Micro, soft, windows: integrating super-resolution viewing capabilities into soft lithographic devices
Christopher
Moraes
Department of Chemical Engineering, McGill University, Montreal, QC, Canada. E-mail: chris.moraes@mcgill.ca
First published on 16th December 2014
Abstract
Microengineered cell culture environments afford experimentalists with the critical ability to study cells in precisely-defined, yet physiologically-realistic environments. A significant, but often overlooked, feature of these technologies is the unique ability to optically probe cellular and sub-cellular processes during culture in these complex environments, thereby obtaining information that would not be possible via conventional techniques. Motivated by the recent presentation of the Nobel prizes for super-resolution imaging and more recent technological breakthroughs in lattice-based light sheet microscopy, in this research highlight we survey recent innovations in the design of microfluidic cell culture platforms, that will ultimately allow experimentalists to probe biological activity with high-spatial and temporal-resolution. These advances will provide new technology-driven windows into biological processes and mechanisms.
Microengineered cell culture systems may be used to recreate physiologically realistic environments, that can accurately predict cellular response to candidate therapeutic compounds for drug discovery applications, or to dissect the effects of microenvironmental cues on complex cellular processes.1 For example, recent work by Kamm and co-workers in Lab on a Chip demonstrates a microfabricated high-throughput screening system to study the angiogenic activity of human endothelial cells, in response to varied chemical stimuli.2 This approach requires the fabrication of an engineered endothelium on a three-dimensional hydrogel matrix, and subjecting these layers to gradients of angiogenesis-promoting factors. The combination of gradient formation and culture in a three-dimensional, degradable matrix is essential to driving angiogenesis, and the microfluidic system enables real-time monitoring of this complex process. While this work is focused on identifying therapeutic dose parameters and activity mechanisms, another recent contribution from the Ehrbar lab in Integrative Biology applies a similar gradient-generating technology in poly(ethylene glycol) hydrogels to determine the role of soluble and matrix-tethered gradients on mesenchymal stem cell morphogenesis during tissue regeneration.3 Both studies recreate gradient features of the in vivo microenvironment, which cannot be provided with a Petri dish culture, and leverage the spatial control afforded by microfluidics to (i) create physiologically realistic microenvironmental cultures, and (ii) enable a rapid and increased-throughput study of complex cellular processes.
While microfluidic technologies can enable greatly improved experimental throughput over conventional systems, they also significantly reduce the amount of biological material available for analysis, often limiting the use of conventional end-point assays for studies of gene and protein expression. Instead, optical imaging is the de facto standard with which to study cell function in these systems, and hence, implicitly constrains the design of the experimental setup. Fortunately, silicone-based microfluidic systems are easily adapted to imaging, as the materials are transparent to visible light, and the devices can be fabricated directly on standard imaging coverslips.2 Similarly, when fabricated with micro-scale resolution in the vertical plane, most artificial and natural biomaterial scaffolds can be made optically transparent. Hence, these technologies are compatible with most microscopy platforms. Moreover, they should be theoretically compatible with recent developments in super-resolution imaging approaches, capable of observing sub-100 nm scale structures. Although current super-resolution imaging does impose stringent constraints on sample preparation and device format, integrating these two technologies may ultimately provide the best of both worlds: extreme spatial-resolution readouts of cellular processes, within physiologically realistic culture environments.
The recent presentations of the 2014 Nobel Prizes in Chemistry have brought renewed focus on the field of super-resolution microscopy. Eric Bietzig, Stefan W. Hell, and W. E. Moerner were each recognized as Nobel Laureates for their sustained contributions towards beating the diffraction limit, the long-held belief that the spatial resolution of optical microscopes was limited to around 0.2 micrometers. The high spatial-resolution approaches developed by these researchers, including stimulated emission depletion (STED) microscopy, photoactivated localized microscopy (PALM), and stochastic optical reconstruction microscopy (STORM), have been investigated since the mid-1990s, and have been reviewed in detail elsewhere.4 Commercialization and widespread adoption of these technologies is now enabling scientists to study interactions and relationships between individual proteins within cells, and when combined with microfabrication technologies, these powerful approaches can provide critical insights into biological structure-function relationships at the single-molecule level.
In a recent demonstration of combined super-resolution imaging and microengineered environmental cultures, Tijore et al. applied micropatterning approaches to direct mesenchymal stem cell shape, and employed STORM techniques to understand how the distribution pattern of focal-adhesion associated integrins are associated with cardiomyogenic differentiation.5 Briefly, human mesenchymal stem cells were micropatterned at high aspect ratios on glass coverslips, using standard microcontact printing technologies, and driven towards cardiomyogenic commitment. The researchers found that when cells were confined to high-aspect ratio adhesive patterns, super-resolution microscopy-based nanoscale analysis of active integrin-β1 revealed that integrin clusters were distributed uniformly within the focal adhesion complex. In contrast, unpatterned cells expressed the active integrin-β1 clusters around the periphery of focal adhesions (Fig. 1). These results provide an extraordinary multi-scale insight into the relationship between global cell shape and local integrin recruitment at the single-protein level, which may provide a molecular basis to understand cell-matrix interactions, and ultimately inform the design of scaffolds to direct stem cell differentiation.
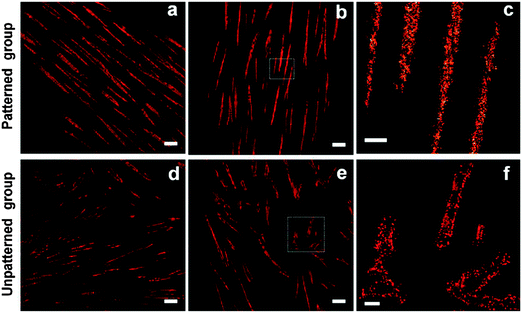 | ||
| Fig. 1 Super-resolution imaging of micropatterned human mesenchymal stem cells undergoing cardiomyogenic differentiation. Integrin clusters were found to be uniformly distributed with focal adhesions when cells were constrained to high-aspect ratio micropatterned areas (a–c). In contrast, integrins were organized around the periphery of focal adhesions in an unpatterned culture (d–f). Scale bars = 2 μm (a, b, d, and e), and 500 nm (c and f). Reprinted with permission from ref. 5, copyright 2014 American Chemical Society. | ||
Another recent example of integration between super-resolution imaging technologies and microfabricated systems is the use of microfluidic arrays of hydrodynamic traps to capture single cells, and facilitate their imaging at a spatial resolution of 25 nm, using PALM.6 These approaches may be particularly useful for super-resolution imaging in cells that are typically non-adherent: preventing cells from moving during the lengthy imaging process is of critical importance in obtaining distortion-free images. The position of centromere histones were then tracked during cell division of captured bacterial cells (Fig. 2). Although this preliminary work simply demonstrates the technical capabilities of this approach, the authors note that these experiments demonstrate that the device itself does not limit the attainable image resolution. As both microfluidics and super-resolution imaging technologies grow more mature, we expect that integration of these approaches will provide new and unique insight into the spatial molecular dynamics underlying biological processes.
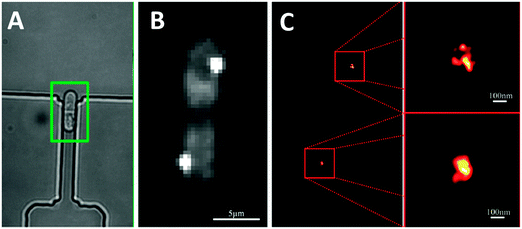 | ||
| Fig. 2 (A) Microfluidic hydrodynamic trapping array used to immobilize non-adherent bacterial cells during the cell division process. (B) Conventional epifluorescence and (C) super-resolution PALM microscopy used to image the fluorescently labelled centromeric histone H3 protein during division. Adapted with permission from ref. 6. | ||
Although super high-resolution microscopy techniques provide unique capabilities in elucidating biological mechanisms, they are often limited in terms of temporal resolution. Since increased spatial resolution requires more measurements, takes more time and requires researchers to pump additional damaging radiation into the biological samples, these techniques can cause premature photobleaching and phototoxicity, which will therefore either limit the temporal imaging window or alter the physiological state of the specimen. Hence, obtaining both high-spatial and high-temporal resolution images remains challenging. To address this problem, the Bietzig lab recently developed ‘lattice light-sheet microscopy’,7 and this technique may have particular relevance to the microfabrication and bioengineering communities. Unlike conventional confocal microscopy, in which a single objective both excites fluorescent probes and detects the emission signals, light-sheet microscopy8 uses orthogonal excitation and detection objectives. A light sheet is formed using a cylindrical lens, which is then used to excite fluorophores in a thin cross-section of the sample. The light sheet is swept through the thickness of the sample, and images of emitted light patterns are collected by the orthogonal detection objective. This technique enables rapid scanning of three-dimensional samples, and limits the amount of incident photo-activating light, thereby minimizing toxicity and photobleaching effects. Light-sheet microscopy systems are now available to researchers either commercially, or via open-source DIY communities.9
Bietzig and co-workers extended this approach towards super-resolution microscopy, by incorporating principles of structured illumination microscopy (SIM), and the use of multiple “nondiffracting” Bessel beams to create a structured illumination light sheet narrow enough for sub-cellular imaging. Reconstructing multiple SIM images can then be used to generate super-resolution images, while minimizing sample exposure to incident radiation, at a time scale rapid enough to visualize real-time biological processes. They demonstrated the use of this high-spatial and high-temporal resolution imaging technique on 20 different biological processes, and were able to visualize 3D nuclear lamins, dynamic organelle rearrangement, 3D tracking of microtubules during mitosis, and neutrophil motility in a collagen mesh (Fig. 3), amongst several others. This capability to visualize single-molecule interactions on the time scale of seconds should provide tremendous insight into previously unknown or unsuspected biological activity mechanisms.
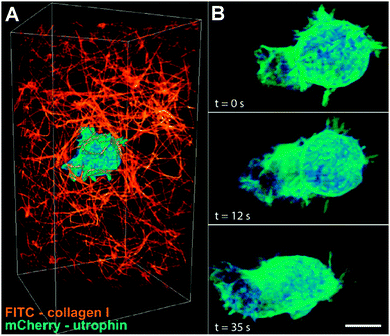 | ||
| Fig. 3 Lattice light-sheet microscopy enables high-spatial and high-temporal images of sub-cellular structures. In these example images, a cell is shown migrating through a collagen matrix, over a 35 second time span. Adapted with permission from ref. 7. | ||
Integrating light-sheet microscopy-based approaches into microfluidic systems would significantly enhance our ability to understand dynamic cellular processes under defined, yet realistic, microenvironmental conditions. However, this presents a greater challenge than integrating other existing super-resolution techniques, because of the inherent geometric limitations of planar microfluidic systems. Fabrication constraints typically allow optical access through the top and bottom surfaces, making it a challenge to establish orthogonal excitation and detection light paths. Although not yet explicitly designed for this purpose, advances in optofluidic technologies may play a critical role in designing light guides capable of conducting these super-resolution studies in microscale platforms (reviewed elsewhere10). Perhaps a simpler approach, however, lies in a recent technical innovation, developed by Koh et al., in which microscale epoxy prisms with mirrored surfaces are fabricated, and then embedded into microfluidic channels (Fig. 4).11 Briefly, triangular-profile trenches are etched into silicon using an anisotropic etching process, and then replicated in PDMS. A second replication step generates a negative template, which is then filled with a UV-curable epoxy. The cured prism is then released from the PDMS mold, and an aluminum mirror finish is created via physical deposition. These structures can then be integrated directly into microfluidic devices to direct light-paths towards desired locations. In this work, the researchers used this technology to create side-views of particles within a microfluidic channel. However, the possible applications for these simple micro-scale optical components in designing microfluidic culture devices that can be fully integrated into advanced imaging setups, such as light-sheet microscopy, are plentiful.
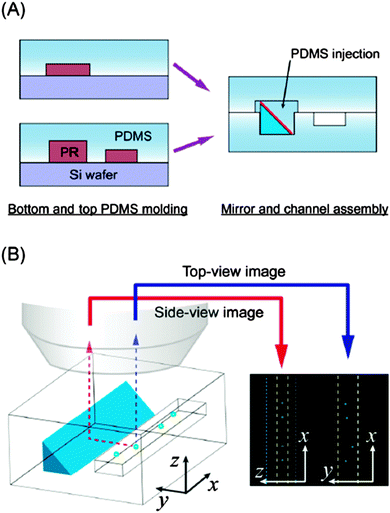 | ||
| Fig. 4 Simple microengineered optical elements may provide the critical integration elements needed to integrate novel imaging setups with microengineered cell culture environments. (A) A microengineered mirror prism is incorporated into a microfluidic channel beside a channel of interest. (B) The mirror reflects light at 90 degrees to the plane of incidence, allowing collection of ‘side images’ of cells flowing within a channel. The figure is used with permission from ref. 11. | ||
Continued improvements, adoption and availability of super-resolution microscopy tools will undoubtedly result in further examples of integrated biological studies, capable of providing a unique insight into biological systems. Although still in the early stages, the integration between microengineered tools with advanced imaging modalities promises a powerful platform for novel, groundbreaking biological inquiry.
References
- C. Moraes, G. Mehta, S. C. Lesher-Perez and S. Takayama, Ann. Biomed. Eng., 2012, 40, 1211–1227 CrossRef PubMed.
- C. Kim, J. Kasuya, J. Jeon, S. Chung and R. D. Kamm, Lab Chip, 2015, 15, 301–310 RSC.
- P. S. Lienemann, Y. R. Devaud, R. Reuten, B. R. Simona, M. Karlsson, W. Weber, M. Koch, M. P. Lutolf, V. Milleret and M. Ehrbar, Integr. Biol., 2014 10.1039/C4IB00152D.
- S. W. Hell, Nat. Biotechnol., 2003, 21, 1347–1355 CrossRef CAS PubMed.
- A. Tijore, S. Hariharan, H. Yu, C. R. I. Lam, F. Wen, C. Y. Tay, S. Ahmed and L. P. Tan, ACS Appl. Mater. Interfaces, 2014, 6, 15686–15696 CAS.
- L. Bell, A. Seshia, D. Lando, E. Laue, M. Palayret, S. F. Lee and D. Klenerman, Sens. Actuators, B, 2014, 192, 36–41 CrossRef CAS PubMed.
- B.-C. Chen, W. R. Legant, K. Wang, L. Shao, D. E. Milkie, M. W. Davidson, C. Janetopoulos, X. S. Wu, J. A. Hammer, Z. Liu, B. P. English, Y. Mimori-Kiyosue, D. P. Romero, A. T. Ritter, J. Lippincott-Schwartz, L. Fritz-Laylin, R. D. Mullins, D. M. Mitchell, J. N. Bembenek, A.-C. Reymann, R. Böhme, S. W. Grill, J. T. Wang, G. Seydoux, U. S. Tulu, D. P. Kiehart and E. Betzig, Science, 2014, 346, 1257998 CrossRef PubMed.
- P. J. Keller, A. D. Schmidt, J. Wittbrodt and E. H. K. Stelzer, Science, 2008, 322, 1065–1069 CrossRef CAS PubMed.
- P. G. Pitrone, J. Schindelin, L. Stuyvenberg, S. Preibisch, M. Weber, K. W. Eliceiri, J. Huisken and P. Tomancak, Nat. Methods, 2013, 10, 598–599 CrossRef CAS PubMed.
- Y. Zhao, Z. S. Stratton, F. Guo, M. I. Lapsley, C. Y. Chan, S. S.-C. Lin and T. J. Huang, Lab Chip, 2013, 13, 17–24 RSC.
- J. Koh, J. Kim, J. H. Shin and W. Lee, Appl. Phys. Lett., 2014, 105, 114103 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |
