Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lignin repolymerisation in spruce autohydrolysis pretreatment increases cellulase deactivation†
Thomas
Pielhop
*a,
Gastón O.
Larrazábal
b,
Michael H.
Studer
c,
Simone
Brethauer
c,
Christoph-M.
Seidel
a and
Philipp
Rudolf von Rohr
a
aInstitute of Process Engineering, ETH Zurich, Sonneggstrasse 3, CH-8092 Zurich, Switzerland. E-mail: pielhopt@ipe.mavt.ethz.ch; vonrohr@ipe.mavt.ethz.ch
bInstitute for Chemical and Bioengineering, ETH Zurich, Vladimir-Prelog-Weg 1, CH-8093 Zurich, Switzerland
cSchool of Agricultural, Forest and Food Sciences, Bern University of Applied Sciences, Länggasse 85, CH-3052 Zollikofen, Switzerland
First published on 29th April 2015
Abstract
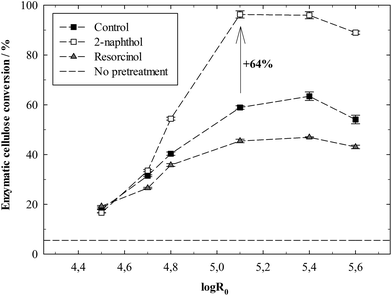
This study presents a modified autohydrolysis pretreatment which helps to overcome the recalcitrance of softwood for enzymatic hydrolysis of its cellulose. Autohydrolysis pretreatments of spruce wood were performed with 2-naphthol, which prevents lignin repolymerisation reactions, thereby increasing the enzymatic digestibility of cellulose by up to 64%. The negative influence of repolymerised lignin structures on enzymatic hydrolysis was confirmed by the addition of resorcinol in autohydrolysis, which is known to promote repolymerisation reactions and decreased the biomass digestibility. Several analyses were performed to study the underlying mechanism of this effect on hydrolysis, indicating that cellulolytic enzymes are adsorbed and deactivated especially by repolymerised lignin structures, which accounts for the high differences in biomass digestibility. It was shown that lignin repolymerisation significantly increases its specific surface area through modification of the lignin nanostructure, which is supposed to increase the unproductive binding of enzymes.
1. Introduction
Lignocellulosic biomass like wood, agricultural side products or energy crops is a potential renewable source for the production of chemicals and fuels. An intensively studied approach is the biochemical upgrading route by enzymatic hydrolysis of cellulose and hemicellulose to monomeric sugars, followed by fermentation to the final product. The lignin fraction of the biomass can be burned for the production of process heat and power but is also a prospective source for the production of aromatic chemicals due to the abundance of aromatic structures in the lignin polymer.The lignin and its entanglement with cellulose and hemicellulose however, protects the biomass from external influences like enzymatic attacks. Lignin can hinder the enzymatic hydrolysis by acting as a physical barrier, restricting the accessibility of cellulose to enzymes,2 but also by non-productive binding of cellulolytic enzymes.2–4 The hindrance by lignin is one of the main reasons why a pretreatment capable of breaking down the lignocellulosic structure, or even removing the lignin, is necessary prior to the enzymatic hydrolysis of the biomass.
Autohydrolysis pretreatment methods like hot-water and steam pretreatment are attractive regarding their cost-savings potential.5 They do not require acid, base or solvent chemicals and accordingly simplify a biorefinery process. The need for neutralisation chemicals is reduced6 and the removal of a lignin solvent, which can be inhibitory to enzymes and fermentative microorganisms,5 is not necessary. In particular steam pretreatments are of commercial relevance, as they allow for high biomass loadings and can help defibrating7 the biomass by the rapid release of pressure (steam explosion). In fact, steam explosion is one of only a few cost-effective pretreatment technologies that have been advanced to pilot scale and commercialised application.8
Softwood is an interesting feedstock, being a fast and straight growing tree and the dominant lignocellulosic feedstock available in the Northern hemisphere, therefore viewed as a potential source for fermentable carbohydrate in the United States, Canada and Scandinavia.7 However, hot-water and steam pretreatments on softwood are not effective for subsequent enzymatic hydrolysis.9 Softwood like spruce is known to be especially recalcitrant to enzymatic hydrolysis, having even been called a “worst-case scenario” as a feedstock.10 The high lignin content7 and its great degree of cross-linking11 are a major obstacle for the disintegration of the wood structure. Next to that, lignin that had been isolated from pretreated softwood was observed to exhibit stronger enzyme-lignin interactions and inhibitory effects than lignin from other sources.12
To the extent of our knowledge, only two pretreatment methods allow for high cellulose conversions from softwood with moderate enzyme dosages. The addition of an acid catalyst like sulfur dioxide (SO2)9,13 and organosolv pretreatments9,14 which remove lignin from the biomass. The complexity of those processes however is a challenge and operations that remove lignin are seen as too expensive for the bioconversion of lignocellulose into fuels.11 While organosolv pretreatments lead to an outstanding digestibility due to the almost complete lignin removal from softwood, processes involving SO2 just partly remove lignin as lignosulphonates but sulphonate the remaining lignin, increasing its hydrophilicity.18,19 This is supposed to be beneficial for enzymatic hydrolysis by reducing hydrophobic lignin-enzyme interactions18–20 and reflects the importance of lignin conditioning in softwood pretreatment.
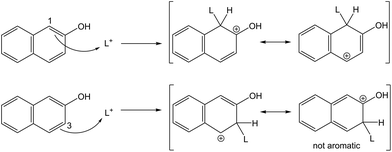
In autohydrolysis pretreatments, hemicellulose is easily solubilised and removed, while lignin is practically not removed and remains in the solid phase together with cellulose.5 This is one reason why autohydrolysis pretreatments are not effective for softwood whose lignin forms a particularly big obstacle for enzymatic hydrolysis. Another important factor might be repolymerisation reactions (sometimes also referred to as condensation reactions) of the lignin which take place during the pretreatment. In autohydrolysis, organic acids like acetic acid are released from hemicellulose6 leading to moderate acidic conditions of typically pH 2–4, depending on the type of biomass, load and pretreatment severity. Researchers have found that under acidic treatments of wood, carbonium ions are formed in the lignin molecule, that are responsible for the repolymerisation reactions.17,21,22 In today's chemical terminology, those three-coordinate carbon ions are denoted as carbenium ions,23 but will be referred to as carbocations here to avoid misconceptions with existing literature. Those carbocations have been identified as intermediates in lignin depolymerisation reactions, especially in the cleavage of β-arylether linkages as shown in Fig. 1 (route a–c). On the other hand, the electrophilic carbocations are also supposed to form, through substitution, stable C–C bonds with the electron rich carbon atoms of the aromatic rings present in lignin17,21,22 (Fig. 1 route a and d). High-molecular weight and repolymerised lignin structures are formed.21,24,25 It has further been reported that also the phenolic extractives of wood may react in that way with lignin.25 We suspect that such repolymerised lignin structures form an additional obstacle for the enzymatic hydrolysis of cellulose.
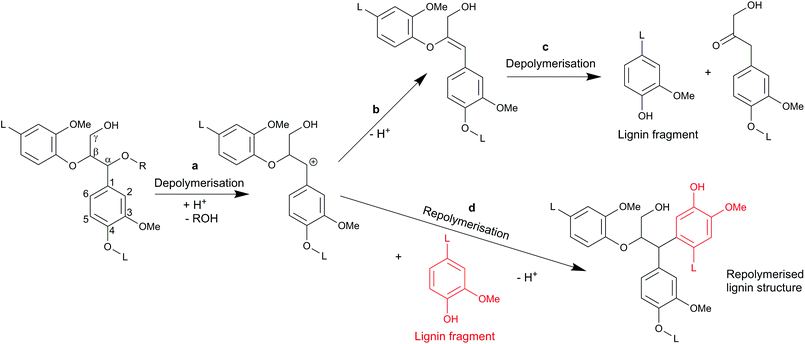 | ||
| Fig. 1 Scheme for reactions of lignin in acidic media adapted from Voitl et al.15 (a) cleavage of β-O-4 bond and formation of carbocation;16 (b) elimination of β-proton giving rise to an enol ether;16 (c) acid hydrolysis of enol ether;16 (d) repolymerisation reaction.17 | ||
Guaiacyl units in lignin can undergo repolymerisation reactions in autohydrolysis more easily26 and it has been suggested that – even in the presence of other monolignols – these reactions preferably take place among the guaiacyl units themselves.27 It is interesting to note that different studies28,29 reported how a decrease in the Syringyl(S)/Guaiacyl(G) ratio of lignin in biomass also gave rise to a significant decrease in glucose release by enzymatic hydrolysis after an autohydrolysis pretreatment. Remarkably, the S/G ratio had no influence on sugar release when no pretreatment was carried out. Next to the more difficult cleavage of bonds in samples with low S/G ratios28 (less β-O-4 and more 5–5 and β-5 linkages30), an increase in repolymerisation reactions during pretreatment with increasing guaiacyl content might play a role in this effect.
The S/G ratio in softwoods is exceptionally low,30 suggesting that repolymerisation reactions play an important role in autohydrolysis. A method for suppressing undesired lignin repolymerisation reactions is the use of carbocation scavengers. Wayman and Lora21 first reported that certain aromatic compounds can act as scavengers in the autohydrolysis of aspen and increase the yield of organic solvent-extractable lignin, allowing to obtain a highly delignified pulp. The scavengers are believed to compete with the aromatic rings present in the lignin for the formed carbocations and prevent repolymerisation reactions. 2-naphthol was found to be a very effective additive for preventing repolymerisation, yielding a lignin of lower molecular weight1,22 with greatly enhanced extractability.21,22,24,31
We have recently shown that the use of carbocation scavengers in the autohydrolysis of lignocellulosic biomass can also improve its enzymatic hydrolysability.32 The cellulose conversion in the enzymatic hydrolysis of spruce was improved up to 46% by adding 2%w/w (relative to biomass load) of 2-naphthol to the hot-water pretreatment. Surprisingly, the enhanced digestibility was not based on an increased lignin removal during the pretreatment. The effect was therefore attributed to a potentially different lignin structure in the pretreated biomass.
The present manuscript addresses two questions of an autohydrolysis pretreatment with carbocation scavengers. First, what is its potential for overcoming the high recalcitrance of softwood, i.e. to what extent can sugar yields be enhanced? Second, in which way do lignin structures that repolymerise in autohydrolysis hinder the enzymatic hydrolysability?
2. Experimental
2.1. Enhancing softwood digestibility
2.2. Effect of lignin repolymerisation on enzyme deactivation
3. Results and discussion
3.1. Enhancing softwood digestibility
In contrary to 2-naphthol, the addition of resorcinol at low concentrations was found to promote the formation of repolymerised lignin and increase its molecular weight.1,21 Resorcinol is a strong nucleophile with its aromatic ring being activated by two OH groups which are ortho and para directing towards electrophilic substitution. The resulting three activated positions allow for several substitutions to occur (up to three43) and increase the cross-linking of lignin fragments, as shown in Fig. 3.
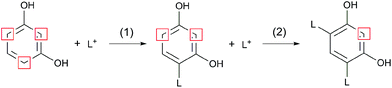 | ||
| Fig. 3 Reaction scheme adapted from Lora and Wayman1 showing the first (1) and second (2) reaction of resorcinol with a lignin carbocation (L+). Positions most reactive towards electrophilic aromatic substitution are highlighted. | ||
Pretreatment severities between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R0 = 4.5 and 5.6 as defined in (ESI eqn (4)†) were tested in the experiments with the additives. A severity of 5.1 was enough so as to release all acids from the hemicellulose and decreased the pH in the liquor to around pH 3. Increasing severity did not decrease pH any further (Fig. S1†). Hemicellulose (represented as mannan) content in all pretreated biomasses was low (Fig. S2a†) as it was easily dissolved, around 76% are already removed at a severity of 4.5. Cellulose content gradually decreased with increasing severity as more cellulose got hydrolysed and dissolved (Fig. S2a†). The AIL content rises accordingly, but no influence of the additives on the removal of lignin from the biomass is noticeable (Fig. S2b†). ASL just makes up a minor fraction of the lignin. Its content was increased with 2-naphthol and resorcinol (Fig. S2b†), probably due to their aromatic molecules being integrated into the lignin structure, thereby increasing UV-absorption in the ASL measurement. Besides, the suppression of lignin repolymerisation with 2-naphthol might also increase its solubility and thereby ASL content.
R0 = 4.5 and 5.6 as defined in (ESI eqn (4)†) were tested in the experiments with the additives. A severity of 5.1 was enough so as to release all acids from the hemicellulose and decreased the pH in the liquor to around pH 3. Increasing severity did not decrease pH any further (Fig. S1†). Hemicellulose (represented as mannan) content in all pretreated biomasses was low (Fig. S2a†) as it was easily dissolved, around 76% are already removed at a severity of 4.5. Cellulose content gradually decreased with increasing severity as more cellulose got hydrolysed and dissolved (Fig. S2a†). The AIL content rises accordingly, but no influence of the additives on the removal of lignin from the biomass is noticeable (Fig. S2b†). ASL just makes up a minor fraction of the lignin. Its content was increased with 2-naphthol and resorcinol (Fig. S2b†), probably due to their aromatic molecules being integrated into the lignin structure, thereby increasing UV-absorption in the ASL measurement. Besides, the suppression of lignin repolymerisation with 2-naphthol might also increase its solubility and thereby ASL content.
Fig. S3† shows the yields of mannose and glucose being dissolved in the pretreatment liquor from the biomass. The pretreatment additives had no influence on the sugar yields in the pretreatment liquor. It can thus be assumed they did not affect sugar dissolution and degradation.
The highest total glucose yield, summing up the glucose yields from enzymatic hydrolysis and pretreatment liquor, was 81% and reached at a severity of 5.1 with 2-naphthol (Fig. S4a†). The gradual degradation of cellulose in the pretreatment (compare Fig. S2a†) reduced the yields, especially at very high severities. Next to that, in particular the fast degradation of mannose reduced the total sugar yields, which sum up all yields from cellulose and hemicellulose (Fig. S4b†). The highest total sugar yield of 58% was likewise reached at a severity of 5.1 with 2-naphthol.
Considering the fact that the not-yet optimized pretreatment with scavengers allowed for total glucose yields of 81%, an additional recovery of the hemicellulose fraction should allow for comparable or even higher sugar yields in a simple autohydrolysis process. Moreover, preliminary experiments show that 2-naphthol is even more effective in (dilute) acid pretreatments, as carbocations are especially formed under acidic conditions17 and considerable lignin repolymerisation can be expected. Further preliminary experiments show that 2-naphthol is also effectively applicable to a pure steam pretreatment by simple mixing of the biomass with the 2-naphthol powder before the pretreatment.
Pretreatment severities that allowed for nearly complete cellulose conversions (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R0 > 4.8) resulted in 2-naphthol conversions of 95% and higher (Fig. S5†). This corresponds to a remaining 2-naphthol concentration of approximately 0.5%w/w of the cellulose in the pretreated biomass. Considering the further processing after pretreatment, it has to be noted that the activity of cellulases can be inhibited by monomeric46 or oligomeric47 phenolic compounds like 2-naphthol. Enzymatic hydrolysis experiments of Avicel (microcrystalline cellulose) in the presence of 2-naphthol did however not reveal an inhibition of enzymes, even at very high 2-naphthol concentrations of 35%w/w of cellulose (Fig. S6†). Next to that, phenolic compounds have also been suggested to exert an inhibitory effect in fermentation.48 The fermentation of glucose in the presence of 2-naphthol revealed that the growth of yeast organisms can indeed be inhibited by 2-naphthol, which was estimated by absorption measurements of the fermentation broth (Fig. S7a†) that correlate with the concentration of yeast cells in the liquid.49 2-naphthol concentrations below 1%w/w of glucose however allowed for complete glucose conversions (Fig. S7b†) and did neither influence ethanol yields in a 24 h fermentation (Fig. S7c†). It can thus be assumed that a process with 2-naphthol conversions of more than 95% as presented here should not be afflicted by noticeable yeast inhibition.
R0 > 4.8) resulted in 2-naphthol conversions of 95% and higher (Fig. S5†). This corresponds to a remaining 2-naphthol concentration of approximately 0.5%w/w of the cellulose in the pretreated biomass. Considering the further processing after pretreatment, it has to be noted that the activity of cellulases can be inhibited by monomeric46 or oligomeric47 phenolic compounds like 2-naphthol. Enzymatic hydrolysis experiments of Avicel (microcrystalline cellulose) in the presence of 2-naphthol did however not reveal an inhibition of enzymes, even at very high 2-naphthol concentrations of 35%w/w of cellulose (Fig. S6†). Next to that, phenolic compounds have also been suggested to exert an inhibitory effect in fermentation.48 The fermentation of glucose in the presence of 2-naphthol revealed that the growth of yeast organisms can indeed be inhibited by 2-naphthol, which was estimated by absorption measurements of the fermentation broth (Fig. S7a†) that correlate with the concentration of yeast cells in the liquid.49 2-naphthol concentrations below 1%w/w of glucose however allowed for complete glucose conversions (Fig. S7b†) and did neither influence ethanol yields in a 24 h fermentation (Fig. S7c†). It can thus be assumed that a process with 2-naphthol conversions of more than 95% as presented here should not be afflicted by noticeable yeast inhibition.
Next to increasing the biomass digestibility, the prevention of lignin repolymerisation could at the same time add value to the obtained lignin fraction. Such a lignin, with a more uniform, lower molecular weight and introduced functionalities by the scavenger like aryl groups could e.g. be interesting for the use as a blend in polymers.31 The production of aromatic monomers from lignin could be enhanced as well due to less aromatic C–C bonds that are difficult to cleave.
3.2. Effect of lignin repolymerisation on enzyme deactivation
As presented in the previous section, the suppression or increase of lignin repolymerisation did not influence lignin removal from the biomass in autohydrolysis. Still, its enzymatic digestibility was considerably affected. Two possible mechanisms were considered being responsible for this effect. On the one hand, repolymerised lignin structures may “enwrap” or partly cover the cellulose fibres and thereby block the access of enzymes. On the other, it is known that enzymes can be deactivated by irreversible binding2 and possibly even denaturing39,50 on lignin. Those processes could occur more pronounced on repolymerised lignin structures.In order to study both possible mechanisms, biomass samples with a varying degree of repolymerised lignin were prepared and underwent several experimental procedures, as outlined in Fig. 5. Autohydrolysis treatments of spruce were carried out with varying 2-naphthol concentrations between 0 and 16%w/w of the biomass (compare Table S1†) at a high severity of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R0 = 5.4, which had allowed for a considerable effect of the scavenger on biomass digestibility (see Fig. 4).
R0 = 5.4, which had allowed for a considerable effect of the scavenger on biomass digestibility (see Fig. 4).
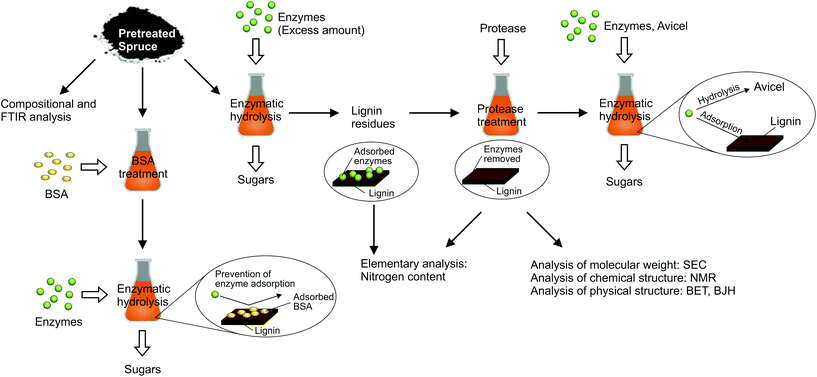 | ||
| Fig. 5 Overview of experimental procedures for studying cellulase deactivation on spruce pretreated with different 2-naphthol concentrations. | ||
The 2-naphthol concentration in autohydrolysis had neither a noticeable influence on pH (Fig. S8†) nor on the formation of dissolved byproducts and sugar degradation products detectable by HPLC (the main constituents acetic acid, HMF and furfural were quantified; Fig. S9†). Hence, those products were most likely not involved in reactions with the scavenger or lignin repolymerisation.
The increased integration of 2-naphthol into the lignin structure with increasing concentration in autohydrolysis could be confirmed by FTIR analysis of the pretreated biomass. With increasing 2-naphthol concentration, a strengthening of IR signals at 750 and 815 cm−1 can be observed (Fig. S11†). This is characteristic for 1,2-disubstituted naphthalenes51,52 like 2-naphthol substituted at position C-1 (as illustrated in Fig. S11†). The finding also shows that 2-naphthol preferably undergoes a single electrophilic substitution on position C-1 and acts as a blocking agent for lignin fragments in that way, since otherwise the strengthening of further signals (e.g. from 1,2,3-trisubstituted naphthalenes at 790 cm−1 (ref. 51)) should be observable. In the whole scanning range of 4000–600 cm−1, no further significant signal changes could be observed (Fig. S11†), also indicating that 2-naphthol did not influence functional groups of the lignin detectable by FTIR.
The size-exclusion chromatography (SEC) analysis of lignin that had been isolated from the pretreated biomass gives information about changes in its molecular weight distribution (isolation and chromatography procedure provided in the ESI†). The use of 2-naphthol distinctly decreased the molecular weight and also lead to a narrower molecular weight distribution, as shown exemplarily in Fig. S12.† This indicates it was effectively preventing lignin repolymerisation, which generally leads to an increased molecular weight and a more heterogeneous lignin structure.22 In addition, the lignin isolation procedure revealed that the organic solvent extractability of the lignin was enhanced by the use 2-naphthol, a characteristic that has been attributed to its less repolymerised structure.21,22,24 The prevention of lignin repolymerisation by 2-naphthol was further disclosed by 13C–1H heteronuclear single quantum coherence (HSQC) nuclear magnetic resonance (NMR) measurements of the isolated lignins (isolation and NMR procedure provided in the ESI†). The quantity of β-O-4 structures as well as the proportions of unsubstituted C-2, C-5 and C-6 carbon atoms in the guaiacyl rings can be quantified based on the HSQC spectra31,53 and are shown in Table S2.† The use of 2-naphthol (16%w/w of biomass) did practically not affect the C-5/C-2 signal ratio, while the C-6/C-5 signal ratio was increased by 76% compared to the control, indicating a different substitution pattern of the aromatic nuclei. Under acidic conditions, repolymerisation reactions can occur predominantly between the C-6 site and the carbocation at the C-α position of adjacent lignin structures in softwood lignin54,55 (compare Fig. 1), probably accounting for the reduced C-6 signal of the control. No signals from β-O-4 structures could be observed in the pretreated samples, likely meaning those bonds had completely been cleaved. Next to that, the NMR spectra can again show the incorporation of 2-naphthol into the lignin structure31 (Fig. S13†).
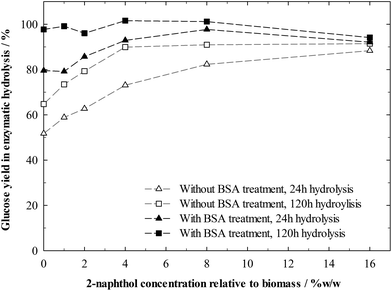
Without BSA treatment, increasing 2-naphthol concentration in the pretreatment up to 4%w/w steadily increased sugar yields in hydrolysis (Fig. 6). 4%w/w of 2-naphthol were sufficient to reach yields of 90% after 120 h of hydrolysis. Treating the biomass before hydrolysis with BSA however, allowed for a virtually complete cellulose conversion after 120 h of hydrolysis, independent of the 2-naphthol concentration. This reveals that the cellulose in all samples had been equally accessible. Lignin is not blocking digestible cellulose, preventing enzymes from accessing it, but repolymerised lignin seems to intensify the enzyme deactivation.
BSA treated samples, pretreated with low amounts of 2-naphthol (0–4%w/w) were digested slower, as can be seen from the hydrolysis conversions after 24 h. This indicates that, while certainly effective, the applied amounts of BSA could not occupy all binding sites on samples with more repolymerised lignin and more enzymes were deactivated.
Therefore, the lignins were first isolated by enzymatic hydrolysis from the pretreated biomass with an excess dose of enzymes. The progress of this hydrolysis over time with a successive increase of the enzyme dose is shown in Fig. S14.† Increasing 2-naphthol concentration in pretreatment continuously increased the speed of the cellulose digestion, suggesting that less enzymes were deactivated and the hydrolysis could proceed faster.
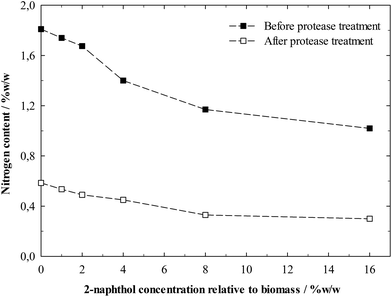
The biomass residues after hydrolysis consisted primarily of lignin as nearly all cellulose had been digested. However, enzymes from the hydrolysis were expected to be still adsorbed on the lignin, so the material was “cleaned” for the following Avicel hydrolysis by hydrolysing the adsorbed proteins with a protease treatment. The nitrogen content of the lignin residues before and after the protease treatment is shown in Fig. 7. This serves as an indirect indicator of the protein content, which can be increased by enzyme contamination.3,39,59 The nitrogen content was significantly decreased by the protease treatment, showing that basically proteins, which can effectively be removed by proteases, account for the nitrogen content. The amount of proteins bound to the residues before protease treatment decreased with increasing amount of 2-naphthol in the pretreatment. This proves that enzyme adsorption plays a major role in the deactivation process and the pretreatment with 2-naphthol decreases the ability of lignin to bind enzymes in hydrolysis.
It is interesting to note that the protease treatment did not reduce the nitrogen content of all samples to the same level. A possible explanation could be that the access of protease to the adsorbed enzymes was restricted by the structure of the more repolymerised lignins.
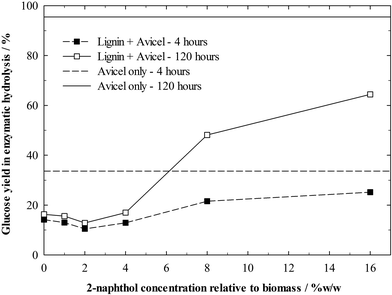
The isolated and “enzyme cleaned” lignin residues could then be added to the enzymatic hydrolysis of Avicel. Hydrolysis experiments were carried out with concentrations of 1%w/w Avicel and 1%w/w lignin, so as to have similar conditions as in the hydrolysis of the pretreated biomass and being able to assess the impact of enzyme deactivation by the lignin. However, due to the higher digestibility of pure cellulose, the enzyme dosing was decreased to 10 FPU g−1 cellulose. A control experiment without lignin addition shows that Avicel itself is well digestible with a glucose yield of 96% after 120 h (Fig. 8). Even though all lignin residues inhibited the hydrolysis of Avicel to some extent, the inhibition by the residual lignins from samples treated with low concentrations of 2-naphthol (0–4%w/w) was particularly remarkable. The glucose yield after 120 h was decreased up to 87% relative to the yield from pure cellulose due to the presence of lignin. The very small progress in the hydrolysis of these samples between 4 and 120 h indicates that the enzyme deactivation occurs very rapidly and enzymes bind quickly to lignin. The least repolymerised lignin decreased glucose yields only by 32% after 120 h, demonstrating the high impact of repolymerised lignin structures on enzyme deactivation.
Surprisingly, samples treated with 0 to 4%w/w 2-naphthol inhibited the Avicel hydrolysis similarly. A role in this effect might play, as mentioned before, a restricted accessibility of inner lignin structures in more repolymerised lignin samples or other factors which are still unknown.
Steam pretreatment has been suggested to increase lignin hydrophobicity due to the loss of hydroxyl and carboxylic acid functions, increasing the non-productive binding of cellulases to lignin in that way.13 However, recent studies with lignin isolated from spruce wood did indeed observe an increased enzyme binding capacity after steam pretreatment, but could not observe an increase in hydrophobicity.59 At the same time, a 57% increase in lignin molecular weight was observed due to repolymerisation in the steam pretreatment, which calls an influence of repolymerisation on hydrophobicity into question. This is consistent with results from this work, which showed that suppressing lignin repolymerisation did not have an influence on functional groups detectable by FTIR (Fig. S11†). The degree of lignin hydrophobicity is largely determined by its functional groups, that is to say the present study did not reveal an influence of repolymerisation on hydrophobicity neither. 2-naphthol itself has a very hydrophobic character, so its introduction into the lignin structure which was disclosed before (compare Fig. S11†) would rather be disadvantageous from this point of view. Resorcinol is very hydrophilic, still, its introduction into the lignin structure decreased yields in hydrolysis after all (compare Fig. 4). All things considered, other factors besides hydrophobicity should be taken into account when explaining the deactivating effect of repolymerised lignin on enzymes.
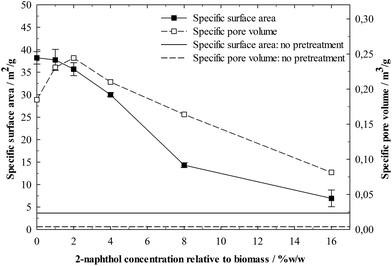
It is also possible that lignin repolymerisation influences lignin–enzyme interactions through modification of the physical lignin structure. This is strongly suggested by the BET analysis of the isolated lignins’ specific surface areas (Fig. 9). Samples treated with low concentrations of 2-naphthol show a significantly higher specific surface area and the use of 16%w/w 2-naphthol could decrease the specific surface area by five times compared to the control. The lignin repolymerisation did considerably increase its specific surface area, which generates more potential for enzymes to adsorb and very likely accounts for the poorer digestibility in enzymatic hydrolysis.
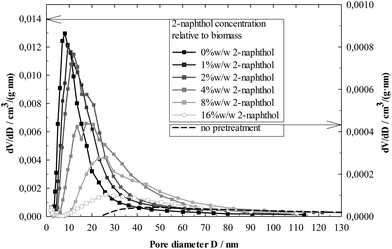
Further insight into the modification of the physical lignin structure can be gained from the BJH analysis of the lignin pore volume distribution (Fig. 10). Higher 2-naphthol concentrations distinctly increased pore diameters, shifting the distribution maxima from less than 10 nm (control) to about 30 nm (16%w/w 2-naphthol). The BJH analysis as well provides information on the specific pore volume, shown in Fig. 9. A maximum can be found at a concentration of 2%w/w 2-naphthol, but higher concentrations steadily decreased the specific pore volume of the samples up to 44% of the control. Though no information is available on the actual pore forms (BJH analysis assumes cylindrical pores), the decrease in specific pore volume together with the increase of the average pore diameter indicates that also the number of pores is decreased with high concentrations of 2-naphthol. Summing up, lignin repolymerisation seems to lead to smaller and also to an increased number of pores.
The mechanisms that lead to the formation of those nanostructures have still to be elucidated. The wood cell wall has a complex hierarchical organisation, starting with cellulose microfibrils of 3 nm that group together to form macrofibrils 20–30 nm in diameter.60 Those macrofibrils are embedded in hemicellulose and enwrapped by lignin.61 BET specific surface area measurements of pretreated but non-hydrolysed biomass gave very low values <1 m2 g−1. Not until cellulose was removed in the lignin isolation procedure, higher specific surface areas between 7 and 38 m2 g−1 (Fig. 9) were measured, revealing a matrix of lignin structures in which the cellulose fibres were embedded and which seems to be modified during the pretreatment. Lignin that had not underwent a pretreatment and was isolated from raw spruce by ball milling and enzymatic hydrolysis, showed a very low specific surface area and pore volume (Fig. 9) and comparatively high pore diameters (Fig. 10). This indicates that the observed porous lignin structures with small pores are formed during the pretreatment.
The BJH analysis of the most repolymerised lignin (0%w/w 2-naphthol) revealed pore structures with diameters smaller than 4 nm, so smaller than the diameter of typical macrofibrils. This suggests the possibility of lignin cross linking (e.g. through repolymerisation) occurring within or crossing the cellulose macrofibrils (compare Fig. 11). In that way, an advancing cross linking could lead to a more “fine meshed” network of the lignin matrix, which could explain the simultaneous decrease in pore size and increase in specific surface area as observed in samples pretreated with low concentrations of 2-naphthol. Another (contrary) approach to explanation is based on the coalescence of lignin, e.g. through physical condensation. It has been reported that in autohydrolysis and acidic pretreatments at temperatures above the lignin glass transition temperature, lignin can coalesce and migrate into the bulk liquid phase, resulting in lignin redeposition in the form of droplets on the cell wall surface that have a negative impact on cellulose hydrolysis.62–64 Lignin coalescence might also lead to the modification of the lignin matrix and the formation of porous structures as observed in this work, which were found to have a negative impact on hydrolysis as well.
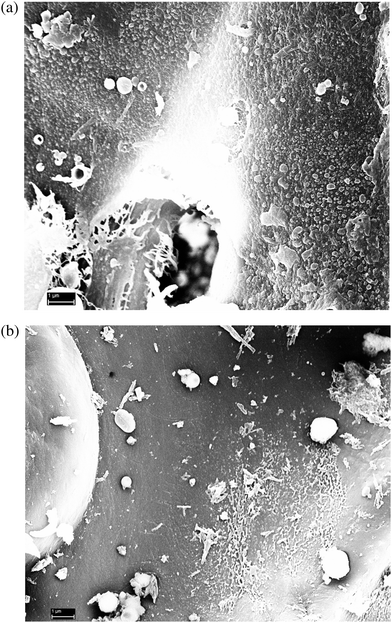
SEM analysis of the lignin residues did indeed reveal coalesced/condensed-like structures. The sample pretreated without 2-naphthol showed numerous droplets which were dispersed all over the biomass surface (Fig. 12a, further images provided in Fig. S15a†) and certainly had a negative impact on hydrolysis. The droplets had a broad size distribution ranging from around 30 nm up to several μm. In contrast, the sample pretreated with 16%w/w 2-naphthol exhibited much less of those structures (Fig. 12b, further images provided in Fig. S15b†). The presence of spherical ball-shaped structures can be observed in both samples and is regarded as typical for the redeposition of lignin from the liquid phase on the biomass.62,64 It is also conceivable however, that the observed structures were formed when hydrophobic lignin emerges from the “inner” of the biomass upon contact with the bulk aqueous phase. It remains to be clarified as well if such structures are formed by physical coalescence or chemical repolymerisation reactions. In every way, the modification of the physical lignin structure seems to play the major role in explaining the effect of 2-naphthol on enzymatic hydrolysis.
4. Conclusions
The high recalcitrance of softwood to processing by enzymatic hydrolysis seems to be of a twofold character. In the first place, autohydrolysis pretreatments with very high severities or acidic pretreatments are needed to obtain a highly accessible cellulose. On the other hand, those harsh pretreatment conditions will always be accompanied by extensive lignin repolymerisation. The lignin repolymerisation seems to modify its nanostructure in a way that significantly increases its specific surface area. This poses an additional obstacle to hydrolysis, as it increases the binding and deactivation of enzymes. The prevention of lignin repolymerisation by carbocation scavengers in such pretreatments can help to overcome this problem and allow for the required harsh pretreatment conditions. Next to increasing the biomass digestibility, such a process could add value to the obtained lignin fraction by avoiding lignin-lignin repolymerisation reactions that render these products nearly impossible to upgrade. An improved valorisation of both cellulose and lignin seems possible by inhibiting lignin repolymerisation in the first stage of most biorefinery concepts – the pretreatment.Acknowledgements
The authors gratefully acknowledge support from the Swiss National Science Foundation through grant #4066-136709. The authors thank Genencor for the donation of enzymes and Christian Bährle (Paul Scherrer Institute) for the provision of wood samples and helpful discussions. Robert Büchel and Vito Giampetro from ETH Zurich are acknowledged for help with the BET/BJH respectively SEM analysis.Notes and references
- J. H. Lora and M. Wayman, J. Appl. Polym. Sci., 1980, 25, 589 CrossRef CAS PubMed.
- R. Esteghlalian Ali, V. Srivastava, N. Gilkes, J. Gregg David and N. Saddler John, in Glycosyl Hydrolases for Biomass Conversion, ed. M. E. Himmel, J. O. Baker and N. Saddler John, American Chemical Society, 2000, vol. 769, ch. 6, p. 100 Search PubMed.
- A. Berlin, M. Balakshin, N. Gilkes, J. Kadla, V. Maximenko, S. Kubo and J. Saddler, J. Biotechnol., 2006, 125, 198 CrossRef CAS PubMed.
- H. Palonen, F. Tjerneld, G. Zacchi and M. Tenkanen, J. Biotechnol., 2004, 107, 65 CrossRef CAS PubMed.
- P. Alvira, E. Tomás-Pejó, M. Ballesteros and M. J. Negro, Bioresour. Technol., 2010, 101, 4851 CrossRef CAS PubMed.
- N. Mosier, C. Wyman, B. Dale, R. Elander, Y. Y. Lee, M. Holtzapple and M. Ladisch, Bioresour. Technol., 2005, 96, 673 CrossRef CAS PubMed.
- X. Pan, D. Xie, N. Gilkes, D. Gregg and J. Saddler, Appl. Biochem. Biotechnol., 2005, 124, 1069 CrossRef.
- Y. Zheng, Z. Pan and Z. Zhang, Int. J. Agric. & Biol. Eng., 2009, 2, 51 CAS.
- A. Limayem and S. C. Ricke, Prog. Energy Combust. Sci., 2012, 38, 449 CrossRef CAS PubMed.
- Y. Lu, B. Yang, D. Gregg, J. Saddler and S. Mansfield, Appl. Biochem. Biotechnol., 2002, 98–100, 641 CrossRef CAS.
- R. P. Chandra, R. Bura, W. E. Mabee, A. Berlin, X. Pan and J. N. Saddler, in Biofuels, ed. L. Olsson, Springer, Berlin, Heidelberg, 2007, vol. 108, ch. 64, p. 67 Search PubMed.
- S. Nakagame, R. P. Chandra and J. N. Saddler, Biotechnol. Bioeng., 2010, 105, 871 CAS.
- S. Nakagame, R. P. Chandra, J. F. Kadla and J. N. Saddler, Bioresour. Technol., 2011, 102, 4507 CrossRef CAS PubMed.
- X. Pan, C. Arato, N. Gilkes, D. Gregg, W. Mabee, K. Pye, Z. Xiao, X. Zhang and J. Saddler, Biotechnol. Bioeng., 2005, 90, 473 CrossRef CAS PubMed.
- T. Voitl, M. V. Nagel and P. Rudolf von Rohr, Holzforschung, 2009, 64, 13 Search PubMed.
- L. Knut and L. Rolf, Acta Chem. Scand., 1972, 24, 2005 Search PubMed.
- K. V. Sarkanen and C. H. Ludwig, Lignins: occurrence, formation, structure and reactions, Wiley-Interscience, New York, 1971 Search PubMed.
- J. Y. Zhu, X. J. Pan, G. S. Wang and R. Gleisner, Bioresour. Technol., 2009, 100, 2411 CrossRef CAS PubMed.
- L. Shuai, Q. Yang, J. Y. Zhu, F. C. Lu, P. J. Weimer, J. Ralph and X. J. Pan, Bioresour. Technol., 2010, 101, 3106 CrossRef CAS PubMed.
- C. A. Mooney, S. D. Mansfield, M. G. Touhy and J. N. Saddler, Bioresour. Technol., 1998, 64, 113 CrossRef CAS.
- M. Wayman and J. H. Lora, Tappi, 1978, 61, 55 CAS.
- J. Li, G. Henriksson and G. Gellerstedt, Bioresour. Technol., 2007, 98, 3061 CrossRef CAS PubMed.
- P. Muller, Pure Appl. Chem., 1994, 66, 1077 CrossRef.
- M. Wayman and J. H. Lora, Tappi, 1978, 88, 88 Search PubMed.
- E. Sjöström, Wood chemistry: fundamentals and applications, Academic Press, Washington DC, USA, 2nd edn, 1981 Search PubMed.
- H. Trajano, N. Engle, M. Foston, A. Ragauskas, T. Tschaplinski and C. Wyman, Biotechnol. Biofuels, 2013, 6, 110 CrossRef CAS PubMed.
- S. Cao, Y. Pu, M. Studer, C. Wyman and A. J. Ragauskas, RSC Adv., 2012, 2, 10925 RSC.
- M. H. Studer, J. D. DeMartini, M. F. Davis, R. W. Sykes, B. Davison, M. Keller, G. A. Tuskan and C. E. Wyman, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 6300 CrossRef CAS PubMed.
- X. Li, E. Ximenes, Y. Kim, M. Slininger, R. Meilan, M. Ladisch and C. Chapple, Biotechnol. Biofuels, 2010, 3, 1 CrossRef PubMed.
- E. Dorrestijn, L. J. J. Laarhoven, I. W. C. E. Arends and P. Mulder, J. Anal. Appl. Pyrolysis, 2000, 54, 153 CrossRef CAS.
- J. Li and G. Gellerstedt, Ind. Crops Prod., 2008, 27, 175 CrossRef CAS PubMed.
- T. Pielhop, M. H. Studer and P. Rudolf von Rohr, Switzerland Pat, WO/2013/068092, 2013 Search PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, NREL/TP-510-42618, National Renewable Energy Laboratory, Golden, CO, 2008 Search PubMed.
- A. Sluiter, R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton, NREL/TP-510-42619, National Renewable Energy Laboratory, Golden, CO, 2005 Search PubMed.
- R. P. Overend, E. Chornet and J. A. Gascoigne, Philos. Trans. R. Soc. London, Ser. A, 1987, 321, 523 CrossRef CAS.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton, NREL/TP-510-42623, National Renewable Energy Laboratory, Golden, CO, 2006 Search PubMed.
- M. Selig, N. Weiss and Y. Ji, NREL/TP-510-42629, National Renewable Energy Laboratory, Golden, CO, 2008 Search PubMed.
- B. Adney and J. Baker, NREL/TP-510-42628, National Renewable Energy Laboratory, Golden, CO, 1996 Search PubMed.
- J. Rahikainen, S. Mikander, K. Marjamaa, T. Tamminen, A. Lappas, L. Viikari and K. Kruus, Biotechnol. Bioeng., 2011, 108, 2823 CrossRef CAS PubMed.
- T. Tamminen and B. Hortling, in Advances in Lignocellulosics Characterization, ed. D. S. Argyropoulos, Tappi Press, Atlanta, GA, 1999, ch. 1, p. 1 Search PubMed.
- S. Brethauer, M. H. Studer, B. Yang and C. E. Wyman, Bioresour. Technol., 2011, 102, 6295 CrossRef CAS PubMed.
- F. Radt, Elsevier Encyclopedia of Organic Chemistry, Series III Carboisocyclic organic compounds, Elsevier, New York, 1950 Search PubMed.
- R. B. Durairaj, Resorcinol: Chemistry, Technology and Applications, Springer, Berlin, 2005 Search PubMed.
- M. Galbe and G. Zacchi, Appl. Microbiol. Biotechnol., 2002, 59, 618 CrossRef CAS PubMed.
- Q. Nguyen, M. Tucker, F. Keller and F. Eddy, Appl. Biochem. Biotechnol., 2000, 84–86, 561 CrossRef CAS.
- E. Ximenes, Y. Kim, N. Mosier, B. Dien and M. Ladisch, Enzyme Microb. Technol., 2010, 46, 170 CrossRef CAS PubMed.
- A. Tejirian and F. Xu, Enzyme Microb. Technol., 2011, 48, 239 CrossRef CAS PubMed.
- E. Palmqvist and B. Hahn-Hägerdal, Bioresour. Technol., 2000, 74, 25 CrossRef CAS.
- L. W. Bergman, in Two-Hybrid Systems: Methods and Protocols, ed. P. N. MacDonald, Humana Press, Totowa, NJ, 2001, vol. 177, ch. 2, p. 9 Search PubMed.
- J. Börjesson, M. Engqvist, B. Sipos and F. Tjerneld, Enzyme Microb. Technol., 2007, 41, 186 CrossRef PubMed.
- J. G. Hawkins, E. R. Ward and D. H. Whiffen, Spectrochim. Acta, 1957, 10, 105 CrossRef CAS.
- M. Wayman and J. H. Lora, J. Appl. Polym. Sci., 1980, 25, 2187 CrossRef CAS PubMed.
- L. Zhang and G. Gellerstedt, in Proceedings of the Sixth European Workshop on Lignocellulosics and Pulp, 2000, 7 Search PubMed.
- Pulp and Paper Chemistry and Technology - Volume 2 Pulping Chemistry and Technology, ed. E. Monica, G. Gellerstedt and G. Henriksson, de Gruyter, Berlin, 2009 Search PubMed.
- G. Gellerstedt and G. Henriksson, in Monomers, Polymers and Composites from Renewable Resources, ed. B. M. Naceur and G. Alessandro, Elsevier, Oxford, 2008, p. 219 Search PubMed.
- T. Eriksson, J. Börjesson and F. Tjerneld, Enzyme Microb. Technol., 2002, 31, 353 CrossRef CAS.
- C. A. Haynes and W. Norde, Colloids Surf., B, 1994, 2, 517 CrossRef CAS.
- B. Yang and C. E. Wyman, US Pat, US20110076725 A1, 2011 Search PubMed.
- J. L. Rahikainen, R. Martin-Sampedro, H. Heikkinen, S. Rovio, K. Marjamaa, T. Tamminen, O. J. Rojas and K. Kruus, Bioresour. Technol., 2013, 133, 270 CrossRef CAS PubMed.
- V. Bucur, Delamination in Wood, Wood Products and Wood-Based Composites, Springer, Netherlands, 2011 Search PubMed.
- E. M. Rubin, Nature, 2008, 454, 841 CrossRef CAS PubMed.
- H. Li, Y. Pu, R. Kumar, A. J. Ragauskas and C. E. Wyman, Biotechnol. Bioeng., 2014, 111, 485 CrossRef CAS PubMed.
- B. S. Donohoe, S. R. Decker, M. P. Tucker, M. E. Himmel and T. B. Vinzant, Biotechnol. Bioeng., 2008, 101, 913 CrossRef CAS PubMed.
- M. J. Selig, S. Viamajala, S. R. Decker, M. P. Tucker, M. E. Himmel and T. B. Vinzant, Biotechnol. Prog., 2007, 23, 1333 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4gc02381a |
| This journal is © The Royal Society of Chemistry 2015 |