 Open Access Article
Open Access ArticleLab-in-a-tube systems as ultra-compact devices
S.
Sánchez†
*
Max-Planck Institute for Intelligent Systems, Heisenbergstr. 3, 70569 Stuttgart, Germany. E-mail: sanchez@is.mpg.de
First published on 8th December 2014
Abstract
In this Focus article, I will give an overview on the current and future interests of our multidisciplinary research group. One of our main interests is to develop highly integrated on-chip components towards ultra-compact devices for biosensing technologies (lab-in-a-tube). Our other activities are focused in developing self-powered devices that can generate either motion of a fluid or autonomous propulsion. We are particularly interested in three-dimensional (3D) nanofabrication technologies and stimuli responsive soft materials.
The interest in cross-disciplinary topics to make compact devices is growing significantly every year. Engineering of elastic and stimuli responsive materials, energy harvesting materials, self-powered pumps and micro-nanomotors using new and emerging technologies with bio-mimicking characteristics or with healthcare applications is increasing in demand.
Our group has an interest in the design of miniaturized devices that bridge functional materials and bio-related applications. We have explored compact on-chip sensors for a new paradigm technology shrinking the Lab-on-a-Chip towards the Lab-in-a-Tube concept. A large part of our activities has been – and will be – focused on self-powered micro-nanomotors and pumps designed by various techniques including photolithography, electro-deposition, and 3D printing. We are very interested in soft and responsive materials and the use of biological components combined with artificial parts for the development of micro-bio-robots.
Therefore, over the following Focus articles, we will be selecting and highlighting cutting-edge, pioneering and ground-breaking reports on the above mentioned fields. Here, I will present a short overview on recent activities in lab-in-a-tube work.
Compact on-chip microtubes for bio-related applications
Lab-on-a-chip devices aim at scaling down several components and integrating them into compact devices. What if we could integrate some of those components into a small tube? This would eventually miniaturize an entire laboratory into an ultra-compact architecture. My group and others have put some efforts into that concept, based on the shrinkage of “lab-on-a-chip” to “lab-in-a-tube” micro-analytical systems, which can delicately control the positioning of single cells inside micro-tubular structures integrated on-chip.1 Moreover, this system would enable the reduction of many steps of the analytical process such as the sampling separation and interaction of cells with small amounts of chemicals. The use of the microtubes both as reaction chambers for live studies and as detection systems is of great novelty. The “lab-in-a-tube” concept represents a multifunctional device that enables the observation of single cell behavior inside transparent microtubes and can be employed for several biological applications as well as fundamental understanding of biological behaviours while mimicking in vivo cellular micro-environments.Transparent biocompatible microtubes for cell studies
Many of the studies on mammalian cells are carried out on planar two-dimensional (2D) substrates. However, cells behave differently in 2D and 3D. Cells normally proliferate and differentiate in confined spaces and circulatory cells move through small capillaries during metastasis. There are several methods to mimic the 3D complexity of tissues. Over the last few years, some reports demonstrated the suitability of roll-up nanotech2 to engineer 3D micro-scaffolds mimicking in vivo environments such as vessels.Rolling up thin oxide layers, whose diameters can be deterministically tuned on demand, allows for the direct observation of single cell features and single cell behaviours.3
Huang et al. reported the first use of rolled-up microtubes for guiding the growth of cells in vitro.4 The transparent oxide microtubes were used to confine yeast cells and guide their growth and budding (Fig. 1(a)). Schulze et al. took a step forward by culturing primary mouse motor neurons and CAD cells, demonstrating the morphological differentiation of neurons.5 The tubular micro-patterns support axon guidance forming complex square-shaped neurite networks (Fig. 1(b, d–f)). This work attracted much attention from the community and has been recently followed by others.6
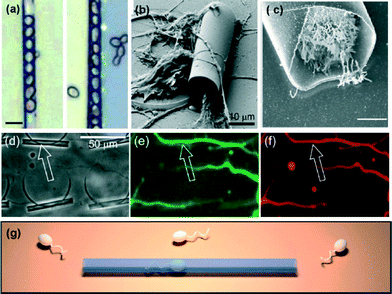 | ||
| Fig. 1 Different cell types encapsulated into rolled-up microtubes. (a) Optical images of yeast cells inside transparent oxide microtubes. Scale bar: 10 μm. (b) SEM image of motor neurons extending their neurites onto/into a microtube. (c) SEM of a HeLa cell inside the cavity of a microtube. Scale bar: 5 μm. (d–f) Neurite outgrowth guided by microtubes producing straightly aligned axons and dendrites. (d) Phase contrast, and (e) AlexaFluor488 fluorescently stained axons. (f) Aligned neurites stained by AlexaFluor568. (g) Illustration of sperm swimming into a transparent microtube, which acts as a highway for aligning the motion of sperm cells on-chip. Reprinted from ref. 4 (a), ref. 5 (b, d–f), ref. 9 (c), and ref. 7 (g), with permission. | ||
In another recent publication, Magdanz et al. fabricated an on-chip system that mimics tubular micro-environments for the study of spermatozoa motion in confinement (Fig. 1(g)).7 The influence of tube diameter on the velocity, directionality, and linearity of spermatozoa was investigated. The motivation relies on the fact that in vitro experiments often lack comparability to in vivo conditions. This was resolved by using microtubular channels, comparable to the journey of sperm cells to the oocyte.
Oxide microtubules of 2 μm were used for reproducing dentinal tubules in vitro, where E. faecalis were able to penetrate and reproduce. This system allows examination of the mechanisms of bacterial adhesion and invasion into tubular structures for dental investigations and applications.8
Just this year, two publications reported more biophysical insights into cell–micropattern interactions with single-cell resolution and high imaging resolution.
The potential of the rolled-up technology for cellular studies was recently extended to HeLa cancer cells and non-transformed retinal pigment epithelial RPE1 cells, and high-resolution techniques were used for imaging chromosome segregation errors (CSE) and cell divisions in 3D contexts (Fig. 2(a–c)).9 The results showed that below a tube diameter threshold, physical constraints led to a large increase in CSEs for both cell lines, perturbed metaphase plate formation and delayed mitosis. Interestingly, extreme confinement prevented RPE1 cells from entering mitosis, but the HeLa cells divided under the same physical constrains. This is an important discovery and technology for the investigation of cancer progression.
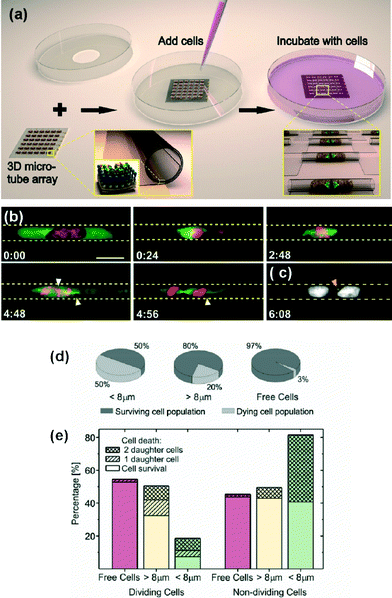 | ||
| Fig. 2 Study of cell behaviours in confined tubular spaces. (a) Schematic depicting the 3D tubular platform array. The zoomed-in image in the bottom left shows the rolled-up nanotechnology fabrication of transparent SiO/SiO2 microcavities on a transparent substrate. The insert indicates that the surface of the microcavities was biofunctionalized with a self-assembled monolayer of octadecanylphosphonic acid, and yellow, brown and blue spheres, to which fibronectin (on top of the blue spheres) was covalently coupled. The zoomed-in image in the bottom right shows the encapsulation of individual proliferating cells inside the microcavities. (b) Spatial confinement of HeLa cells leads to mitotic delays in prometa-/metaphase and chromosome segregation errors (CSEs) in cavities of 9 μm diameter. GFP-tubulin is expressed in green and H2B-mCherry in red. (c) Formation of micronuclei in the arising daughter cells (orange arrowhead). (d) Overall survival of U2OS cells in confined spaces of different diameters. (e) Further discrimination between U2OS cells that divide or show no cell division. Reprinted from ref. 9 (a–c) and ref. 10 (d–e), with permission. | ||
Koch et al. observed nuclei elongation of U2OS confined cells upon tube diameter decrease.10 The cells adopt the shape of the micro-structures, and their survival and mitosis is affected by the extent of confinement (Fig. 2(d–e)). The microtube technology can be employed to test maximum deformability of cell nuclei and as a tool for studying the cell mechanics in 3D.
Similar to what Whitesides reported for 2D patterns,11 this work demonstrated that cells require a minimum space for survival and division in 3D.10 The authors concluded that extreme squeezing of the cell nucleus can lead to abnormal cell cycle progression.
Label-free optical sensors
In addition to transparency and biocompatibility for bio-applications, the lab-in-a-tube systems can combine several functionalities, such as optical or electrochemical sensing, among others.1Large arrays of micro-tubular resonators were designed for capturing fibroblast NIH3T3 mouse cells and their optofluidic sensing was provided by analysing micro-photoluminescence spectroscopy.12 The presence of cells inside the cavity of the transparent oxide microtubes had a reproducible and very sensitive effect on sharpening the quality factor (Q-factor) and shifting the whispering gallery modes (WGMs) (Fig. 3(a)). The authors hypothesized that the optical measurement and observation of cellular events could be taken while pumping the fluid through microtubes integrated in microfluidic devices. Related results did not take long to appear. Indeed, Harazim et al. reported in Lab on a Chip the fabrication and characterization of high Q (2900) rolled-up optofluidic ring resonators fully integrated into lab-on-a-chip devices (Fig. 3(b)).13 The authors described a technology platform for the integration process from the fabrication of microtubular resonators to the final device. They obtained a minimum detection limit of 3.4 × 10−4 per refractive index unit (RIU) and a maximum sensitivity of 880 nm RIU−1, which was the highest value measured for any optofluidic ring resonator at the time of publication.
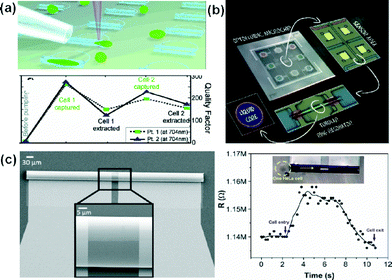 | ||
| Fig. 3 Label-free detection systems using the lab-in-a-tube concept. (a) Illustration of the pumping system of NIH3T3 cells into oxide microtubes. The lower panel shows the calculated Q-factor of each spectrum for two different points (Pt. 1 and Pt. 2) at 704 nm. Before a cell capture, after a cell capture, and after evacuation of the cell is shown for two cells pumped consecutively. (b) Sequence of integration of optofluidic tubular ring resonators in microfluidic chips. (c) SEM image of the 3D tubular microsensor 250 μm in length. Left panel: optical image of a single HeLa cell (yellow dash cycle, top) about to go through the tubular cavity of the sensor (yellow arrow indicates the direction) and the corresponding real-time changes in the resistance read-out (bottom). Reprinted from ref. 12 (a), ref. 13 (b) and ref. 17 (c), with permission. | ||
Electrochemical ultra-compact tubular sensors
Self-assembled rolled-up tubular structures enable the inclusion of microelectrodes14–16 on the inner surface, towards lab-in-a-tube systems. The self-rolling process leads to the development of 3D tubular microsensor, which can be integrated into PDMS microfluidic chips. In particular, we are interested in biodetection using label free impedimetric sensors (Fig. 3(c)).17 The compact microsensors demonstrated a limit of detection (0.1 nM for KCl) two orders of magnitude lower than planar conductivity detection systems. In addition, these sensors are capable of detecting and observing – under an optical microscope – single HeLa cells in flowing conditions.Rather than a photoresist, the use of Ge, which is etched slowly by water, leads to more compact and stable tubular structures during the rolling process.14 This rolling-up methodology based on water makes the device compatible for further bio-applications, including bio-functionalization of surfaces, cytometry, cell sorting/manipulation and biosensing, among others. The transparency of TiO2 enables the simultaneous optical detection and observation of bio-organisms and labeled molecules flowing through the microtube. This is of special interest for applications in the field of cellular biology, because cells can be monitored both electrically and optically.
Self-powered on-chip micropumps
Controlling the fluid flow at the micro scale is a challenge in the field of micro-nanodevices. Particular attention has been given to pumps that can precisely modulate flow rate without any external source of power, giving precise spatial and temporal control over the flow rate.Sen’s group demonstrated catalytically induced flow-induced patterns in H2O2 solution above a Au surface containing Ag circles and rings,18 and most recently enzyme-powered micropumps that use catalase, urease, lipase, and GOx to generate fluid flows.19 Bachtold et al. observed the motion of neutral, positive, and negatively charged particles near a Pt/Au bimetallic micro-pump and were able to estimate the direction and magnitude of the electric field and fluid flow around the pump.20 Solovev et al. immobilised catalytic microtubes on-chip to act as micropumps. These micro-tubes contain an inner Pt layer, which decomposes H2O2 into H2O and O2 microbubbles that are continuously expelled out of the tubes. The integrated catalytic pumps are activated at H2O2 concentrations as low as 0.0009% v/v. The motion of the flow surrounding the micropumps can be tracked by using tracer particles. Longer tubes require lower concentrations of H2O2 than shorter tubes to start pumping.21
Due to the conceptual similarities between catalytic micropumps and catalytic micromotors – which is one of my main interests – this section will be extended in upcoming Focus articles, highlighting new integrated catalytic and bio-catalytic micropumps.
Conclusions
The synergy between materials science, engineering and biology opens many multidisciplinary fields and topics with great potential in healthcare, energy, sensing, etc. Among them, ultra-compact devices and 3D architectures may be very important in the near future in cell adhesion, migration and other cell biology fields. Self-powered compact devices such as micropumps and microrobotics are emerging fields with applications ranging from sensing–actuating devices, drug delivery, and environmental remediation. This Focus article was presented as a self-introductory summary of our activities in a new concept of on chip integration, the so-called: Lab-in-a-Tube.Acknowledgements
S. Sánchez thanks all the people who contributed to this project over the last 4 years and the European Research Council (ERC) for Starting Grant “Lab-in-a-tube and Nanorobotics biosensors; LT-NRBS” [no. 311529] for financial support.Notes and references
- E. J. Smith, W. Xi, D. Makarov, I. Mönch, S. Harazim, V. A. B. Quiñones, C. K. Schmidt, Y. Mei, S. Sanchez and O. G. Schmidt, Lab Chip, 2012, 12, 1917 RSC.
- O. G. Schmidt and K. Eberl, Nature, 2001, 410, 168 CrossRef CAS PubMed.
- S. M. Harazim, W. Xi, C. K. Schmidt, S. Sanchez and O. G. Schmidt, J. Mater. Chem., 2012, 22, 2878 RSC.
- G. Huang, Y. Mei, D. J. Thurmer, E. Coric and O. G. Schmidt, Lab Chip, 2009, 9, 263 RSC.
- S. Schulze, G. Huang, M. Krause, D. Aubyn, V. A. B. Quiñones, C. K. Schmidt, Y. Mei and O. G. Schmidt, Adv. Eng. Mater., 2010, 12, B558 CrossRef.
- M. Yu, Y. Huang, J. Ballweg, H. Shin, M. Huang, D. E. Savage, M. G. Lagally, E. W. Dent, R. H. Blick and J. C. Williams, ACS Nano, 2011, 5, 2447–2457 CrossRef CAS PubMed.
- V. Magdanz, B. Koch, S. Sanchez and O. G. Schmidt, Small, 2014 DOI:10.1002/smll.201401881.
- B. W. Sigusch, S. Kranz, S. Klein, A. Völpel, S. Harazim, S. Sanchez, D. C. Watts, K. D. Jandt, O. G. Schmidt and A. Guellmar, Dent. Mater., 2014, 30, 661–668 CrossRef CAS PubMed.
- W. Xi, C. K. Schmidt, S. Sanchez, D. H. Gracias, R. E. Carazo-Salas, S. P. Jackson and O. G. Schmidt, Nano Lett., 2014, 14, 4197–4204 CrossRef CAS PubMed.
- B. Koch, S. Sanchez, C. K. Schmidt, A. Swiersy, S. P. Jackson and O. G. Schmidt, Adv. Healthcare Mater., 2014, 3, 1753–1758 CrossRef CAS PubMed.
- C. S. Chen, M. Mrksich, S. Huang, G. M. Whitesides and D. E. Ingber, Science, 1997, 276, 1425 CrossRef CAS.
- E. J. Smith, S. Schulze, S. Kiravittaya, Y. Mei and S. Sanchez, Nano Lett., 2011, 11, 4037 CrossRef CAS PubMed.
- S. M. Harazim, V. A. Bolanos Quinones, S. Kiravittaya, S. Sanchez and O. G. Schmidt, Lab Chip, 2012, 12, 2649 RSC.
- C. C. S. Bof Bufon, J. D. Cojal González, D. J. Thurmer, D. Grimm, M. Bauer and O. G. Schmidt, Nano Lett., 2010, 10, 2506–2510 CrossRef CAS PubMed.
- D. Grimm, C. C. Bof Bufon, C. Deneke, P. Atkinson, D. J. Thurmer, F. Schäffel, S. Gorantla, A. Bachmatiuk and O. G. Schmidt, Nano Lett., 2012, 13, 213–218 CrossRef PubMed.
- I. Mönch, D. Makarov, R. Koseva, L. Baraban, D. Karnaushenko, C. Kaiser, K.-F. Arndt and O. G. Schmidt, ACS Nano, 2011, 5, 7436–7442 CrossRef PubMed.
- C. S. Martinez-Cisneros, S. Sanchez, W. Xi and O. G. Schmidt, Nano Lett., 2014, 14, 2219–2224 CrossRef CAS PubMed.
- T. R. Kline, W. F. Paxton, Y. Wang, D. Velegol, T. E. Mallouk and A. Sen, J. Am. Chem. Soc., 2005, 127, 17150–17151 CrossRef CAS PubMed.
- S. Sengupta, D. Patra, I. Ortiz-Rivera, A. Agrawal, S. Shklyaev, K. K. Dey, U. Córdova-Figueroa, T. E. Mallouk and A. Sen, Nat. Chem., 2014, 6, 415–422 CrossRef CAS PubMed.
- A. A. Farniya, M. J. Esplandiu, D. Reguera and A. Bachtold, Phys. Rev. Lett., 2013, 111, 168301 CrossRef.
- A. A. Solovev, S. Sanchez, Y. Mei and O. G. Schmidt, Phys. Chem. Chem. Phys., 2011, 13, 10131–10135 RSC.
Footnote |
| † Now also at: Institute for Bioengineering of Catalonia (IBEC), 08028 Barcelona, Spain, Institució Catalana de Recerca i Estudis Avançats (ICREA), 08010 Barcelona, Spain. |
| This journal is © The Royal Society of Chemistry 2015 |
