Making it stick: the role of structural design in implantable technologies
Wontae
Lee
,
Richard L.
Leask
and
Christopher
Moraes
*
Department of Chemical Engineering, McGill University, Canada. E-mail: chris.moraes@mcgill.ca
First published on 8th October 2015
Abstract
Designing technologies that work within the human body requires innovation at the interface of biology, engineering, and material sciences. The human body presents a surprisingly hostile environment towards technologies designed to improve health, and recent approaches to these problems have leveraged the links between material form and function to improve implantable systems. The use of physical structure has emerged as a key design parameter in developing these systems, and has recently been applied to make significant progress in the field. Here, we highlight recent studies that demonstrate the innovative use of structure in the design of technologies meant to operate within the human body, with a specific focus on improving their biointegration, delivery, and functionality.
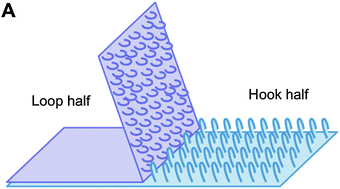
One, two, buckle my shoe; three, four, shut the door. Recited in primary schools across the world, the nursery rhyme is now out-dated, as the traditional buckle on children's shoes has been replaced with Velcro, an easy-to-use mechanical fastening system. Velcro is composed of two complementary materials, one containing a series of loops and the other hooks, and allows for the rapid and repeatable attachment and separation of the two pieces (Fig. 1). In a recent Science Advances publication, Radisic and co-workers leveraged this design approach, and fabricated bioscaffolds composed of a honeycomb mesh (loops) and T-shaped posts (hooks), similar to conventional Velcro (DOI: 10.1126/sciadv.1500423). Individual bioscaffold sheets, fabricated from a flexible and biodegradable polymer were designed as a culture surface for cardiac cells. 3D cardiac tissues with immediate and synchronised contraction were created from the horizontal and vertical assembly of individual scaffold sheets. This approach enables the adhesive ‘patch’ to work in wet and dynamic environments such as inside the human body, where chemical-based adhesives may fail. Moreover, the scalability of this structural design approach allows for the construction of patient-specific grafts that may ultimately be used to patch damaged hearts.
Whereas the Velcro inspired bioscaffold enables assembly of 3D tissues within the human body, there are a broad spectrum of challenges facing all implantable technologies, and the physical design approach employed in engineering Velcro-tissues may be a fruitful avenue with which to address these challenges. For technologies that need to operate in vivo over a long term, scientists must find a way to avoid the foreign body response, in which the body's immune system actively rejects the implanted material. Even the process of delivering and retaining technologies at in vivo sites present unique challenges, as surgery-based approaches are time and resource intensive, and associated with significant risks and discomfort. Finally, given the costs associated with these types of interventions, improving functionality in implantable systems is paramount. Here, we highlight recent innovations in the development of implantable technologies, each of which have leveraged structural design principles to improve their biointegration, delivery, and functionality.
Implantation of a material often triggers a series of inflammatory responses, wherein the recruitment of host immune cells to the site results in the formation of a fibrotic capsule around the implanted technology, isolating and preventing it from carrying out its intended function. Collectively, this process is referred to as the foreign body response (FBR), and has significantly impeded the long-term integration and clinical success of implantable technologies. Present strategies to mitigate the foreign body response include developing immune-compatible material surface coatings, treatment with anti-inflammatory drugs, and the use of angiogenic drugs to promote vascularisation and prevent fibrotic capsule isolation (DOI: 10.1208/s12248-010-9175-3). In contrast with these primarily chemical treatments, matching the mechanical compliance of vascular grafts to host arteries has been linked to improved patency (DOI: 10.1016/0741-5214(87)90148-0). Similarly, Minev et al. hypothesised that the mechanical mismatch between stiff implantable technologies and soft tissues was responsible for triggering the FBR. To test this, they engineered soft neural implants with integrated microfluidic channels to deliver drugs to spatially-precise regions within the brain (DOI: 10.1126/science.1260318). These implants, when inserted into the spinal cords of healthy rats, demonstrated superior biointegration when compared with their stiff, plastic counterparts. Over the course of six weeks, rats with stiff implants displayed impaired motor functions and deformations in spinal cord shape. In contrast, rats with soft implants demonstrated no differences from sham-operated control groups. Remarkably, these soft implants were able to restore motion in paralysed rats following spinal cord injury, through pulsed stimulation with serotonin replacement drugs and electrical stimulation. Hence, designing the physical properties of implantable surfaces could be used to prevent the foreign body response, resulting in improved biointegration.
While physical stiffness clearly plays a role in the FBR, recent evidence suggests that geometric size and shape may also be significant in avoiding rejection. Implantable systems are often fabricated with sizes defined to improve characteristic transport of molecules. For example, encapsulating alginate microspheres are used to prevent rejection of donor pancreatic islets when treating Type I diabetes. Commonly fabricated with diameters less than 1 mm, fibrotic capsule formation around the transplanted microspheres continues to impede the long-term success and clinical translatability of this technology. In a recent study in Nature Materials, Anderson and co-workers investigated the effect of size of spherical materials on triggering foreign body responses (DOI: 10.1038/nmat4290). Alginate hydrogel microspheres were fabricated in a range of sizes and implanted into the intraperitoneal space of mice. Surprisingly, improved biointegration was observed when microsphere diameters exceeded 1.5 mm, a size that is outside of the conventional range. This experiment was repeated with spheres of hydrogel, ceramic, metal, and plastic with consistent results: significantly less cellular deposition occurred on large (1.5–2 mm) spheres when compared to smaller spheres (0.5 mm), indicating that FBR is implant-size dependent (Fig. 2). Using these design principles, rat pancreatic islet cells were encapsulated in alginate hydrogel microspheres, and transplanted into diabetic mice where blood glucose levels were monitored over the course of 175 days. As expected, while the standard sized capsule group (0.5 mm) was only able to maintain normoglycaemia for 30 days, the large sized capsule group (1.5 mm) was able to maintain healthy blood glucose levels for the duration of the experiment. This study clearly shows the critical relationship between implant size and the foreign body response, and demonstrates how structural design may be used to develop implantable technologies with sustained, long-term biointegration. While it is known that cells sense and respond to their microenvironment, further experiments that investigate why certain structures are better tolerated in vivo are needed to better understand and avoid the foreign body response.
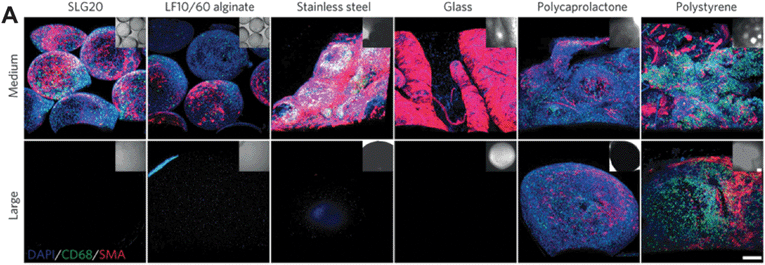 | ||
| Fig. 2 Size dependence of implanted material dictates the foreign body response and subsequent fibrotic capsule formation across material stiffnesses; medium corresponds to 0.5 mm and large corresponds to 1.5–2 mm. Figure adapted with permission from Veiseh et al., Nat. Mater., 2015, 14, 643, DOI: 10.1038/nmat4290. | ||
Implantable technologies are predominantly inserted by surgical means, and hence depend on surgeon and operating room availabilities, and are associated with postoperative complications. Taken together, these factors make traditional delivery via surgery undesirable, and minimally invasive alternatives are needed. In a recent Nature Materials publication, the Segura and Di Carlo groups addressed this problem with an innovative approach to structuring implanted technologies (DOI: 10.1038/nmat4294). Here, microporous scaffolds that self-anneal in situ were fabricated, resulting in two structural conformations: a deliverable form in which the material can be injected into the body in a non-invasive manner, and a functionally active form in vivo that promotes tissue regeneration and wound healing. The scaffolds are assembled from poly(ethylene) glycol based hydrogel microspheres (Fig. 3B) that are engineered to promote cell migration, cell adhesion, and hydrogel degradation during tissue regeneration. These microspheres self-anneal in the presence of exogenous factor XIII, a naturally occurring enzyme that functions as a stabilising factor in blood coagulation. Once on site, the hydrogel microspheres conform to the shape of the wound, and self-anneal, much like the formation of a blood clot. This approach results in scaffolds that precisely match the geometry of the in vivo implant location, eliminating the need to consider scaffold size and shape (Fig. 3A). In vitro, the scaffolds demonstrated the ability to promote cell adhesion, proliferation, and 3D network formation. In vivo, the scaffolds enabled significantly faster wound closure in mouse models when compared to their non-porous (Fig. 3C) and non-annealed hydrogel building block counterparts. Through an innovative structural approach, this study demonstrates the potential of a minimally invasive delivery technology in improving tissue regeneration.
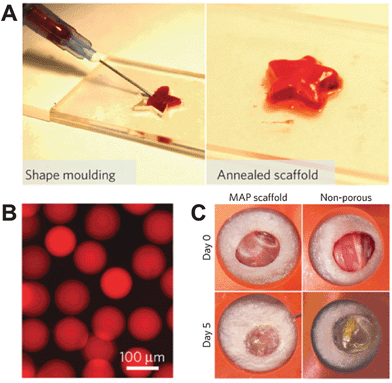 | ||
| Fig. 3 Microporous self-annealing scaffold from hydrogel microsphere building blocks. (A) Hydrogel building blocks conform to the shape of their environment before annealing in situ. (B) Fluorescence microscopy image of individual hydrogel microspheres. (C) Microporous (MAP) scaffold demonstrates superior wound closure in mouse models in vivo than non-porous counterparts. Figure adapted with permission from Griffin et al., Nat. Mater., 2015, 14, 737, DOI: 10.1038/nmat4294. | ||
Rather than assembling the scaffold from separate components in situ, recent work by Huebsch et al. in Nature Materials took a similar ‘in vivo modification’ design approach, but instead developed a system to create voids in a cell-laden hydrogel material following injection and polymerization in situ (DOI: 10.1038/nmat4407). By designing a novel time-degradable pore-forming material, they hypothesized that timed void formation could be used in combination with cell proliferation and migration rates to control the timing of cell infiltration and tissue regeneration. Furthermore, this approach would decouple the differentiation-driving effects of changing matrix elasticity in the slowly-degrading bulk matrix, from the need to have space for the cells to migrate into and remodel. This structural approach was used to demonstrate stiffness-driven bone formation, but more broadly allows for porosity, matrix elasticity and matrix adhesivity to be independently and dynamically tuned for in vivo cell-therapy applications.
While the initial placement of the implantable technology at a specific location is important, maintaining its spatial position over time is just as essential. For example, localising therapeutic vaccines may be beneficial, as it enables immune cells to be exposed to high concentrations of antigen, and thereby build up a strong immune response. One vaccine therapy that has gained notable traction in recent years is the use of autologous whole cancer cells for the immunotherapeutic treatment of cancer. Whole cancer cell vaccines offer the advantage of presenting multiple antigens, and the subsequent recruitment of a diverse range of immune cells elicits a potent and robust anti-tumour immune response (DOI: 10.3390/vaccines3020344). In this approach, patient-derived cancer cells are harvested, irradiated, and genetically engineered to overexpress immune system stimulants. However, short-term cell survival rates and poor localisation post-transplantation, along with the high costs associated with genetic manipulation have limited the success and adoption of these techniques. Recently in Nature Communications, Bencherif et al. addressed these issues using a cryo-formed macroporous delivery structure (DOI: 10.1038/ncomms8556). Irradiated tumour cells were seeded within macroporous gels that had been modified with RDG peptides to enable cell attachment and spreading, improving both cell viability and localisation. Immune response stimulants were incorporated into the porous network, and were released over time, eliminating the need for genetic manipulation. The macroporous structure enabled the entire platform to be compressed without fracture or cell death, and injected subcutaneously, after which the cryogel reverted back to its original state. In vivo experiments in mouse melanoma models, a common preclinical system, demonstrated the efficacy of the vaccination platform, as mice treated with the cryogel vaccine showed prolonged survival rates when compared to vaccines without the cryogel structure. This study highlights the use of structural design in the creation of an effective delivery platform, in which both the placement and retention of the implanted technology is improved.
3D printing is emerging as a powerful tool, as it enables the fabrication of precise 3-dimensional structures, a feat unobtainable by traditional manufacturing methods. The use of 3D printing in implantable technologies has been demonstrated, perhaps most notably by Hollister and co-workers (DOI: 10.1056/NEJMc1206319), in which a bioresorbable 3D printed tracheal splint was used to treat a newborn baby with tracheobronchomalacia. An otherwise terminal condition, where softening of the airway leads to its periodic collapse, the patient-tailored 3D printed splint was able to restore normal breathing and ultimately save the patient's life. 3D printing has more recently been used to improve technologies that are not conventionally considered ‘implantable’, but certainly enter the body: orally-administered medication. The FDA recently approved the world's first 3D-printed drug, Spritam levetiracetam, a treatment for seizures in patients with epilepsy. The 3D printing platform enabled the creation of a highly porous, high dose formulation with faster dissolution rates than existing orally disintegrating tablets. Proposed as an alternative to pills that must be swallowed, orally disintegrating tablets are helpful in treating paediatric and elderly populations where swallowing and precise dose management may be difficult. The structural design capabilities of the 3D printer enabled novel drug macrostructures with significantly improved dissolution rates over conventional powder compaction manufacturing techniques.
The initial FDA approval for 3D printed medications could be the tip of the iceberg, as 3D printed structures may enable multiple drugs to be printed in a single tablet. Presently, in the treatment of hypertensive patients with Type II diabetes, nifepidine and captopril are taken together in separate tablets. Recently, Khaled et al. showed that independent drug release profiles could be obtained in a single tablet containing three drugs using a compartmentalised structure (DOI: 10.1016/j.ijpharm.2015.07.067). Once inside the body, the joining layer dissolves, and captopril is released by osmosis, while nifedipine and glipizide are released by diffusion. One can envision future strategies that leverage this approach, in which layered structures could be used to deliver multiple doses or precisely timed sequential drug treatments. These studies illustrate how 3D printing may be used to fabricate structures unobtainable by conventional manufacturing approaches, and as the power and resolution of 3D printers grow, more creative and intricate structural designs that introduce new functionalities to printed medications can be anticipated.
Taken together, these studies highlight the importance of structure in the development of technologies designed to function within the human body, and demonstrate how structural design principles may be leveraged to engineer implantable technologies with improved biointegration, delivery, and functionality. Similar to the manner in which Velcro revolutionised the fastening industry, innovating around the physical and structural design of implantable technologies has tremendous potential to make them stick.
| This journal is © The Royal Society of Chemistry 2015 |