENORBIO (Rede Nordeste de Biotecnologia) and Fundação Cearense de Apoio ao Desenvolvimento Científico – FUNCAP. A research grant to INNN by DST India and a research felowship to DKN by CSIR India are gratefully acknowledged.Notes and references
- B. D. Smith, G. L. Smith, A. Hurria, G. N. Hortobagyi and T. A. Buchholz, J. Clin. Oncol., 2009, 27, 2758–2765 CrossRef PubMed.
- P. Boyle and B. Levin, World Cancer Report, 2008, IARC Press, Lyon, 2008, p. 510 Search PubMed.
- J. Ferlay, I. Soerjomataram, M. Ervik, R. Dikshit, S. Eser, C. Mathers, M. Rebelo, D. M. Parkin, D. Forman and F. Bray, GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet], International Agency for Research on Cancer, Lyon, France, 2013, http://globocan.iarc.fr, accessed 10 April 2014 Search PubMed.
- A. Reichstein, S. Vortherms, S. Bannwitz, J. Tentrop, H. Prinz and K. Müller, J. Med. Chem., 2012, 55, 7273–7284 CrossRef CAS PubMed.
- E. N. da Silva Júnior, M. C. B. V. Souza, A. V. Pinto, M. C. F. R. Pinto, V. F. Ferreira, R. F. S. Menna-Barreto, R. S. F. Silva, D. V. Teixeira, C. A. de Simone and S. L. de Castro, Eur. J. Med. Chem., 2008, 43, 1774–1780 CrossRef PubMed.
- M. L. Bolognesi, N. Calonghi, C. Mangano, L. Masotti and C. Melchiorre, J. Med. Chem., 2008, 51, 5463–5467 CrossRef CAS PubMed.
- E. N. da Silva Júnior, C. F. de Deus, B. C. Cavalcanti, C. Pessoa, L. V. Costa-Lotufo, R. C. Montenegro, M. O. de Moraes, M. C. F. R. Pinto, C. A. de Simone, V. F. Ferreira, M. O. F. Goulart, C. K. Z. Andrade and A. V. Pinto, J. Med. Chem., 2010, 53, 504–508 CrossRef PubMed.
- E. Pérez-Sacau, R. G. Díaz-Peñate, A. Estévez-Braun, A. G. Ravelo, J. M. Garcia-Castellano, L. Pardo and M. Campillo, J. Med. Chem., 2007, 50, 696–706 CrossRef PubMed.
- I. Garkavtsev, V. P. Chauhan, H. K. Wong, A. Mukhopadhyay, M. A. Glicksman, R. T. Peterson and R. K. Jaina, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 11596–11601 CrossRef CAS PubMed.
- C. J. Li, Y. Z. Li, A. V. Pinto and A. B. Pardee, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 13369–13374 CrossRef CAS.
- R. P. Verma, Anti-Cancer Agents Med. Chem., 2006, 6, 489–499 CrossRef CAS.
- F. Prati, E. Uliassi and M. L. Bolognesi, Med. Chem. Commun., 2014, 5, 853–861 RSC.
- F. S. Emery, M. C. F. R. Pinto, C. A. de Simone, V. R. S. Malta, E. N. da Silva Júnior and A. V. Pinto, Synlett, 2010, 13, 1931–1934 Search PubMed.
- E. N. da Silva Júnior, C. A. de Simone, A. C. B. de Souza, C. N. Pinto, T. T. Guimarães, M. C. F. R. Pinto and A. V. Pinto, Tetrahedron Lett., 2009, 50, 1550–1553 CrossRef PubMed.
- A. V. Pinto, C. Neves Pinto, M. C. F. R. Pinto, R. M. Santa Rita, C. Pezzella and S. L. de Castro, Arzneim.-Forsch., 1997, 47, 74–79 CAS.
- S. L. de Castro, F. S. Emery and E. N. da Silva Júnior, Eur. J. Med. Chem., 2013, 69, 678–700 CrossRef CAS PubMed.
- E. N. da Silva Júnior, C. F. de Deus, B. C. Cavalcanti, C. Pessoa, L. V. Costa-Lotufo, R. C. Montenegro, M. O. de Moraes, M. C. F. R. Pinto, C. A. de Simone, V. F. Ferreira, M. O. F. Goulart, C. K. Z. Andrade and A. V. Pinto, J. Med. Chem., 2010, 53, 504–508 CrossRef PubMed.
- E. N. da Silva Júnior, M. A. B. F. de Moura, A. V. Pinto, M. C. F. R. Pinto, M. C. B. V. de Souza, A. J. Araújo, C. Pessoa, L. V. Costa-Lotufo, R. C. Montenegro, M. O. de Moraes, V. F. Ferreira and M. O. F. Goulart, J. Braz. Chem. Soc., 2009, 20, 635–643 CrossRef PubMed.
- C. Viegas Júnior, A. C. Danuello, V. S. Bolzani, E. J. Barreiro and C. A. M. Fraga, Curr. Med. Chem., 2007, 14, 1829–1852 CrossRef.
- E. H. G. da Cruz, P. H. P. R. Carvalho, J. R. Corrêa, D. A. C. Silva, E. B. T. Diogo, J. D. de Souza Filho, B. C. Cavalcanti, C. Pessoa, H. C. B. de Oliveira, B. C. Guido, D. A. da Silva Filho, B. A. D. Neto and E. N. da Silva Júnior, New J. Chem., 2014, 38, 2569–2580 RSC.
- K. C. G. Moura, P. F. Carneiro, M. C. F. R. Pinto, J. A. da Silva, V. R. S. Malta, C. A. de Simone, G. G. Dias, G. A. M. Jardim, J. Cantos, T. S. Coelho, P. E. A. da Silva and E. N. da Silva Júnior, Bioorg. Med. Chem., 2012, 20, 6482–6488 CrossRef CAS PubMed.
- V. F. Ferreira, A. Jorqueira, A. M. T. Souza, M. N. da Silva, M. C. B. V. de Souza, R. M. Gouvêa, C. R. Rodrigues, A. V. Pinto, H. C. Castro, D. O. Santos, H. P. Araújo and S. C. Bourguignon, Bioorg. Med. Chem., 2006, 14, 5459–5466 CrossRef CAS PubMed.
- E. H. G. da Cruz, C. M. B. Hussene, G. G. Dias, E. B. T. Diogo, I. M. M. de Melo, B. L. Rodrigues, M. G. da Silva, W. O. Valença, C. A. Camara, R. N. de Oliveira, Y. G. de Paiva, M. O. F. Goulart, B. C. Cavalcanti, C. Pessoa and E. N. da Silva Júnior, Bioorg. Med. Chem., 2014, 22, 1608–1619 CrossRef CAS PubMed.
- T. T. Guimarães, M. C. F. R. Pinto, J. S. Lanza, M. N. Melo, R. L. do Monte-Neto, I. M. M. de Melo, E. B. T. Diogo, V. F. Ferreira, C. A. Camara, W. O. Valença, R. N. de Oliveira, F. Frézard and E. N. da Silva Júnior, Eur. J. Med. Chem., 2013, 63, 523–530 CrossRef PubMed.
- Y. Zhang, B. Srinivasan, C. Xing and J. Lü, Anticancer Res., 2012, 32, 3689–3698 CAS.
- R. T. Blickenstaff, W. R. Hanson, S. Reddy and R. Witt, Bioorg. Med. Chem., 1995, 3, 917–922 CrossRef CAS.
- V. Tomar, G. Bhattacharjee, Kamaluddin, S. Rajakumar, K. Srivastava and S. K. Puri, Eur. J. Med. Chem., 2010, 45, 745–751 CrossRef CAS PubMed.
- F. L. Ansari, S. Umbreen, L. Hussain, T. Makhmoor, S. A. Nawaz, M. A. Lodhi, S. N. Khan, F. Shaheen, M. I. Choudhary and Atta-ur-Rahman, Chem. Biodiversity, 2005, 2, 487–496 CAS.
- L. F. Fieser and M. Fieser, J. Am. Chem. Soc., 1948, 70, 3215 CrossRef CAS.
- D. K. Nair, R. F. S. Menna-Barreto, E. N. da Silva Júnior, S. M. Mobin and I. N. N. Namboothiri, Chem. Commun., 2014, 50, 6973–6976 RSC.
- J. S. Sun, A. H. Geiser and B. Frydman, Tetrahedron Lett., 1998, 39, 8221–8224 CrossRef CAS.
- C. Salas, R. A. Tapia, K. Ciudad, V. Armstrong, M. Orellana, U. Kemmerling, J. Ferreira, J. D. Maya and A. Morello, Bioorg. Med. Chem., 2008, 16, 668–674 CrossRef CAS PubMed.
- A. V. Pinto, M. C. F. R. Pinto and C. G. T. de Oliveira, An. Acad. Bras. Cienc., 1982, 54, 108–114 Search PubMed.
- C. E. M. Carvalho, V. F. Ferreira, A. V. Pinto, M. C. F. R. Pinto and W. Harrison, Dyes Pigm., 2002, 52, 209–214 CrossRef CAS.
- E. B. T. Diogo, G. G. Dias, B. L. Rodrigues, T. T. Guimarães, W. O. Valença, C. A. Camara, R. N. de Oliveira, M. G. da Silva, V. F. Ferreira, Y. G. de Paiva, M. O. F. Goulart, R. F. S. Menna-Barreto, S. L. de Castro and E. N. da Silva Júnior, Bioorg. Med. Chem., 2013, 21, 6337–6348 CrossRef CAS PubMed.
- E. N. da Silva Júnior, M. C. B. V. de Souza, A. V. Pinto, M. C. F. R. Pinto, M. O. F. Goulart, F. W. A. Barros, C. Pessoa, L. V. Costa-Lotufo, R. C. Montenegro, M. O. de Moraes and V. F. Ferreira, Bioorg. Med. Chem., 2007, 15, 7035–7041 CrossRef PubMed.
- P. H. Di Chenna, V. Benedetti-Doctorovich, R. F. Baggio, M. T. Garland and G. Burton, J. Med. Chem., 2001, 44, 2486–2489 CrossRef CAS PubMed.
- E. Winter, P. D. Neuenfeldt, L. D. Chiaradia-Delatorre, C. Gauthier, R. A. Yunes, R. J. Nunes, T. B. Creczynski-Pasa and A. D. Pietro, J. Med. Chem., 2014, 57, 2930–2941 CrossRef CAS PubMed.
- R. Shivahare, V. Korthikunta, H. Chandasana, M. K. Suthar, P. Agnihotri, P. Vishwakarma, T. K. Chaitanya, P. Kancharla, T. Khaliq, S. Gupta, R. S. Bhatta, J. V. Pratap, J. K. Saxena, S. Gupta and N. Tadigoppula, J. Med. Chem., 2014, 57, 3342–3357 CrossRef CAS PubMed.
- F. J. Smit and D. D. N’Da, Bioorg. Med. Chem., 2014, 22, 1128–1138 CrossRef CAS PubMed.
- E. Blanco, E. A. Bey, C. Khemtong, S. G. Yang, J. Setti-Guthi, H. Chen, C. W. Kessinger, K. A. Carnevale, W. G. Bornmann, D. A. Boothman and J. Gao, Cancer Res., 2010, 70, 3896–3904 CrossRef CAS PubMed.
- S. Y. Jeong, S. J. Park, S. M. Yoon, J. Jung, H. N. Woo, S. L. Yi, S. Y. Song, H. J. Park, C. Kim, J. S. Lee, J. S. Lee and E. K. Choi, J. Controlled Release, 2009, 139, 239–245 CrossRef CAS PubMed.
- SMART and SAINT, Area Detector Control Integration Software, Bruker Analytical X-ray Instruments, Inc., Madison Wisconsin, USA, 1999 Search PubMed.
- G. M. Sheldrick, SADABS, Program for Empirical Absorption Correction of Area Detector Data, University of Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS.
- T. J. Mosmann, Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1018940. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4md00371c |
| This journal is © The Royal Society of Chemistry 2015 |