Research highlights: cell separation at the bench and beyond
Anja
Kunze
,
James
Che
,
Armin
Karimi
and
Dino
Di Carlo
*
Department of Bioengineering, California NanoSystems Institute, Jonsson Comprehensive Cancer Center, University of California Los Angeles, 420 Westwood Plaza, 5121 Engineering V, Box 951600, Los Angeles, California 90095, USA. E-mail: dicarlo@ucla.edu
First published on 18th December 2014
Abstract
We highlight recent progress in applying micro- and nanotechnology enabled cell separations to life sciences and clinical use. Microfluidic systems operate on a scale that matches that of cells (10–100 μm) and therefore allow interfacing and separations that are sensitive at this scale. Given the corresponding dimensions, it is not surprising that a wide array of microfluidic cell separation technologies have been developed using hydrodynamic, electrical, magnetic and optical forces, and have been applied to a range of biological and clinical problems in sample preparation. Passive separation approaches have distinct advantages for point of care applications or when downstream cell-based therapies are envisioned. We highlight a recent approach that allows for passive hydrodynamic filtering of cells over almost two orders of magnitude in flow conditions, which allowed the researchers to interface with a standard manual pipettor, creating a “microfluidic pipette tip”. In a second work, passive separation by size yields distinct populations of mesenchymal stem cells that can be used therapeutically. The researchers report on other biophysical separations that would be expected to refine these cell populations further for the most efficacious cell-based therapies. In an intriguing twist, we highlight a creative idea in which stem cell populations could potentially also be extracted from a patient with less invasive surgeries, performing the separation using magnetic nanoparticles in vivo without bulk tissue disruption. New cell separation technologies will continue to be demonstrated, however, a major research thrust appears to be now developing these technologies to address unique application niches in point-of-care sample preparation for research and diagnostics or cell-based therapies.
Microfluidic pipette tip for combined solution transfer and cell filtration
Many microfluidic approaches have been demonstrated to remove cells from a sample and retrieve cell-free fluid for downstream assays.1–3 However, such approaches usually require precise control of flow conditions and electronically controlled pumping systems. For some formats in which solutions are transferred, like pipetting using handheld pneumatic pipettes or pipettors, flow conditions are usually not well controlled. However, the addition of filtration capabilities would be valuable, and eliminate sample preparation steps such as centrifugation.S. Choi et al.4 previously developed a hydrodynamic filtration technique (hydrophoresis) that allowed for cell positioning and filtration only in a range of lower flow rates, requiring a high precision syringe pump for device operation. As the Reynolds number (Re) increased, the particle trajectories were perturbed due to the coupling of fluid inertia effects with hydrophoresis.5 The impact of the flow rate created a limitation for robust and precise manipulation of particle position.
To address this issue, the group presented a combined hydrophoresis inertial microfluidic platform that enables cell removal and liquid-medium recovery over a range of flow rates, without the need for expensive and highly precise flow control, and with a facile interconnection to a pipette tip or syringe and manual operation. A rejection efficiency of 99.3% of cells at a throughput of 1.63 × 106 cells s−1 was demonstrated in their work.
The design was able to cover a wide range of flow conditions by using both hydrophoretic and inertial microfluidic mechanisms, such that cell focusing positions for both techniques were spatially aligned. A high aspect ratio channel with a sequence of expansion and contraction channels and an inclined angle relative to the vertical direction was used in this experiment (Fig. 1a). The rotational flow induced by the slanted grooves on the sides of the channel causes the particles to focus along the long side walls of the channel. The hydrophoresis equilibrium position (Lp) is determined by the width of the contraction channel (wc), channel height (h), particle diameter (d), and Re.5 In this configuration, the particles satisfying wc ≤ 4d were focused to two equilibrium positions near the side wall at low Re. Importantly, the high aspect ratio channel (>3) enables particles to focus to two equilibrium positions along the same side walls of the channel due to inertial effects at higher Re.6,7 For Re from 0.7–36.0, the inclined-wall device was able to achieve relatively precise focusing of 6.1 μm diameter polystyrene particles (Fig. 1b).
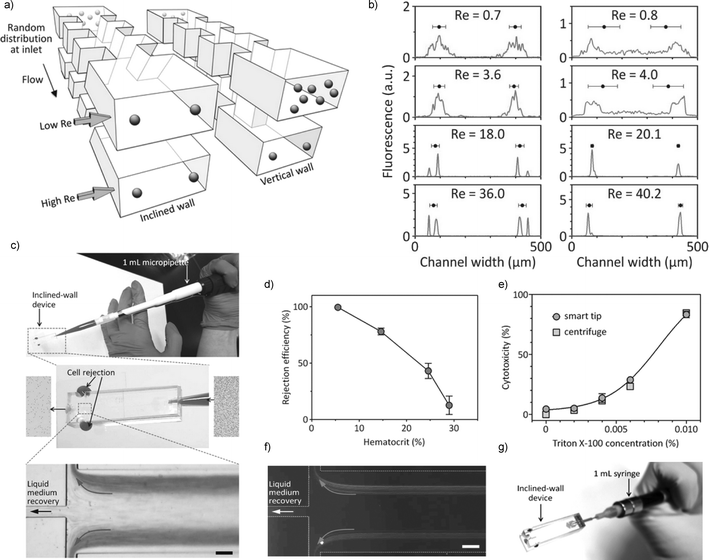 | ||
| Fig. 1 Pipette-enabled cell separation across flow rates. (a) Schematic of the microfluidic device for particle focusing – the width of the expansion and contraction channels were we = 60 μm and wc = 20 μm, respectively. The channel heights (h) for the inclined-wall and the vertical-wall devices are 72.5 μm and 62.9 μm, respectively. In the inclined-wall device, randomly distributed particles are focused near the side walls by hydrophoresis at low Re and by inertial forces at high Re. (b) Fluorescence intensity profile across the width of the channel outlet – left: the inclined-wall device, right: the vertical-wall device. (c) Liquid medium recovery using the microfluidic pipette tip add-on inclined-wall device. Interconnection was to a 1 mL micropipette. (d) Effect of hematocrit on rejection efficiency – blood samples with different hematocrit concentrations are injected into the tip by positive displacement operations (throughput of 144.6 ± 12.7 μL min−1 for blood cell rejection). (e) Dose–response curve for Jurkat cells exposed to Triton X-100 in comparison with centrifuge-based sample preparation methods (p = 0.11 using a two-tailed paired t-test), n = 3 different devices. (f) Channel containing a solution with food dye for better visualization of the streams. (g) The microfluidic pipette tip connected to the inclined-wall device. Interconnection was to a 1 mL syringe (positive displacement). Figure adapted from Song et al.5 with permission. | ||
Improved focusing efficiency was observed as the particle size increased (σ = 57.7, 24.8, and 17.8 μm for particle diameters of 4.1 μm, 6.1 μm and 9.9 μm, respectively). The smaller particles experience less steric interaction, which may not be sufficient to keep the particles out of channel spanning rotational flows. RBCs in Dulbecco's phosphate-buffered solution (DPBS) with a final concentration of 3.4 × 108 cells mL−1 was also examined. The RBCs, which have a Stokes' radius of 2.58 μm,8 satisfied the hydrophoresis equilibrium criterion and also exhibited focusing positions close to the side walls regardless of Re.
The authors were able to use the relative flow-rate insensitivity of the device to filter cell solutions from a pipette tip depressed by a pipettor. Diluted blood sample was loaded into the 1 mL pipette tip and the tip was connected to the PDMS device, which was operated manually by pushing the plunger of a pipette or syringe (Fig. 1c, f, g). An average volumetric flow rate of Qav = 29.3 ± 3.3 μL min−1 and an average pressure Pav = 90 kPa were obtained by compressing the air inside the air displacement micropipette. A high rejection efficiency (ratio of the number of rejected cells divided by the initial number of cells) of 88.9 ± 2.7% was obtained (Fig. 1d). A seven fold increase in volume throughput was achieved when 10 channels were stacked on top of each other in a parallel device, without compromising device performance (a rejection efficiency of 85.9% with an improved throughput of 1.1 × 106 cells s−1 was obtained while collecting cell-free liquid medium with a volume recovery of 20.3%). In a separate experiment, Jurkat cells were exposed to various concentrations of Triton X-100, a cytotoxic surfactant, for 30 min and the lysed cell content was characterized by separating out the remaining live cells (Fig. 1e). LDH released from the porated cells was recovered at a rate of 197.9 ± 22.5 μL min−1 with a liquid recovery of 18.7 ± 0.9%.
In summary, the proposed hydrophoresis inertial platform enabled cell removal and liquid-medium recovery across a range of flow conditions without the need for high precision and costly equipment for device operation. Further improvement in the rejection efficiency and throughput will be needed in the future towards a “microfluidic-enabled pipette tip” that operates seamlessly in seconds as a user pipettes.
Physical separations for improved cell-based therapies
Significant advances have emerged in the study and generation of multipotent mesenchymal stromal cells (MSCs),10 but there has been difficulty in characterizing and isolating these rare cell types from heterogeneous cell populations. In particular, distinguishing multipotent MSC subpopulations from precommitted osteogenic progenitor cells within derived MSCs remains a challenge. However, the intricacies of cellular gene expression, signaling cascades, and other complex biochemical interactions may be encompassed by a measurable set of biophysical parameters. Physical parameters are also beneficial for separations destined for cell therapies because exogenous factors that require additional regulation can be minimized. To address such ideas, Lee et al. assessed the use of biophysical traits as indicators of multipotency.11First, the group made use of inertial microfluidic Dean vortices in a spiral channel device to sort cells by size (Fig. 2a). As previously characterized by the authors,12 the device is able to fractionate four size ranges of adult bone marrow-derived MSCs (aMSCs) at high throughput (>107 cells h−1) and viability (>90%). All experiments were performed on cells with low (Dlo, ~16.8 μm) or high diameters (Dhi, ~25.7 μm) with minimal overlap. Fetal MSCs (fMSCs) were consistently small and were not sorted in this fashion. Based on size alone, all large diameter aMSCs were found to be bipotent (osteogenic and chondrogenic), but not all small aMSCs and fMSCs were multipotent.11
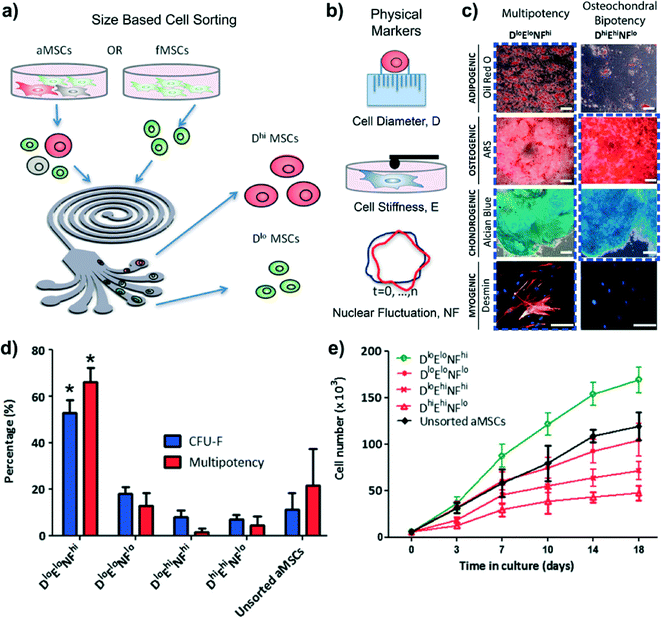 | ||
| Fig. 2 Inertial microfluidic dean vortices for rare cell isolation. (a) aMSCs are heterogeneous in size and were first size sorted using a microfabricated spiral microchannel into four fractions (outlets 1, 2, 3, and 4) of decreasing cell diameter. Subpopulations of the largest diameter cells (outlet 1; ~25 μm, Dhi) and subpopulations of the smallest diameter cells (outlet 4; ~16 μm, Dlo) were used for subsequent experiments for each donor source. The corresponding unsorted aMSCs from the same donor (~21 μm) were used as the control. fMSCs exhibit less dispersion in size (~16 μm, Dlo) and could not be further fractionated as a function of diameter. (b) After size separation, the Dlo and Dhi MSC subpopulations were categorized in terms of cell size, stiffness, and relative nuclear fluctuation. (c) Multi-lineage differentiation of MSC subpopulations along fat, bone, cartilage, and muscle lineages in vitro. Only DloEloNFhi MSCs were multipotent (>60%) and consistently exhibited differentiation along all four lineages, whereas MSCs that exhibited either high stiffness (Ehi) or low nuclear fluctuation (NFlo) were limited to differentiation along only the osteogenic and chondrogenic lineages (scale bar = 50 μm). The DloEloNFhi MSCs also showed higher colony formation efficiency, CFU-F (d), and higher proliferation rate (e). Figure adapted from Lee et al.11 with permission. | ||
Although size alone was not sufficient to distinguish between subpopulations, size-based sorting helped facilitate further biophysical analysis (Fig. 2b). Using atomic force microscopy, large aMSCs were found to be stiffer (460.3–1100 Pa) than their smaller counterparts (329.6 Pa for aMSCs and 321.3 Pa for fMSCs) and also less potent (r = −0.787, P < 0.01).11 All stiff cells (Ehi) with E > 375 Pa exhibited osteochondral bipotency, but not all compliant subpopulations (Elo) with E < 375 Pa were multipotent.
Temporal fluctuations in nuclear lamina were also examined, using transfections with tagged nuclear membrane protein EGFP-LaminB1. Large MSCs exhibited lower relative nuclear fluctuation NF (0.90–1.12%) whereas small MSCs had higher NF, which also correlated with multipotency (r = 0.852, P < 0.05).11
Transcripts and markers associated with osteogenic differentiation were found in DhiEhiNFlo groups but were down-regulated in DloEloNFhi groups, and markers for all three mesenchymal lineages were primarily found in DloEloNFhi MSCs (Fig. 2c). Moreover, DloEloNFhi MSCs demonstrated larger numbers of colony forming units (Fig. 2d) as well as higher proliferation rates (Fig. 2e). The authors conclude that small (D < 18 μm) and compliant (E < 375 Pa) cells with high nuclear fluctuations (NF > 1.2%) are necessary and sufficient characteristics for multipotency (DloEloNFhi).11
Finally, the group demonstrated enhanced bone mineralization in NOD/SCID mice when using osteoprogenitor MSC groups (DloEloNFlo, DloEhiNFhi, and DhiEhiNFlo) seeded into osteoinductive polymer scaffolds.11 Since committed osteoprogenitor cells may be isolated with their device by size alone, advances in bone regeneration may be within reach. With regards to muscle regeneration, the group found that DloEloNFhi MSCs gave rise to more myofiber-like tissues in mice with cardiotoxin-damaged skeletal muscle tissue.11 Consequently, the selection of cells based on diameter, elastic modulus, and nuclear fluctuations may enable diverse applications in tissue regeneration.
Although characterizations of some parameters may still be time-consuming, this work is an impressive step toward label-free isolation of cell subpopulations and sets the framework for cell classification based purely on multivariate biophysical traits. Further improvements in this field may greatly enhance biological studies and therapeutic applications.
Separating cells from the living brain with nanomagnets
Multipotent stem cells obtained from a patient can be used to produce patient-specific cell models to test therapeutic approaches in vitro.13 The major steps in this process include: (i) isolating and extracting tissue from the donor, and (ii) differentiating multipotent cells to a specific cell type in which disease arises. When it comes to the central nervous system, this approach faces technical challenges. The isolation of tissue from the brain requires a high risk surgery and the target location of ideal cell populations is difficult to reach.14Lui and co-workers developed a magnetic nanoparticle-aided surgical technique, in which magnetic nanoparticles (nanomagnets) are injected into the brain to bind to and extract the target ependymal cells. After attachment to or endocytosis by ependymal cells, the nanomagnets are isolated from the brain tissue through magnetic agitation and syringe pump driven cell collection9 (Fig. 3a). The nanomagnets consist of an iron oxide core (Massart's method), a silica shell (reverse micelle method) and fluorescein isothiocyanate. The silica shell is further functionalized with CD133 antibodies (EDC/NHS chemistry), which allowed Lui and co-workers to specifically target their nanomagnets to associate with the ependymal cell layer.
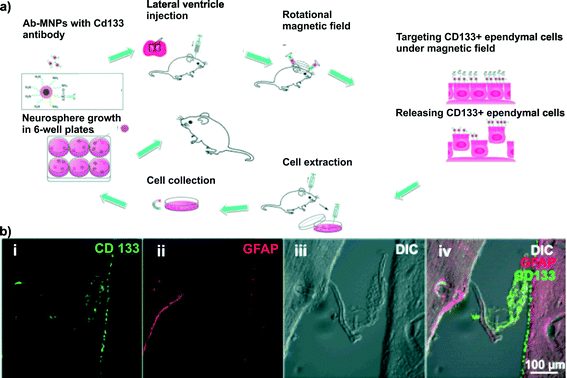 | ||
| Fig. 3 In situ extraction of brain-derived neural stem cells using magnetic nanoparticles. (a) Process steps required for neural stem cell isolation from anesthetized living rat brains. During a brain surgery, functionalized magnetic nanoparticles were injected into the sub-ventricular zone to specifically bind to ependymal cells, an assumed source of neural stem cells. Rotational magnetic fields are externally applied to agitate and release ependymal cells with endocytosed nanomagnets, which are further cultured in vitro. (b) Under optimal parameters, 6 h incubation time for nanomagnet uptake (2000 μg mL−1 in 5 μL of PBS) and an additional 15 min magnetic agitation, ependymal cells (CD133) break apart from the sub-ventricular tissue layer. Scale bar: 100 μm. Figure adapted from Lui et al.9 with permission. | ||
The nanomagnets were administered into the SVZ of anesthetized rats, through two stereotaxically drilled, 200 μm diameter holes (bregma: +0.05 cm, midline: ±0.1 cm) in the skull. Then, nanomagnets were allowed to interact with the SVZ tissue over 1 to 24 hours. To loosen the nanomagnet-loaded ependymal cells, a magnetic probe was inserted into the skull and the ependymal cells were magnetically agitated. Although nanomagnetic force driven cell separation could be easily integrated, Lui and co-workers instead retrieved loosened ependymal cells through suction.
Under optimal parameter selection for the nanomagnet uptake (6 h) and magnetic agitation (15 min), between 80 and 390 neural stem cells per experiment were extracted and further cultured in well-plates (Fig. 3b). The neural stem cell culture yielded an average neurosphere generation rate of 4.49 ± 2.0 (SE, n = 4), reaching 100 μm in diameter after 9 days in vitro. Additionally, Lui and co-workers demonstrated through immunocytolabeling the existence of neurons, astrocytes, oligodendrocytes and neural progenitor cells within their spheres.
In summary, nanomagnetic cell separation from living tissue is a new approach to sample previously inaccessible purified cell populations without gross tissue removal. One potential application may be to establish patient-derived in vitro cell models to adjust clinical treatments, although induced pluripotent cell models from non-invasively obtained cells (e.g. fibroblasts, or leukocytes) may also serve this role in the future.
References
- M. Kersaudy-Kerhoas and E. Sollier, Lab Chip, 2013, 13, 3323–3346 RSC.
- J. El-Ali, P. K. Sorger and K. F. Jensen, Nature, 2006, 442, 403–411 CrossRef CAS PubMed.
- R. Fan, O. Vermesh, A. Srivastava, B. K. H. Yen, L. Qin, H. Ahmad, G. A. Kwong, C.-C. Liu, J. Gould, L. Hood and J. R. Heath, Nat. Biotechnol., 2008, 26, 1373–1378 CrossRef CAS PubMed.
- S. Choi and J.-K. Park, Small, 2009, 5, 2205–2211 CrossRef CAS PubMed.
- S. Song and S. Choi, Appl. Phys. Lett., 2014, 104, 074106 CrossRef PubMed.
- S. C. Hur, H. T. K. Tse and D. Di Carlo, Lab Chip, 2010, 10, 274–280 RSC.
- J. Zhou, P. V. Giridhar, S. Kasper and I. Papautsky, Lab Chip, 2013, 13, 1919–1929 RSC.
- A. C. Groom and J. C. Anderson, J. Cell. Physiol., 1972, 79, 127–137 CrossRef CAS PubMed.
- C. N. P. Lui, Y. P. Tsui, A. S. L. Ho, D. K. Y. Shum, Y. S. Chan, C. T. Wu, H. W. Li, S. C. E. Tsang and K. K. L. Yung, Angew. Chem., Int. Ed., 2013, 52, 12298–12302 CrossRef CAS PubMed.
- I. A. Titushkin, J. Shin and M. Cho, Crit. Rev. Biomed. Eng., 2010, 38, 393–433 CrossRef.
- W. C. Lee, H. Shi, Z. Poon, L. M. Nyan, T. Kaushik, G. V. Shivashankar, J. K. Y. Chan, C. T. Lim, J. Han and K. J. Van Vliet, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E4409–E4418 CrossRef CAS PubMed.
- W. C. Lee, A. A. S. Bhagat, S. Huang, K. J. Van Vliet, J. Han and C. T. Lim, Lab Chip, 2011, 11, 1359–1367 RSC.
- H. I. Kornblum, Stroke, 2007, 38, 810–816 CrossRef PubMed.
- N. Sanai, A. D. Tramontin, A. Quinones-Hinojosa, N. M. Barbaro, N. Gupta, S. Kunwar, M. T. Lawton, M. W. McDermott, A. T. Parsa, J. Manuel-Garcia Verdugo, M. S. Berger and A. Alvarez-Buylla, Nature, 2004, 427, 740–744 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |
