 Open Access Article
Open Access ArticleSelf-assembly via microfluidics
Lei
Wang
ab and
Samuel
Sánchez
*bcd
aSchool of Chemical Engineering and Technology, Harbin Institute of Technology, 92 West Dazhi Street, Harbin, 150001, China
bSmart nano-bio-devices Laboratory, Institute for Bioengineering of Catalonia (IBEC), Baldiri Reixac, 10-12, Barcelona 08028, Spain. E-mail: ssanchez@ibecbarcelona.eu
cInstitució Catalana de Recerca i Estudis Avançats (ICREA), Psg. Lluís Companys, 23, 08010 Barcelona, Spain
dMax Planck Institute for Intelligent Systems, Heisenbergstr. 3, 70569 Stuttgart, Germany. E-mail: sanchez@is.mpg.de
First published on 21st October 2015
Abstract
The self-assembly of amphiphilic building blocks has attracted extensive interest in myriad fields in recent years, due to their great potential in the nanoscale design of functional hybrid materials. Microfluidic techniques provide an intriguing method to control kinetic aspects of the self-assembly of molecular amphiphiles by the facile adjustment of the hydrodynamics of the fluids. Up to now, there have been several reports about one-step direct self-assembly of different building blocks with versatile and multi-shape products without templates, which demonstrated the advantages of microfluidics. These assemblies with different morphologies have great applications in various areas such as cancer therapy, micromotor fabrication, and controlled drug delivery.
Introduction
The past few decades have witnessed the rapidly increasing demand for novel active materials with complex architectures, such as soft matter and multifunctional materials, due to their promising applications ranging from drug encapsulation/delivery to cell imaging and cancer therapy. The construction of these materials has emerged as a hot topic, especially using small amphiphilic molecules or colloidal NPs as building blocks due to their unique properties. Until now, there have been great advances in the area of controlled assembly of building blocks into various larger/ordered high-hierarchical structures. There are some traditional methods to obtain the controlled assembly, such as using templates, electric or magnetic fields, and directional physical or chemical binding. However, these methods cannot provide good control over the kinetics of the self-assembly process, which would be a key aspect for the design of multifunctional structures with large degrees of complexity. Microfluidics has become a powerful platform to manipulate fluids and adjust the hydrodynamics of flows at the microscale, which can be a novel strategy to control the kinetic process of the self-assembly.In this paper, we focus exclusively on recent self-assembly using microfluidics within two major categories: lipid-based systems and NPs-based systems.
Lipid-based systems
Microfluidics (MFs) has emerged as a powerful platform for material synthesis and self-assembly during the recent years, due to its several advantages, such as enhanced heat- and diffusional mass-transfer, a well-defined and predictable interfacial region between different flows caused by laminar flow, and easy control over the hydrodynamic conditions in the microchannel. Hence, MFs has been applied for the continuous assembly of molecular amphiphiles, with its unprecedented control over the sizes and morphologies of assemblies. There are many types of building blocks that can be utilized in this system, such as surfactants, lipids, polymers, chitosan,1 lipid–polymer hybrid nanoparticles,2 and block copolymer (BCP)-tethered NPs (BNPs). To our best knowledge, the pioneering paper on controlled lipid vesicle self-assembly in microfluidic channels with hydrodynamic focusing was published in 2004 (Fig. 1).3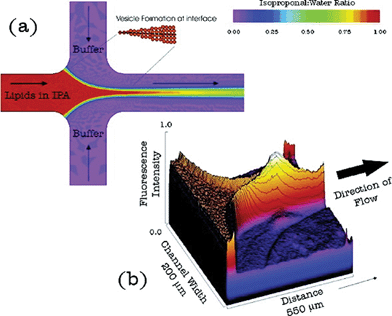 | ||
| Fig. 1 (a) Schematic of liposome formation process in the microfluidic channel. Color contours represent the concentration ratios of isopropanol to aqueous buffer. (b) 3-D color contour map of DiIC18 fluorescence intensity at a focused region during liposome formation. Adapted from ref. 3. Copyright© Publ. 2004 Am. Chem. Soc. | ||
In that early publication, a stream of lipid tincture was hydrodynamically focused at a microchannel cross junction between two aqueous buffer streams. The formation of liposomes was energetically favourable at points in the system where the concentration of the mixture of isopropyl alcohol and buffer solution reached a critical condition where lipid solubility was low. Hence, liposomes, with the diameter range of 100–300 nm, were successfully fabricated and utilized for the encapsulation of different molecules. From the results presented in that work, we can see that giant unilamellar lipid vesicles are rather challenging to fabricate using normal methods or common flow focusing methods. To solve this problem, recently, Ces et al.4 fabricated giant unilamellar vesicles with a diameter of ca. 60 μm based on lipid self-assembly on the interface of water-in-oil droplets, and studied the mechanical properties of the resultant lipid bilayers. This work offered a high-throughput generation of giant unilamellar liposomes. However, there is still no direct self-assembly method, i.e., without droplet templates, in microfluidics to obtain lipid assemblies of large sizes.
Nanoparticles (NPs)-based systems
To the best of our knowledge, the first report on the controlled self-assembly of a NPs-based system was reported in 2008,5 regarding polymer-stabilized quantum dots assembled into mesoscale aqueous spherical assemblies. In those two-step flow-focusing regimes, self-assembly was triggered by the addition of water to a blended solution of polystyrene-coated QDs and amphiphilic polystyrene-block-poly(acrylic acid) stabilizing chains in the first step, and terminated in a second step by adding more water in the downstream to quench the process of self-assembly. That work realized the assembly of polymer-stabilized quantum dots and free polymer together to obtain products of solid micelles with a diameter ranging from 40–100 nm, without products with other morphologies, like hollow vesicles.In 2010, Prof. Langer's group gave a demonstration of self-assembly of monodisperse lipid–polymer and lipid–quantum dot (QD) NPs in a single mixing step.6 In that work, the product NPs contained either a polymeric core for drug encapsulation or a QD core for imaging purposes (Fig. 2), a hydrophilic polymeric shell, and a lipid monolayer at the interface of the core and the shell, which offered great potential for drug delivery and imaging in clinical areas. Additionally, the authors proved the effect of mixing conditions on the size distribution and the formation process of the assemblies. Similarly, Hassan et al.7 reported a proof of concept for the continuous multi-step microfluidic assisted self-assembly of fluorescent (SiO2 NPs), plasmonic (AuNPs), and magnetic nanostructures (Fe2O3 NPs) to produce lab-on-a-particle architectures (Fig. 3a). Their products were solid micelles with an average diameter around 250 nm (Fig. 3b and c).
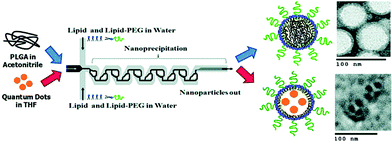 | ||
| Fig. 2 Scheme of the self-assembly process of lipid–polymeric NPs, with representative TEM images and corresponding illustrative images of microfluidic synthesized NP component layers. Adapted from ref. 6. Copyright© 2010, American Chemical Society. | ||
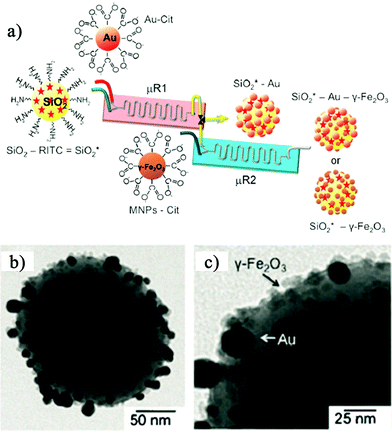 | ||
| Fig. 3 (a) Scheme of a two-step microfluidic synthetic procedure for the assembly of multifunctional nanoparticles/fluorescent silica sphere assemblies. RITC = Rhodamine isothiocyanate, Cit = citrate. (b) and (c) TEM images of SiO2*–Au–γ-Fe2O3 nanostructures. Adapted from ref. 7. Copyright© Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission. | ||
Recently, Prof. Zhihong Nie's group demonstrated the continuous self-assembly of colloidal amphiphiles (CAMs) consisting of BNPs in MFs,8 where the self-assembly of BNPs was typically triggered by focusing a solution containing BNPs in tetrahydrofuran (THF) sheathed by two pure water flows (Fig. 4a).
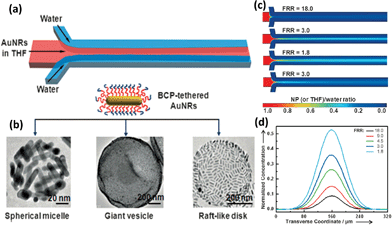 | ||
| Fig. 4 (a) Scheme of hydrodynamic self-assembly of amphiphilic NPs tethered with BCPs using microfluidic flow focusing devices. (b) TEM images of assemblies obtained at different hydrodynamic conditions in microchannels. (c) Simulated NPs and THF concentration distribution in the focused stream at various flow rates. (d) Simulated NP concentration profiles as a function of the transverse coordinate of the microchannel. Adapted from ref. 8. Copyright© Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission. | ||
There was laminar flow in the microchannels due to the small Reynolds number (<≈0.1–10, Fig. 4c), where diffusion was the predominant factor of the mixing between different fluids. In such a case, the diffusive mixing along the transverse direction of the microchannel led to the environment change for the amphiphilic building blocks, i.e. the change of hydrophilic–hydrophobic interaction, thus leading to the self-assembly of BNPs. Taking building blocks of BCP-tethered Au nanorods (BNRs) as an example, different structures, like micelles, giant vesicles, and disks (Fig. 4b) were easily and reproducibly obtained by simply tuning the flow rates of different fluids (hydrodynamic conditions), being one of the most significant advantages of that system. When the flow rate of the water phase was set as a constant, the morphologies of the assemblies changed gradually from small assemblies of micelles to giant vesicles, with the growth of the flow rate of the THF flow. The size of the assemblies increased from ca. 100 nm to ca. 500 nm. When the flow rate of THF kept increasing, the assemblies became raft-like disks, likely due to the bigger shearing force generated with the growth of flow rate. Additionally, the size of giant vesicles can be finely tuned by the flow rates as well. With these hollow structures, where drugs could be loaded, the giant vesicles were utilized in NIR-controlled drug release, due to the photothermal effect of Au NRs. This system of self-assembly based on polymer-modified Au NRs can also be achieved when the shape of the building blocks changed.9 When Au NPs were modified with BCPs, and utilized as new building blocks to self-assemble in the microchannel, vesicular structures were easily obtained. The authors systematically studied the continuous assembly of building blocks in MFs based on experiments and simulations. These building blocks assembled via the mixing of the THF solution and water, and constituted into the membrane of the vesicles. The size of the vesicles can also be easily adjusted. The hydrodynamic conditions (e.g., flow rates of the fluids and diffusion coefficient of building blocks) that affected the diffusion and mixing of the fluids had great impact on the self-assembly process. The conclusion was that larger building blocks with a slow diffusion rate generated larger assemblies because of the continuous recruitment of the building blocks. Hence, this work gave a full investigation of the hydrodynamic effect on the assemblies.
Furthermore, the building blocks not only can be of different shapes, but also can be of different types, which was demonstrated in another report.10 In that work, hybrid Janus-like vesicles (HJVs) were obtained using a single-step template-free method based on microfluidics. These HJVs are asymmetrically functionalized with BCPs-tethered Au NPs, Au NRs, and Pt NPs in two distinct halves of their membranes, where one half is the polymer-rich domain, the other half is the nanoparticle-rich domain. This method also provided good control of the size of HJVs (in the range of 400–2600 nm), besides, the surface coverage of each domain can be easily tuned as well, by changing the concentration of free polymers. Based on the Janus hollow structure, the HJVs were successfully combined with autonomous propulsion and the encapsulation and controlled release of active compounds into a single system. That is because the introduction of Pt NPs into one domain of the HJVs allowed the vesicles to self-propel in the presence of hydrogen peroxide; and the controlled release of payloads from HJVs was realized by irradiation with NIR light, due to the photothermal effect of Au NRs in the vesicular membranes. This work made advancement in the NPs-based self-assembly using microfluidics for drug loading and controlled release.
Another report was recently published regarding the fabrication of a hybrid nanocomposite which uses a pH responsive polymeric material and porous silicon NPs as separate building blocks (Fig. 5), in a flow-focusing microfluidic chip.11 In that work, porous silicon NPs and the solubilized amphiphilic polymers were forced to co-flow with a miscible antisolvent inside a flow-focusing glass capillary chip. The self-assembly of copolymers and porous silicon nanoparticles occurred when the fluids rapidly mixed, which was fast and well controllable by simply tuning the flow rates of different fluids. Although the products were only micelles, without other morphologies, they still showed robust potential in therapeutic applications with their enhanced stability in plasma, narrow size distribution, high cytocompatibility, and pH-controlled release. All the above reports on self-assembly were based on modified building blocks which were obtained before transferring to the chip.
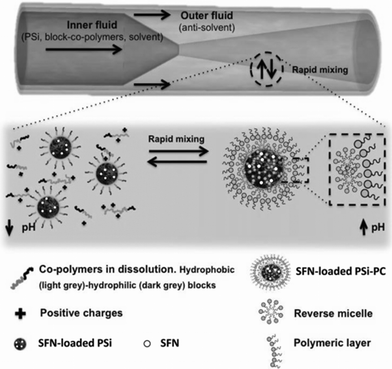 | ||
| Fig. 5 Schematic representation of the microfluidic nanoprecipitation process. From ref. 11. Copyright© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Prof. Langer's group made substantial progress in the programmable synthesis of the same material with different sizes and surface compositions,12 which offered guidance for the direct preparation of building blocks on chip. Based on this concept, the one-step template-free synthesis of metallic nanoparticles and oligomers of an organic material and their self-assembly in one microfluidic chip was realized.13 In that work, the redox reaction between o-phenylenediamine (oPD) and silver nitrate was employed to generate oPD oligomers and Ag NPs. The self-assembly took place when these building blocks interacted with each other in the process of fluids mixing. Products of AgNPs-decorated-PoPD (poly(o-phenylenediamine)) with different morphologies were obtained by easily tuning flow rates, in the meanwhile, the composition of the obtained products could also be adjusted by changing the flow rates of different reagents. To the best of our knowledge, that is the first work to realize the synthesis and self-assembly in one step in one single microfluidic chip.
Additionally, one of the advantages of microfluidics is to offer good control over the kinetics of the assembly process, which was further confirmed by the simulation results using COMSOL Multiphysics 4.3.8 The concentration distribution of BNPs and THF is shown in Fig. 4c and d, from which we can clearly see the diffusive process caused by the increase in flow rates. When the flow rate of the THF solution is low, the strong focusing effect rapidly depleted the central flow, thus leading to the quick quenching of the assembling process and to obtaining relatively smaller assemblies. With the increase in the flow rate of THF, the mixing time becomes longer, thus offering a relatively long time for the building blocks to assemble forming relatively large assemblies. The reports summarized above give us clear guidance for future research and deep insights into the self-assembly mechanisms of NPs-based building blocks via microfluidics. These achievements not only confirmed the high potential of microfluidics in materials science but also broadened new fundamental perspectives using microfluidic techniques and laminar flows to study and control the kinetics of self-assembly processes. Hence, it should be possible from these experimental studies as well as numerical simulations to optimize the parameters, such as the size and charge of NPs, the shearing force, and the mixing time, to easily realize on demand self-assembly of hierarchical nanostructures. But there is still a long way to go, trying to realize the idea of assembly based on microfluidics, i.e., one assembly (e.g. a nanoparticle or a hybrid structure) at each time, or several separate assemblies at several times with different morphologies or properties.
Conclusions
Microfluidics has become a powerful tool to control the self-assembly of different building blocks, especially amphiphilic materials. The recent progress in self-assembly based on two types of building blocks, lipids- and nanoparticles-based building blocks, were briefly summarized in this focus paper. Although much progress has been made in this area in bulk solution, there is still plenty to investigate in microfluidics to well control the properties of assemblies, such as the direct assembly of lipids into GUVs without templates, using BCPs with better bio-compatibility, and to detail the effects on the assemblies caused by chip design, flow type or external fields.Acknowledgements
S. Sánchez thanks the European Research Council (ERC) for Starting Grant “Lab-in-a-tube and Nanorobotics biosensors; LT-NRBS” [no. 311529] for financial support.Notes and references
- F. S. Majedi, M. M. Hasani-Sadrabadi, S. H. Emami, M. A. Shokrgozar, J. J. VanDersarl, E. Dashtimoghadam, A. Bertsch and P. Renaud, Lab Chip, 2013, 13, 204–207 RSC.
- Y. Kim, F. Fay, D. P. Cormode, B. L. Sanchez-Gaytan, J. Tang, E. J. Hennessy, M. Ma, K. Moore, O. C. Farokhzad and E. A. Fisher, ACS Nano, 2013, 7, 9975–9983 CrossRef CAS PubMed.
- A. Jahn, W. N. Vreeland, M. Gaitan and L. E. Locascio, J. Am. Chem. Soc., 2004, 126, 2674–2675 CrossRef CAS PubMed.
- K. Karamdad, R. Law, J. Seddon, N. Brooks and O. Ces, Lab Chip, 2015, 15, 557–562 RSC.
- G. Schabas, H. Yusuf, M. G. Moffitt and D. Sinton, Langmuir, 2008, 24, 637–643 CrossRef CAS PubMed.
- P. M. Valencia, P. A. Basto, L. Zhang, M. Rhee, R. Langer, O. C. Farokhzad and R. Karnik, ACS Nano, 2010, 4, 1671–1679 CrossRef CAS PubMed.
- N. Hassan, V. Cabuil and A. Abou-Hassan, Angew. Chem., Int. Ed., 2013, 52, 1994–1997 CrossRef CAS PubMed.
- J. He, Z. Wei, L. Wang, Z. Tomova, T. Babu, C. Wang, X. Han, J. T. Fourkas and Z. Nie, Angew. Chem., Int. Ed., 2013, 52, 2463–2468 CrossRef CAS PubMed.
- J. He, L. Wang, Z. Wei, Y. Yang, C. Wang, X. Han and Z. Nie, ACS Appl. Mater. Interfaces, 2013, 5, 9746–9751 CAS.
- L. Wang, Y. Liu, J. He, M. J. Hourwitz, Y. Yang, J. T. Fourkas, X. Han and Z. Nie, Small, 2015, 11, 3762–3767 CrossRef CAS PubMed.
- B. Herranz-Blanco, D. Liu, E. Mäkilä, M.-A. Shahbazi, E. Ginestar, H. Zhang, V. Aseyev, V. Balasubramanian, J. Salonen, J. Hirvonen and H. A. Santos, Adv. Funct. Mater., 2015, 25, 1488–1497 CrossRef CAS PubMed.
- P. M. Valencia, E. M. Pridgen, M. Rhee, R. Langer, O. C. Farokhzad and R. Karnik, ACS Nano, 2013, 7, 10671–10680 CrossRef CAS PubMed.
- L. Wang, S. Ma, B. Yang, W. Cao and X. Han, Chem. Eng. J., 2015, 268, 102–108 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |
