Research highlights: translating chips
Janay Elise
Kong
,
Jaekyung
Koh
,
Jonathan
Lin
and
Dino
Di Carlo
*
Department of Bioengineering, California NanoSystems Institute, Jonsson Comprehensive Cancer Center, University of California Los Angeles, 420 Westwood Plaza, 5121 Engineering V, Box 951600, Los Angeles, California 90095, USA. E-mail: dicarlo@ucla.edu
First published on 24th March 2015
Abstract
Microfluidic and microfabricated systems are providing key functionalities in diagnostic and therapeutic scenarios, translating beyond the research laboratory to pre-clinical animal studies and clinical studies with patients. Here, we highlight a recent study making use of miniaturization and automation in the development of a smartphone-integrated point-of-care diagnostic to detect antibodies to infectious diseases in a global health setting. We also review an intraocular implanted diagnostic system for glaucoma that relies on imaging the location of a fluid meniscus in a microchannel to readout pressure within the eye. Developments in low-cost and highly functional consumer electronic systems (e.g. smartphones in both highlighted works) has led to a continuing trend to incorporate such technologies with microfluidic fluid handling capabilities to achieve complete diagnostic solutions. We conclude with another implanted microdevice that delivers drug locally to tumors through electroosmotic flow and electromigration of charged drug species, which allows high drug concentrations near a tumor or resected tumor site while preventing high systemic levels associated with significant side-effects. The maturity of microsystem components are now allowing integration into fully functional systems that are poised to reach the clinic in a variety of forms – diagnostic to therapeutic.
Microfluidic smartphone accessory for immunosurveillance
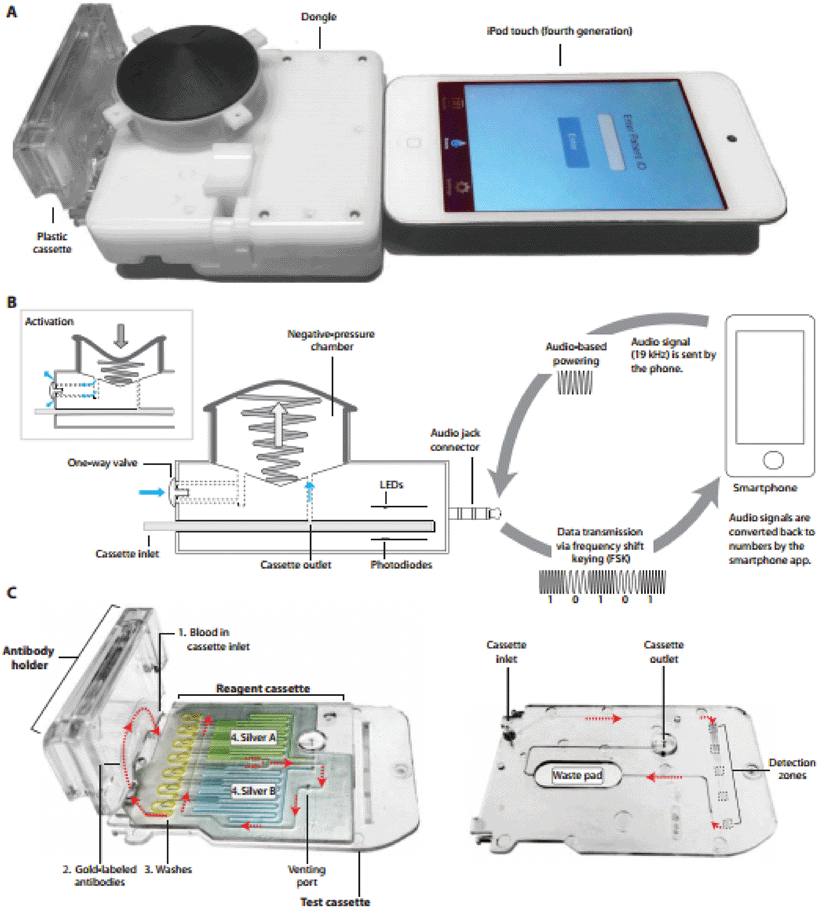
The multifunctionality of smartphones, including powerful computation, high-resolution and sensitive imaging hardware, and compact and long-lasting power supply allows for the integration into a large range of tools, including diagnostics,1,2 that may be suitable in countries with developing infrastructure, where such devices are becoming increasingly prevalent.Laksanasopin et al. focus on designing a smartphone accessory that can be used as a point of care (POC) diagnostic for HIV IgG, treponemal syphilis IgG and IgM, and a nontreponemal syphilis marker – anti-cardiolipin IgG – for use in developing countries.3 They tested this accessory, or “dongle”, on a cohort of local patients in Rwanda. The diagnostic assay run by the dongle makes use of immunorecognition of antigen followed by secondary antibody binding and signal amplification due to silver accumulation on gold nanoparticle seeds bound to the secondary antibodies.4 The smartphone “dongle” shown in Fig. 1a, was designed for low power consumption by the introduction of a “one-push vacuum” that generates a negative pressure within the device (Fig. 1b) to pull the sample through a microfluidic device (Fig. 1c). Other components of the dongle, including LEDs and photodetectors, consume very little power. The optical absorbance of silver precipitation is also measured on the dongle and the data is transferred back to the smartphone. Both the data collected and power for signal measurement is transferred through the audio jack connection on the smartphone (Fig. 1b).
The assay was slightly modified from previous work to accommodate the needs for use in a developing country. An additional detection region was added to allow for a triplex immunoassay, lyophilized gold-conjugated secondary antibodies were packaged in the antibody holder (Fig. 1c) along with a stabilizer and anticoagulant in an individual moisture barrier bag before being shipped to Rwanda, test cassettes were prepared using the stabilizing agent StabiliCoat during physisorption of capture proteins, and lastly wash buffers and silver were preloaded on the reagent cassette (Fig. 1c) each day prior to testing.
The testing was performed on-site at three health centers in Kigali, Rwanda by health care workers (HCW). Ninety-six patients, volunteers enrolled in preventing mother-to-child transmission (PMTCT) and voluntary counseling and testing (VCT) programs, consented to be a part of the study. To perform the test, 2 μl of a diluted blood sample was added to the cassette, which was then inserted into the dongle. Absorbance readings of the level of silver precipitation were performed 15 minutes later and the results for all markers were displayed on the smartphone's interface. HIV antibody detection sensitivity was 100% and specificity was 87%. Treponemal antibody detection sensitivity was 92% and specificity was 92%. Anti-cardiolipin antibody detection sensitivity was 100% and specificity was 79%. There was no significant difference between the dongle assay performance on fingerprick blood and venous whole blood. In a patient survey conducted afterwards, the majority of patients would recommend the dongle assay to others because of the fast turnaround time, potential to offer results for multiple diseases, and simplicity of the procedure.
This novel smartphone attachment provides an alternative to lateral flow-based rapid diagnostic tests (RDTs) which are limited to single antibody detection and generally have lower sensitivity. The high sensitivity and specificity of this POC assay compared to RDTs, along with its low cost, has the potential to provide an impact in resource-limited areas. Further automation of blood sample preparation and reagent/wash introduction would be next steps towards a complete solution.
Intraocular microfluidic pressure sensor
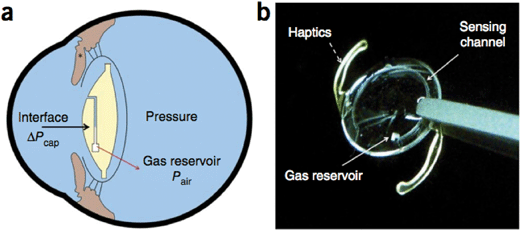
Glaucoma, a disease characterized by progressive loss of retinal ganglion cells, is a leading cause of blindness that affects over 65 million people internationally.5 Though glaucoma is a multifactorial disease, one of the chief risk factors for glaucoma is elevated intraocular pressure (IOP). IOP is not only used as a therapeutic target for glaucoma but is also used for diagnosis and monitoring of the disease. Recent studies have demonstrated significant circadian variations in IOP and some studies have highlighted increased IOP variation as a risk factor for glaucoma progression.6,7 These results, combined with the fact that IOP measurements require skilled technicians and topical anesthesia, demonstrate a need for frequent, low-skill measurements of IOP.In their recent publication, Araci et al. demonstrate an implantable microfluidic device targeted towards self-monitoring of IOP.8 The authors fabricated an intraocular lens integrated with a pressure sensor out of parylene-C-coated PDMS bonded to glass (Fig. 2). The pressure sensor consists of an airtight microfluidic channel connected to an air reservoir on one end and open to the intraocular fluid on the other end. Intraocular fluid enters the microfluidic channel and results in an air–water interface whose position depends on the IOP. The IOP can then be measured using a modified smartphone camera or by an ophthalmologist using a slit lamp.
Using a pressurized chamber, the authors demonstrated that increased external pressure compressed air in the chamber and caused the air–water interface to move closer to the air reservoir. Similarly, decreased external pressure caused the interface to move further from the reservoir. The motion of the interface varied linearly with pressure and repeated measurements demonstrated low variability with a limit of detection at pressure changes of 1 mmHg. Furthermore, the authors demonstrated that the sensitivity of the sensors could be adjusted by changing the air reservoir size with larger reservoirs resulting in greater interface motion for a given pressure change. It is worth noting that the authors observed significant variability in device sensitivity across devices with similar structures.
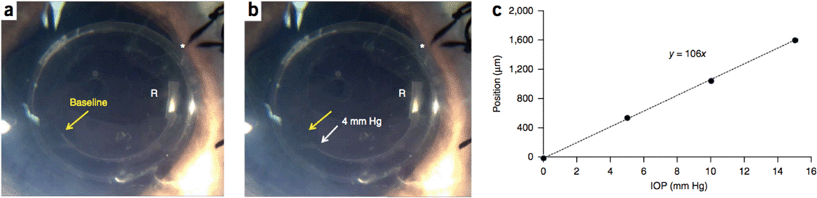
After implantation into porcine eyes, the IOP sensors' air–water interfaces could be visualized using a smartphone camera modified with a 25 mm focal length lens and direct illumination (Fig. 3). Interface position changes were linear with pressure and retained a limit of detection of 1 mmHg change in pressure. The sensitivity of the implanted sensors matched the sensitivity measured in the pressurized chamber, suggesting that calibration prior to implantation is possible. IOPs measured using the pressure sensor agreed with the true IOP with an error of ±0.5 mmHg.
Lastly, the optical performance of the intraocular lens that the IOP sensor is integrated into was compared to that of an intraocular lens without an integrated sensor. US Air Force target images and measurements of modulation transfer function indicated that the sensor introduced no optical distortion even when sensor features fell on the optical axis. Furthermore, ray tracing simulations indicated 100 μm × 100 μm channels produced negligible glare when interacting with light arriving at a 15° angle to the optical axis.
Araci et al. have demonstrated an implantable intraocular sensor capable of measuring changes in IOP. With a simple smartphone-enabled readout that does not require a skilled technician, this device represents a promising step towards self-monitoring of IOP by patients and improvements in glaucoma care. Further work to compensate for drift due to gas leakage and gas dissolution at the interface will be required to validate suitability for long-term implantation and use.
Implanted iontophoretic delivery of chemotherapy
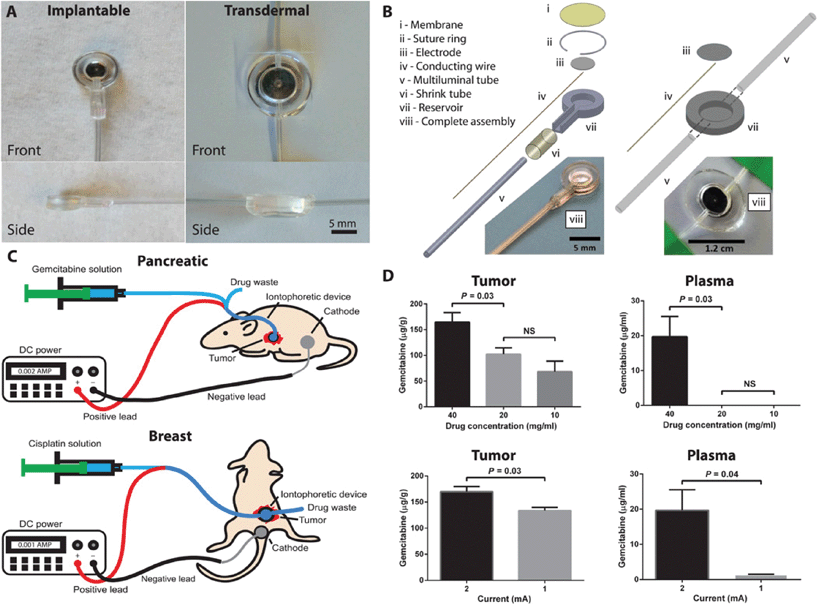
Chemotherapy is widely used and traditionally delivered to cancer patients intravenously or orally. However, the efficacy of chemotherapy is often limited by its low exposure to a tumor site due to impeded drug diffusion in the body. Also, systemic spread of cytotoxic material may lead to side effects, such as vomiting, nausea and hair loss.9,10 To address these issues, Byrne et al. developed an iontophoretic device for local cytotoxic agent delivery to a tumor site.11 When implanted in the proximity of a tumor site, the device boosts the flow of charged drug molecules by electroosmotic forces, thereby increasing drug delivery as well as maintaining a low level of drug systemically. Such an approach may be useful in treating un-resectable tumors or in the residual cavity of resected tumors with complex margins.Two types of devices were designed: implantable for pancreatic tumors and transdermal for breast tumors (Fig. 4A). It has a simple structure with an electrode in direct contact with the drug solution, a polydimethylsiloxane (PDMS) reservoir surrounding the electrode, and an inlet and outlet for drug solution flow (Fig. 4B and C). When an electric field is generated, the drug is transported in the direction normal to the electrode. This active transport is the key difference with many previous implantable drug delivery systems that rely on passive diffusion of degradable materials.
The authors first optimized the conditions of drug concentration and applied current. The device was implanted into an orthotopic patient-derived xenograft (PDX) model of pancreatic cancer, and the drug uptake was measured in the tumor and plasma under different concentrations and currents. With 20 mg ml−1 gemcitabine at 2 mA current, the highest uptake in the tumor with low plasma levels was achieved (Fig. 4D).
After optimization, the authors deployed their device in three distinct orthotopic mouse models of cancer and a canine model. In the pancreatic cancer model, device-delivered gemcitabine resulted in significant tumor regression outperforming IV gemcitabine and no treatment. In the breast cancer model, both device-delivered cisplatin and IV cisplatin resulted in significant tumor growth inhibition compared to no treatment. However, device-delivered cisplatin was better tolerated as shown by the staining of kidneys for a molecular marker of DNA damage and repair. The addition of radiotherapy to the device delivery further improved survival. In a non-tumor-bearing dog model, dogs with the device delivery showed gemcitabine levels 25-fold lower in plasma and 7-fold higher in the pancreas than dogs with IV treatment.
The authors demonstrated that iontophoretic local drug delivery technologies can be successfully applied to pre-clinical animal studies, delivering large amounts of drugs to the tumor site of interest with limited systemic exposure. This work takes us one step further towards the translation of chip-based local drug delivery technologies from basic research into everyday cancer treatment. As an adjunct to surgery or to systemic therapy, the iontophoretic device may enhance the efficacy of cancer treatment and improve the quality of life for patients in a range of clinical scenarios: such as when tumor resection alone is not efficacious, or resection is not warranted because of surgical risk to the patient or wide metastatic spread.
References
- C. D. Chin, et al. Mobile device for disease diagnosis and data tracking in resource-limited settings, Clin. Chem., 2013, 59(4), 629–640, DOI:10.1373/clinchem.2012.199596.
- Q. Wei, et al. Fluorescent Imaging of Single Nanoparticles and Viruses on a Smart Phone, ACS Nano, 2013, 7(10), 9147–9155, DOI:10.1021/nn4037706.
- T. Laksanasopin, et al. A smartphone dongle for diagnosis of infectious diseases at the point of care, Sci. Transl. Med., 2015, 7(273), 273re1, DOI:10.1126/scitranslmed.aaa0056.
- C. D. Chin, et al. Microfluidics-based diagnostics of infectious diseases in the developing world, Nat. Med., 2011, 17(8), 1015–1019, DOI:10.1038/nm.2408.
- H. A. Quigley and A. T. Broman, Br. J. Opthalmol., 2006, 90, 262–267 CrossRef CAS PubMed.
- E. Hughes, P. Spry and J. Diamond, J. Glaucoma, 2003, 12, 232–236 CrossRef PubMed.
- B. Becker, J. Glaucoma, 2000, 9, 487–488 CrossRef CAS PubMed.
- I. E. Araci, et al. An implantable microfluidic device for self-monitoring of intraocular pressure, Nat. Med., 2014, 20(9), 1074–1078, DOI:10.1038/nm.3621.
- A. V. Schally and A. Nagy, Eur. J. Endocrinol., 1999, 141, 1–14 CrossRef CAS PubMed.
- M. A. Moses, H. Brem and R. Langer, Cancer Cell, 2003, 4, 337–341 CrossRef CAS.
- J. D. Byrne, M. N. R. Jajja, A. T. O'Neill, L. R. Bickford, A. W. Keeler, N. Hyder, K. Wagner, A. Deal, R. E. Little, R. A. Moffitt, C. Stack, M. Nelson, C. R. Brooks, W. Lee, J. C. Luft, M. E. Napier, D. Darr, C. K. Anders, R. Stack, J. E. Tepper, A. Z. Wang, W. C. Zamboni, J. J. Yeh and J. M. DeSimone, Sci. Transl. Med., 2015, 7, 273ra14 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |