Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids
R. M.
Wahlström
 * and
A.
Suurnäkki
* and
A.
Suurnäkki
VTT – Technical Research Centre of Finland, P.O. Box 1000, FI-02044, VTT, Espoo, Finland. E-mail: ronny.wahlstrom@vtt.fi; Tel: +358 40 02 54 073
First published on 7th November 2014
Abstract
Biofuels and -chemicals can be produced from carbohydrates in lignocellulosic biomass. For an efficient total enzymatic hydrolysis of the plant cell wall polysaccharides, a pretreatment step is required. Ionic liquids (ILs) have recently gained considerable interest as solvents for cellulose and lignocellulosic biomass and pretreatment of lignocellulose with ILs is currently an extensively studied concept. However, the applicability of ILs in an integrated process, in which enzymatic hydrolysis is done in the same vessel as the IL pretreatment without IL removal and substrate washing between the process steps, suffers from the fact that cellulose-dissolving ILs severely inactivate the cellulases used to catalyse the polysaccharide hydrolysis. This article reviews research on cellulase activity, stability and action in hydrolysis in cellulose-dissolving ILs, and different routes to increase the cellulase performance in these reaction systems. Impressive advances have recently been made in discovering and developing cellulases and other glycosyl hydrolases with increased IL-tolerance. Different cellulase stabilisation techniques and the design of enzyme-friendly cellulose-dissolving ILs are also discussed. In light of the recent developments, the integrated enzymatic hydrolysis of polysaccharides in the presence of ILs may well prove to be a potential route for utilizing lignocellulosic biomass as feedstock in biofuel and -chemical production.
Introduction
The rapidly growing global need for energy, chemicals and materials, price and feedstock security issues, as well as the concern for global warming, directs us from the use of fossil to renewable raw material sources. Lignocellulosic biomass is a renewable, carbon neutral and widely available non-food raw material for the production of fuels, platform chemicals and polymeric materials. Lignocellulosic biomass can be obtained from diverse sources including forestry side-streams, such as logging and wood processing mill residues, removed biomass from forest management and land clearing operations, and agricultural sources such as crop residues (corn stovers, straw), perennial grasses and energy and woody crops.1 It has been estimated that 5–8% of the annual lignocellulose production would be enough to cover the annually used fossil oil.2 Cellulosic ethanol production has, during the 2000s, been demonstrated in several demonstration plants, e.g. by Inbicon in Kalundborg, Denmark and Abengoa in Salamanca, Spain.3 Full-scale commercial plants for the production of cellulosic ethanol have recently become operational, e.g. the Chemtex plant in Crescentino, Italy and the Abengoa plant in Hugoton, Kansas, USA, with several more under construction.The main components of lignocellulosics are cellulose, hemicelluloses and lignin, in various ratios depending on the source of biomass. Together these polymers form a complex matrix, which is highly recalcitrant towards depolymerization. Cellulose and hemicelluloses are polysaccharides. Cellulose is a linear homopolymer consisting of anhydroglucose units linked together by 1,4-β-glycosidic bonds. The anhydroglucose units are distorted 180° to each other so that the smallest repeating unit in the cellulose chain is the anhydrocellobiose unit (Fig. 1). The degree of polymerization (DP) of cellulose can range from 20 (laboratory synthesized) to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (bacterial celluloses).4 Crystalline cellulose has a number of polymorphs, of which cellulose I (natural cellulose) and cellulose II are the most frequently encountered.5 Cellulose II is thermodynamically more stable than cellulose I and can be obtained from cellulose I by regeneration or mercerization. In natural cellulose, the crystalline regions are alternated by non-crystalline regions, which are typically referred to as amorphous cellulose. The cellulose chains organize into small bundles, elementary fibrils, which in turn form microfibrils and further larger fibrillar structures.6 Cellulose is not soluble in water or conventional organic solvents; oligomers of DP 1–6 are soluble in water, whereas oligomers of DP 7–13 are partly soluble in hot water.7 Hemicelluloses display a more heterogeneous structure than cellulose. They generally consist of a polysaccharide backbone, which may carry saccharide branches and other substituents such as acetyl groups and sugar acids.6 Typical saccharides in hemicellulose are glucose, xylose, mannose, galactose, arabinose, rhamnose and glucuronic, galacturonic and 4-O-Me-glucuronic acid. Hemicellulose composition and structure greatly vary depending on its source. The DP of different hemicelluloses varies but is in general an order of magnitude smaller than in cellulose. Unlike cellulose, many hemicelluloses are soluble in alkaline aqueous solutions, or even in neutral water. Lignin is an irregular, branched polymer built up from three different phenylpropanoid monomers, 4-hydroxycinnamyl alcohol, coniferyl alcohol, and sinapyl alcohol, which differ from each other in the degree of methoxylation of the aromatic ring.8 Due to its aromatic constituents, lignin has considerable hydrophobic character, in contrast to the hydrophilic polysaccharides. Lignin and hemicelluloses form covalent bonds with each other, known as lignin-carbohydrate complexes (LCCs).9
000 (bacterial celluloses).4 Crystalline cellulose has a number of polymorphs, of which cellulose I (natural cellulose) and cellulose II are the most frequently encountered.5 Cellulose II is thermodynamically more stable than cellulose I and can be obtained from cellulose I by regeneration or mercerization. In natural cellulose, the crystalline regions are alternated by non-crystalline regions, which are typically referred to as amorphous cellulose. The cellulose chains organize into small bundles, elementary fibrils, which in turn form microfibrils and further larger fibrillar structures.6 Cellulose is not soluble in water or conventional organic solvents; oligomers of DP 1–6 are soluble in water, whereas oligomers of DP 7–13 are partly soluble in hot water.7 Hemicelluloses display a more heterogeneous structure than cellulose. They generally consist of a polysaccharide backbone, which may carry saccharide branches and other substituents such as acetyl groups and sugar acids.6 Typical saccharides in hemicellulose are glucose, xylose, mannose, galactose, arabinose, rhamnose and glucuronic, galacturonic and 4-O-Me-glucuronic acid. Hemicellulose composition and structure greatly vary depending on its source. The DP of different hemicelluloses varies but is in general an order of magnitude smaller than in cellulose. Unlike cellulose, many hemicelluloses are soluble in alkaline aqueous solutions, or even in neutral water. Lignin is an irregular, branched polymer built up from three different phenylpropanoid monomers, 4-hydroxycinnamyl alcohol, coniferyl alcohol, and sinapyl alcohol, which differ from each other in the degree of methoxylation of the aromatic ring.8 Due to its aromatic constituents, lignin has considerable hydrophobic character, in contrast to the hydrophilic polysaccharides. Lignin and hemicelluloses form covalent bonds with each other, known as lignin-carbohydrate complexes (LCCs).9
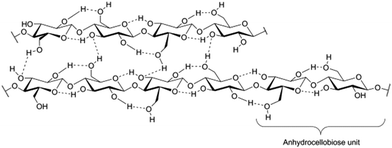 | ||
| Fig. 1 Structure of cellulose and its intra- and interchain hydrogen bonding. The anhydrocellobiose unit is the basic structural element of cellulose. Reproduced with permission from ref. 162. | ||
Lignocellulosics can be converted to fuel molecules or chemicals through various routes or be burned directly for energy, although burning represents the lowest added value to the raw material. Biomass gasification and pyrolysis are techniques for producing charcoal, fuels, heat, energy and chemicals from biomass.10,11 A much studied route for converting lignocellulose to liquid fuel is to hydrolyse its polysaccharides, cellulose and hemicelluloses to monosaccharides and ferment them further to ethanol or other fuel molecules. The conversion of lignocellulose to monosaccharides and then further to liquid fuel is usually achieved in a three-step process consisting of: (1) pretreatment of the lignocellulosic feedstock, (2) hydrolysis of the feedstock polysaccharides to monosaccharides, and (3) microbial fermentation of the liberated monosaccharides to the target product, e.g. ethanol or sugar acids. The pretreatment step is necessary to open up the structure of the usually highly hydrolysis-resistant lignocellulosic matrix. Without pretreatment, enzymatic hydrolysis yields are typically low (∼20% of glucan in feedstock).7
The hydrolysis may be carried out either by enzymatic or mineral acid hydrolysis. Enzymatic hydrolysis offers several advantages over acid hydrolysis: less formation of undesirable by-products, no need for corrosion resistant processing equipment, less acid waste12 and the potential for almost complete conversion.13 In the presence of an acid, glucose is dehydrated to 5-hydroxymethylfurfural (HMF),14 which effectively inhibits the subsequent microbial fermentation.15 Enzymatic cellulose hydrolysis is catalysed by cellulases, i.e. glycosyl hydrolases specialized in the hydrolysis of the 1,4-β-glycosidic bonds. In the total hydrolysis, cellulases are generally applied as cocktails of different cellulase activities. Traditionally, three cellulase activities have been considered: endoglucanases, which catalyse the random cleavage of the cellulose chains especially in the amorphous regions, causing rapid reduction in the cellulose DP while liberating cello-oligomers in the process; cellobiohydrolases (exoglucanases), which catalyse the cleavage of cellobiose from the cellulose chain ends; and β-glucosidases, which catalyse the hydrolysis of the liberated cello-oligomers to glucose. In addition to glycosyl hydrolases, it has recently been suggested that also oxidative enzymes, known as lytic polysaccharide mono-oxygenases (LPMOs, recently reviewed by Horn et al.16), contribute to the total hydrolysis of cellulose. The synergistic action of cellulases in hydrolysis has been studied extensively and at least seven different modes of cellulase synergy have been described.7
Hemicellulose hydrolysis is important both for removing the hemicellulose, which shields the cellulose from hydrolysis, and for producing monosaccharides from hemicellulose for further fermentation. In addition to glucose, also other saccharides, such as xylose17 and galactose,18 may be used as feedstock for various fermentations. A large battery of hemicellulases (e.g. xylanases, mannanases, esterases and α-glucuronidases), which work in synergy, is needed for efficient hydrolysis of diverse hemicelluloses.19 Cellulases and hemicellulases are often closely related, both structurally and regarding their catalytic mechanism. Many glycosyl hydrolases have a modular structure, meaning that the proteins contain a carbohydrate-binding module (CBM), which has been shown to significantly promote the enzymes’ ability to catalyse the hydrolysis of solid substrates.20,21
Ionic liquids (ILs) are by definition salts with melting points <100 °C. Usually ILs consist of an organic and rather bulky cation and either an organic or inorganic anion, which in the simplest case can be a one-atom ion such as a halogen. The low melting point of these salts has mainly been attributed to the mismatched sizes of the cation and the anion and the bulkiness and often asymmetry of the cation, as well as the charges being delocalized on several atoms, leading to weak Coulombic interactions and low crystal lattice energies and preventing crystallization.22,23 ILs have often been called green solvents mainly due to their negligible vapour pressure, which mitigates any emissions of volatile organic compounds (VOCs), and they also have excellent solvent properties due to their capability to dissolve solutes of varying polarity.24,25 In 2002, Swatloski et al. reported the dissolution of cellulose in ILs (in 1-butyl-3-methylimidazolium chloride, [BMIM]Cl, and other related ILs).26 This discovery has been followed by the dissolution of both hardwood and softwood27–30 as well as annual and perennial plants, such as switchgrass31 in ILs.
In 2006, Dadi et al. reported that the dissolution and regeneration of microcrystalline cellulose (MCC) in [BMIM]Cl dramatically accelerated the subsequent enzymatic hydrolysis kinetics,12 which stimulated extensive research on ionic liquid pretreatments of pure cellulosic substrates and later also of natural and industrially more relevant lignocellulose samples. The regeneration procedure of Dadi et al. included a wash of the regenerated substrate between the regeneration and enzymatic hydrolysis steps (Fig. 2). Kamiya et al. proposed another process alternative, the so-called one-pot or in situ hydrolysis, in which IL pretreatment and enzymatic hydrolysis steps were integrated with no separation of IL between the steps.32 It was, however, already in 2003 demonstrated by Turner et al. that cellulose-dissolving ILs ([BMIM]Cl studied) severely inactivate cellulases.33 The one-pot hydrolysis offers some benefits over the two-step regeneration procedure, as will be discussed later, but it is a necessary task to design systems in which the cellulases can remain hydrolytically active in high concentrations of IL to make the one-pot hydrolysis technically attractive.
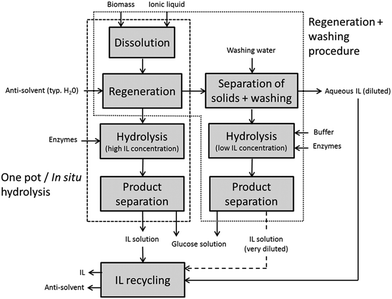 | ||
| Fig. 2 Processing schemes for IL pretreatment with subsequent enzymatic hydrolysis of plant cell wall polysaccharides through the one-pot and the regeneration with washing procedures. | ||
Factors affecting enzymatic hydrolysis of ionic liquid-pretreated biomass
Dissolution of cellulose and lignocellulosic biomass in ionic liquids
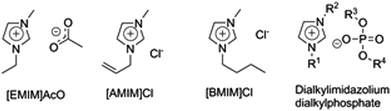
Cellulose is insoluble in water and conventional organic solvents but can, however, be dissolved in certain “exotic” solvent systems by either derivatizing or non-derivatizing dissolution mechanisms. The Lyocell process, in which regenerated cellulose fibers are produced by dissolving cellulose in N-methylmorpholine-N-oxide (NMMO) monohydrate and regenerated by spinning the cellulose solution in a water bath, is in industrial use.34 Cellulose dissolution in the LiCl/N,N-dimethylacetamide (DMAc) and tetrabutylammonium fluoride/dimethylsulfoxide (DMSO) systems is of interest for research applications.35A patent by Graenacher from 1934 is considered the first report on dissolving cellulose in an IL type solution.36 More recently, cellulose dissolution in a pure IL was reported by Swatloski et al. in 2002 who found that cellulose dissolves well in [BMIM]Cl and to some extent in [BMIM]+ ILs with Br− and SCN− anions, but not with BF4− or PF6− anions.26 Another important observation in this study was that the cellulose solubility in [BMIM]+ ILs was extremely water sensitive, with even 1% of added water leading to a complete loss of cellulose solubility. 1-Allyl-3-methylimidazolium chloride ([AMIM]Cl) was the first cellulose-dissolving IL carrying functionalized substituents on the cation (Fig. 3).37,38 In later studies, also non-halogen ILs were observed to dissolve cellulose efficiently, including 1-ethyl-3-methylimidazolium acetate ([EMIM]AcO)27 and dialkylphosphonates, such as 1,3-dimethylimidazolium dimethylphosphate ([DMIM]DMP).39
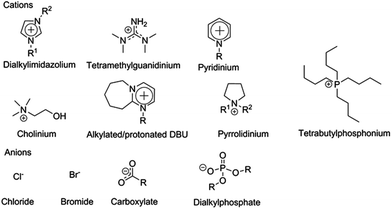
The dissolution and dissolution mechanisms of both cellulose and the complete lignocellulosic matrix have been extensively reviewed in recent years4,40–44 and only the most central points affecting enzymatic hydrolysis will be highlighted in this review. Cellulose-dissolving ILs are typically based on cations such as imidazolium, pyridinium, pyrrolidinium, cholinium, tetrabutylammonium and alkylalkyloxyammonium. Typical anions are halogens, carboxylates, amides, imides, thiocyanates, phosphates, sulphates, sulphonates and dichloroaluminates (Fig. 4). For many years practically all research on cellulose dissolution in ILs was done with imidazolium-based ILs, but recently several new classes of ILs have been described to dissolve cellulose, including ILs based on the organic superbases tetramethylguanidine (TMG)45 and 1,5-diazabicyclo-[4.3.0]non-5-ene (DBN),46 1-hexylpyridinium chloride ([HPy]Cl),47 alkylalkyloxyammonium amino acids,48 and phase-separable ILs based on tetraalkylphosphonium cations, which together with DMSO dissolve cellulose, but upon addition of water form their own, recyclable phase.49
Cellulose-dissolving ILs are, in general, hydrophilic, whereas hydrophobic ILs do not dissolve cellulose or other carbohydrates.50 The basicity of the IL's anion has been considered a key property for cellulose dissolution, as the anion is believed to break up the hydrogen bond network which keeps the cellulose together.51,52 Cellulose solubility in ionic liquid solvent systems has been correlated to e.g. Hildebrand–Hansen solubility parameters and Kamlet–Taft parameters,53,54 which have been found to be especially practical.
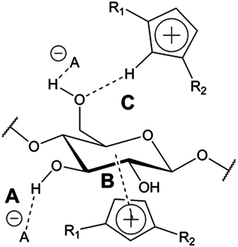
The role of the IL cation in cellulose dissolution appears to be somewhat unclear. The imidazolium cation has been proposed to have hydrophobic interactions with the hydrophobic face of the cellulose (Fig. 5),55,56 although other results suggest only weak interactions between the imidazolium and carbohydrates.57 Also the C-2 proton in the imidazolium has been simulated to interact as a weak hydrogen bond donor with the cellulose hydroxyl groups during dissolution56 and cation acidity has recently been suggested to be an important parameter for predicting cellulose solubility in certain cases.46
Cellulose dissolution rates are greatly affected by the IL's viscosity. Heating naturally reduces the solution viscosity and thus increases cellulose dissolution rates.39 The high viscosity of ILs and especially polymer solutions in ILs not only renders dissolution of cellulose in IL laborious, but also hampers the general processability of IL solutions. Furthermore, the cellulose DP and origin will have an impact on the dissolution behaviour. The dissolution process is very moisture sensitive, which is challenging as moisture can be introduced from air moisture (IL hygroscopicity) and the cellulose itself generally contains some water.
Due to their complex structures, ILs are able to interact with solutes through a variety of different interactions, including dispersive, π–π, n–π, hydrogen bonding, dipolar, and ionic/charge–charge interactions.58 The unique properties of certain ILs to dissolve all the components of lignocellulosic biomass are probably due to this set of possible dissolution interactions that ILs have. [AMIM]Cl is especially rich in π electrons due to the allyl substituent, which in part is likely to explain its high dissolution ability for wood; [AMIM]Cl is able to dissolve not only the polysaccharides but also the lignin due to the extra set of π electrons interacting with the aromatic lignin.27,54 In a comparison between different ILs, [AMIM]Cl was found to be the most efficient for wood dissolution, whereas [EMIM]AcO was the most efficient for dissolving cellulose.27
Lignocellulose pretreatments with ionic liquids
Lignocellulosic feedstocks need to be pretreated prior to enzymatic hydrolysis due to their inherent recalcitrance towards degradation. The pretreatment of biomass has been calculated to be the second largest cost factor in sugar-based biofuel production, after the feedstock itself.59 The main factors contributing to the lignocellulose recalcitrance are cellulose crystallinity, DP and surface accessibility and the presence of hemicellulose and lignin,60 which form a physical barrier around the cellulose.61,62 Furthermore, enzymes can bind non-specifically to lignin.63 The cellulose DP has a direct impact on cellobiohydrolase action as these cellulases need free cellulose chain ends to work on, whereas the DP is not known to influence the action of endoglucanases.7,64 A pretreatment should reduce the substrate recalcitrance by modifying at least one and preferably several of the above mentioned substrate properties. A pretreatment should in the ideal case have low capital and operating costs and be effective on many different substrate types, the side streams (hemicelluloses, lignin) should be easy to recover for further use and the formation of by-products (e.g. furfurals) should be avoided, because these inhibit downstream microbial fermentations.15,60 The pretreated substrates should be efficiently hydrolysed by enzymes at high substrate loadings with short residence times and low enzyme dosage.65 In current processes, the pretreatment is a bottle-neck and no completely satisfying procedure has to date been designed.Conventional pretreatment methods are typically divided into physical (milling), chemical (alkali and acid treatment) and biological pretreatments (treatment with wood-degrading fungi).60 Pretreatments are often combinations of the above mentioned methods, such as e.g. physicochemical pretreatments which include organosolv, ammonia freeze (or fibre) explosion (AFEX), steam explosion and ammonia recycle percolation (ARP). Conventional pretreatment methods have been extensively reviewed in the recent literature and will not be covered here.60,66–68 In the operational demonstration and industrial scale bioethanol plants, the applied pretreatment methods have been steam/hydrothermal pretreatment, in some cases combined with H2SO4 or SO2 catalysis.3
ILs hold great promise for biomass pretreatment and are widely applicable to different types of lignocellulosic feedstocks, due to their unique potential to dissolve the complete lignocellulosic matrix. The first reports of using ILs for cellulose pretreatment were published by Dadi et al., who dissolved and regenerated MCC in [BMIM]Cl and later in [AMIM]Cl.12,69 After optimization, the regenerated cellulose had even 90 times faster initial enzymatic hydrolysis rates than untreated MCC, which was attributed to the transformation of crystalline cellulose I to amorphous cellulose. IL pretreatment of pure cellulosic substrates (especially MCC) has thereafter been widely studied.70–74 MCC serves well as a model substance for studying the effects of IL pretreatment, but cannot be considered an industrially relevant substrate for glucose production. IL pretreatments for the hydrolysis of industrially relevant substrates, such as wheat,75,76 rice straw,77–80 wood,81–85 corn stover,86–88 switchgrass,31,81,82,89 bagasse,47,90–92 cotton waste textiles,93 fibre sludge94 and other lignocellulosic feedstocks, have been extensively studied. In mid-2014, the number of articles dealing with IL pretreatments of lignocellulosics or components thereof is close to 600 and is rapidly growing. Several reviews of this topic have recently been published.44,54,95–97 The most frequently applied ILs in pretreatments are those also known as good cellulose solvents: [EMIM]AcO, [AMIM]Cl, [BMIM]Cl and dialkylimidazolium dialkylphosphate ILs (Fig. 3).
Crystallinity analysis of cellulose regenerated from IL solution has shown that the cellulose precipitates either as amorphous cellulose,12,99 cellulose II,71,81 a mixture of amorphous cellulose and cellulose II,91,100 or a mixture of amorphous cellulose and cellulose I,69,73 depending on dissolution and regeneration conditions and the type of cellulose. Cellulose II has, especially when hydrated, been shown to have a higher enzymatic digestibility than native cellulose I, probably due to changes in the hydrogen bonding network in the cellulose crystallites.101 The crystallinity changes during IL pretreatment have been found to be dependent on the type of substrate and in particular to vary when comparing pure cellulosic and lignocellulose samples.
Many ILs, e.g. cholinium amino acids77,78 and cholinium mono- and dicarboxylates,98 are suitable for hemicellulose and lignin extraction but leave the cellulose mostly unaltered. Pretreatment with these ILs has been reported to be as efficient as with [EMIM]AcO in some cases.98 It has, however, been suggested that lignin cannot to a high degree be extracted from wood by lignin-dissolving ILs, which do not dissolve cellulose, because the lignin is partly trapped inside the lignocellulosic matrix in wood.53
Depolymerization of the biomass polysaccharide fraction has been reported in conjunction with IL treatments and is beneficial for enzymatic hydrolysis, as cellobiohydrolases are directly dependent on finding cellulose chain ends as starting points for hydrolysis.7 Bagasse and MCC pretreatment with [HPy]Cl led to significant depolymerization of the substrate polysaccharides,47 whereas somewhat conflicting results have been reported on cellulose depolymerization during pretreatments in e.g. [EMIM]AcO.47,91 Subjecting the pretreatment mixture to microwave irradiation during pretreatment has been shown to cause significant additional depolymerization in comparison with simple heating; microwaving and sonication have also been noticed to promote the breakdown of the crystalline cellulose structure during pretreatment.70,100,102 Several groups have studied the addition of acid catalysts to the cellulose solutions during IL pretreatment to cause further DP reductions.85,89,103 This procedure can be seen as a combination of pretreatment and acid hydrolysis in IL. Acid hydrolysis in IL solution has recently been reviewed by e.g. Tadesse and Luque.96
Techno-economic evaluations of ethanol production from lignocellulose using IL pretreatment have indicated the IL loss to be the most significant economic parameter in the whole process,59 meaning that also the amount of IL trapped in the regenerated solid substrate is likely to have a high impact on the process economics. Recently, the one-pot hydrolysis procedure and the regeneration with washing procedure were compared in a techno-economic study.104 The cost drivers were significantly different in the two cases. The excessive use of water constituted a large cost in the regeneration and washing procedure, whereas the sugar separation from the IL-containing hydrolysate by a liquid–liquid extraction method was the cost driver in the one-pot procedure. At low or moderate biomass loadings during the pretreatment step, the regeneration procedure was more feasible, whereas both the routes were equally economical at high, 50% biomass loadings. The one-pot procedure was significantly more sustainable regarding water usage. Recovering a pure lignin fraction as a by-product is expected to greatly enhance the economic viability of bioethanol production, although issues such as lignin price and the risk for rapid market saturation are difficult to predict.105
Developing efficient routes for IL recycling is vital if ILs are to be used in biorefineries. To recycle the IL, the simplest proposed procedure is to evaporate the anti-solvent from the IL and then reuse the IL.72,75,84,88,90 A major drawback is the high energy consumption for dehydrating the recycled IL by e.g. evaporation or reverse osmosis.106 Alternatively, IL recovery can be achieved by creating an aqueous-IL biphasic system by mixing the IL phase with concentrated salt solutions.107–109 Serious problems can in both cases be expected with lignin and other components accumulating into the IL.65,110 Recently, phase-separable and cellulose-dissolving tetra-alkylphosphonium ILs, which form their own recyclable IL phase upon addition of water, have been reported.49 The development of cellulose-dissolving acid–base conjugate ILs, such as those based on the TMG or DBN superbases, offers a new recycling alternative through distillation.45,46 This approach would avoid the problems with accumulation of non-precipitated biomass components into the recycled IL.
Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids
Regeneration of cellulose from IL has been shown to be a highly effective pretreatment for enzymatic hydrolysis.12 A thorough washing of the regenerated substrate is, however, needed, as even relatively small amounts of IL in the substrate may induce severe inactivation of the cellulases and also inhibit downstream bioprocessing.73,93 Results from IL pretreatment studies have shown that the cellulose recovery after precipitation may exceed 100%, suggesting that considerable amounts of IL are trapped in the regenerated material.111 Furthermore, the high dilution ratio of the IL caused by the regeneration and subsequent washing presents challenges to economical IL recycling.106 The one-pot procedure proposed by Kamiya et al., in which pretreatment and enzymatic hydrolysis are carried out without removing the IL between the steps, has the potential to avoid excessive IL dilution during regeneration and washing, and to avoid losing IL trapped in the regenerated substrate.32 Reported low enzyme activities in aqueous solutions of cellulose-dissolving ILs are, however, a serious drawback in the one-pot hydrolysis.33General factors affecting enzyme activity in ionic liquid solutions
Biocatalysis in organic solvents was pioneered by Klibanov in the 1980s, showing that enzymes can be used in synthetic applications of organic solvents.112 The first study of the use of an enzyme (alkaline phosphatase) in an IL, aqueous ethylammonium nitrate ([EtNH3]NO3), was published by Magnuson et al. in 1984.113 During the last decade, a number of good reviews have been published on both the fundamentals and applications of biocatalysis in ILs.22,24,25,114–116 ILs differ in many properties from conventional molecular solvents, and it is not yet completely clear to what extent the laws governing biocatalysis in organic solvents can be applied in IL solutions. Water is the natural medium for most enzymes, interacting with them sufficiently to dissolve them, but not to unfold their active structure.24 In buffer, the enzymes show a higher stability due to the “salting out” effect, i.e. repulsions between the charged ions in buffer, and the protein's hydrophobic interior keeps the protein folded. The stabilization energy of folded, active proteins is in most cases very low, calculated to be 30–60 kJ mol−1 in the dissolved state.117 Most enzymes reported to be active in ILs are lipases, which work at water–oil interfaces.114 In many cases, increased enzymatic activity, stability and selectivity have been encountered in IL matrices.25Three types of solution should be taken into account when dealing with enzyme stability and action in ILs: anhydrous hydrophobic ILs, anhydrous hydrophilic ILs, and aqueous hydrophilic ILs. Generally, hydrophilic ILs have been considered to be destabilizing and hydrophobic ILs stabilizing for enzymes.118,119 Whereas hydrophobic ILs stabilize suspended enzymes, carbohydrates have very low solubilities in these ILs,51 thus rendering them of marginal interest as solvents for biocatalytic reactions on dissolved polysaccharides. Hydrophobic ILs do not dissolve enzymes, but rather suspend them.120 Enzyme-dissolving ILs often inactivate the enzymes, with the exception of a few ILs, e.g. [Chol]H2PO4.121 Some hydrophilic ILs may promote refolding of a denatured protein, as has been demonstrated with hen egg white lysozyme in [EtNH3]NO3.122
In the early studies with enzymes in ILs, diverse problems were encountered regarding IL purity, unexpected pH shifts and precipitation of buffer salts.114 Especially, impurities in ILs have led to conflicting results. The main impurities in ILs are often residual halides and water, which cause problems especially in reverse hydrolysis reactions. Very small chloride impurities have been shown to lead to even complete lipase inactivation123 and different purification methods such as washing an IL with an aqueous Na2CO3 solution greatly increased the lipase activity.124
Enzyme stability in hydrophilic solvents has been predicted by the solutions’ Hildebrand solubility parameter (δ), dielectric constant (ε), dipole moment (μ) or octanol-water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P), but applying these parameters in IL systems does not always appear to be straightforward.119 In aqueous salt solutions, the protein stability has with success been related to the Hofmeister series125 or the kosmotropicity and chaotropicity of the IL ions.118,126 IL ions have been found to mainly follow the Hofmeister series in their effect on protein stability, although exceptions exist and especially the effect of larger ions with greater complexity is harder to predict. It is also important to notice that the Hofmeister series is only applicable when the aqueous IL is sufficiently diluted for the ions to be dissociated from each other.118 The imidazolium cations common in cellulose-dissolving ILs have been ordered into the following series based on the destabilising effect on proteins: [BMIM]+ > [EMIM]+ > [DMIM]+.127 Cholinium cations have been found to be stabilizing,127 whereas increasing the cation hydrophobicity has been reported to decrease enzyme melting points.126 Results published by Kaar et al. suggest the enzyme inactivation to be mostly dependent on the anion.119 Nucleophilic anions are possibly able to coordinate to positively charged surface residues on the enzyme and cause conformational changes. It has been found to depend strongly on the anion whether enzyme inactivation is reversible or irreversible. As discussed by Lee et al., enzymes appear to be active in anhydrous ILs containing BF4−, PF6−, bis(trifluoromethylsulphonyl) imide (Tf2N−) or SbF6− anions (i.e. hydrophobic ILs), but not in anhydrous ILs with NO3−, acetate, trifluoroacetate or halide anions (i.e. hydrophilic ILs).123 Large anions spread out their charge on several atoms and are thus believed to form weaker hydrogen bonds with the enzymes and less disruption of the structure.22 Carbohydrate-dissolving ILs have anions which form strong hydrogen bonds, thus also exerting a denaturing effect on enzymes.120
P), but applying these parameters in IL systems does not always appear to be straightforward.119 In aqueous salt solutions, the protein stability has with success been related to the Hofmeister series125 or the kosmotropicity and chaotropicity of the IL ions.118,126 IL ions have been found to mainly follow the Hofmeister series in their effect on protein stability, although exceptions exist and especially the effect of larger ions with greater complexity is harder to predict. It is also important to notice that the Hofmeister series is only applicable when the aqueous IL is sufficiently diluted for the ions to be dissociated from each other.118 The imidazolium cations common in cellulose-dissolving ILs have been ordered into the following series based on the destabilising effect on proteins: [BMIM]+ > [EMIM]+ > [DMIM]+.127 Cholinium cations have been found to be stabilizing,127 whereas increasing the cation hydrophobicity has been reported to decrease enzyme melting points.126 Results published by Kaar et al. suggest the enzyme inactivation to be mostly dependent on the anion.119 Nucleophilic anions are possibly able to coordinate to positively charged surface residues on the enzyme and cause conformational changes. It has been found to depend strongly on the anion whether enzyme inactivation is reversible or irreversible. As discussed by Lee et al., enzymes appear to be active in anhydrous ILs containing BF4−, PF6−, bis(trifluoromethylsulphonyl) imide (Tf2N−) or SbF6− anions (i.e. hydrophobic ILs), but not in anhydrous ILs with NO3−, acetate, trifluoroacetate or halide anions (i.e. hydrophilic ILs).123 Large anions spread out their charge on several atoms and are thus believed to form weaker hydrogen bonds with the enzymes and less disruption of the structure.22 Carbohydrate-dissolving ILs have anions which form strong hydrogen bonds, thus also exerting a denaturing effect on enzymes.120
The inactivating effect of both hydrophilic organic solvents as well as ILs has been suggested to be caused by dehydration of essential water molecules from proteins,24,128 but this inactivation mechanism was deemed unlikely by Kaar et al. when studying a lipase in IL, as rehydration did not return its lost activity.119 It has been proposed that enzyme incubation in hydrophobic ILs such as [BMIM]PF6 could protect the essential water molecules from stripping.129 Having a high number of charged amino acid residues on the protein surface appears to stabilize enzymes in strong salt solutions and ILs.130
Cellulose-dissolving ILs are usually basic which means that the medium pH will not be at the enzyme optimum in IL solutions.131–134 The question whether the pH effect is a major reason for enzyme inactivation e.g. in the case of cellulases is under debate. IL media usually have high viscosities, leading to lower mass transfer rates and slower reactions.135,136 In many cases the reaction system can be diluted with a low-viscosity organic solvent,137 which has also been shown to be applicable for cellulose-solutions with good success for increasing the cellulose dissolution kinetics.138,139 A third general effect of the presence of ILs in a reaction medium is an increased ionic strength, which affects the action of most enzymes. In a study by Engel et al. the cumulative effect of increased viscosity and ionic strength was compared to the inactivation measured at the corresponding concentration of [DMIM]DMP at fixed pH, and it could be concluded that the presence of [DMIM]DMP also had other inactivation mechanisms than those caused by the high viscosity and ionic strength.132
Cellulase activity and stability in ionic liquid solutions
Many reports have been published on the activity, stability and action in hydrolysis experiments of mesophilic cellulases from e.g. the Trichoderma reesei and Aspergillus niger cellulase systems, either with the cellulases in a monocomponent form or as cellulase cocktails (Table 1). Of the monocomponent cellulases studied in ILs, endoglucanases are the most studied. Studies of monocomponent cellobiohydrolases, β-glucosidases and various hemicellulases have also been published. In addition to the mesophilic cellulases, an increasing number of studies have been concentrating on cellulases derived from different extremophilic sources, as thermo-, salt- and alkali-stability appears to be linked to tolerance to cellulose-dissolving ILs. Carboxymethylcellulose (CMC) has been the most employed substrate in the activity and stability measurements for endoglucanases, but studies of the hydrolysis of different solid substrates have also been increasingly reported. Three different types of measures for cellulolytic performance in IL prevail: activity measurements (initial reaction velocity), stability (retained cellulolytic activity after incubation in IL) and long term hydrolysis experiments. Occasionally, cellulase stability in IL solution has also been studied by other, non-reaction based techniques, such as fluorescence spectroscopy or differential scanning calorimetry (DSC).33,136,140 The use of computational methods such as molecular dynamics has also contributed with interesting knowledge on glycosyl hydrolase–IL interactions.141| Substrate | Cellulase | IL(s) | Measurement | Main results | Reference |
|---|---|---|---|---|---|
| Cellulose azure | Trichoderma reesei cellulase | [BMIM]Cl, [BMIM]BF4 | Activity, stability | Cellulose-dissolving ILs were found highly inactivating for the first time, PEG stabilization was beneficial for cellulase stability, fluorescence spectroscopy was used for stability measurements | Turner et al. (2003)33 |
| CMC | Celluzyme 0,7 T (Humicola insolens cellulase) | [BMIM]Cl, [BMIM]BF4, [BMIM]PF6 | Activity, stability | [BMIM]PF6 stabilising, [BMIM]BF4 and [BMIM]Cl inactivating during incubation | Paljevac et al. (2006)142 |
| Regenerated cellulose | Trichoderma reesei cellulase | [EMIM]DEP | Hydrolysis | In situ/one pot cellulose hydrolysis in IL was first introduced, hydrolysis took place in up to 40% (v/v) IL | Kamiya et al. (2008)32 |
| Filter paper, CMC, xylan, steam exploded bagasse | Penicillium janthinellum mutant glycosyl hydrolases | [BMIM]Cl | Hydrolysis, stability | Mutant enzymes active in 20% IL, bagasse was hydrolysed in the presence of IL | Adsul et al. (2009)161 |
| CMC | Bacterial cellulases | 6 different ILs | Activity, stability | CBM pivotal in cellulase IL tolerance, link between salt tolerance and IL-tolerance | Pottkämper et al. (2009)153 |
| Cellulose azure | Trichoderma reesei cellulase cocktail | 8 different ILs | Activity, stability | Significant cellulase stabilization in HEMA, cellulase stability in ILs studied by DSC and tryptophyl fluorescence. | Bose et al. (2010)136 |
| CMC, IL-pretreated corn stover, MCC | Thermotoga maritima, Pyrococcus horikoshii endoglucanases, Trichoderma viride cellulase | [EMIM]AcO | Activity, hydrolysis, stability | Thermophilic endoglucanases exhibited good IL-tolerance | Datta et al. (2010)163 |
| α-Cellulose, CMC, regenerated cellulose, 4-NP-β-cellobioside | Celluclast® 1.5 L | [DMIM]DMP, [EMIM]AcO, [BMIM]Cl, [AMIM]Cl | Activity, hydrolysis, stability | Evaluation of IL solution's viscosity, ionic strength and pH effects on cellulase activity | Engel et al. (2010)132 |
| MCC, regenerated cellulose | Trichoderma reesei cellulase | [EMIM]DEP | Hydrolysis | Cellulase immobilized by glutaraldehyde cross-linking hydrolysed cellulose with increased kinetics in low IL content (2%) | Jones and Vasudevan (2010)177 |
| CMC | Aspergillus niger cellulase | [BMIM]Cl | Activity | High pressure enhanced cellulase activity in the presence of IL, inactivation linearly correlated to water activity in IL | Salvador et al. (2010)143 |
| MCC | Trichoderma reesei cellulase cocktail | Several ILs screened, main work with [DMIM]DMP | Hydrolysis | Different parameters were investigated for enzymatic cellulose hydrolysis in IL solution | Yang et al. (2010)70 |
| 4-NP-glycosides | Thermus thermophilus β-glycosidase, Thermotoga maritima α-galactosidase and Bacillus stearothermophilus α-galactosidase | [DMIM]MeSO4 and [TMIM]MeSO4 | Activity, stability | Strong correlation between thermostability and IL-tolerance was established; both reversible and irreversible inactivation were observed | Ferdjani et al. (2011)160 |
| IL-pretreated switchgrass | Supernatants from thermophilic bacterial consortia | [EMIM]AcO | Hydrolysis, stability | Thermophilic bacterial consortia adapted to switchgrass at 60 °C produced cellulases with good tolerance to IL | Gladden et al. (2011)166 |
| Cotton waste textiles | Commercial cellulase cocktail | [AMIM]Cl | Hydrolysis | Low amounts of residual IL caused severe cellulase inactivation | Hong et al. (2011)93 |
| CMC | Thermoanaerobacter tengcongensis endoglucanase | [BMIM]Cl, [AMIM]Cl | Activity, stability | Thermophilic endoglucanases exhibited good IL tolerance | Liang et al. (2011)165 |
| MCC and various IL-pretreated biomass samples | Cellulase from Continent Biotech (Shanghai) Co. | Various ILs screened, the main work with [EMIM]AcO | Hydrolysis | IL-pretreated lignocellulose was enzymatically hydrolysed in the presence of up to 20% IL with increased yields | Li et al. (2011)102 |
| 4-NP-glycosides | β-glucosidases, xylanase, arabinofuranosidase | [DMIM]DMP, [EMIM]DMP, [EMIM]DEP, [EMIM]AcO | Activity | Enzyme inactivation in various IL solutions was determined | Thomas et al. (2011)159 |
| CMC | Bacillus aquimaris cellulase | [EMIM]MeSO4, [EMIM]Br | Stability | Solvent- and alkali-tolerant cellulase exhibited good IL tolerance | Trivedi et al. (2011)168 |
| CMC, regenerated cellulose and yellow poplar | Celluclast® 1.5L and Aspergillus niger β-glucosidase | [EMIM]AcO | Activity, hydrolysis, stability | Enzymatic hydrolysis of a IL-pretreated biomass sample was demonstrated in the presence of residual IL | Wang et al. (2011)144 |
| Regenerated cellulose and Miscanthus | Trichoderma reesei cellulase, β-glucosidase (Novozym 188) | [DMIM]DMP, [EMIM]AcO, [EMIM]lactate | Activity, hydrolysis, stability | A screening method for enzyme inactivation in IL using green fluorescent protein was reported, results were verified with cellulases | Wolski et al. (2011)146 |
| CMC | Halorhabdus utahensis cellobiohydrolase | [AMIM]Cl, [EMIM]AcO, [EMIM]Cl, [BMIM]Cl | Activity | A large number of charged surface groups on the protein surface linked to salt tolerance | Zhang et al. (2011)171 |
| Cotton cellulose | Trichoderma reesei cellulase | [EMIM]AcO, [EMIM]MeO(H)PO2 | Hydrolysis | Enzymatic cellulose hydrolysis took place in up to 40% (v/v) [EMIM]MeO(H)PO2 in one-pot processing | Auxenfans et al. (2012)184 |
| Cellulose azure | Aspergillus niger endoglucanase | 3 imidazolium ILs and [HEMA]MeSO4 | Activity, hydrolysis, stability | [HEMA]MeSO4 stabilised cellulase towards thermal inactivation | Bose et al. (2012)140 |
| Regenerated cellulose, cellobiose | Trichoderma reesei Cel7A and Cel7B, A. niger β-glucosidase (Novozym 188) | [DMIM]DMP | Activity, hydrolysis, stability | Cellulase cocktail for the hydrolysis of regenerated cellulose in the presence of IL optimized | Engel et al. (2012)150 |
| CMC | Bacterial cellulases | 6 different ILs | Activity, stability | IL tolerance linked to thermo- and halophilicity | Ilmberger et al. (2012)162 |
| Azo-CMC, 4-MUC, MCC, tobacco cell wall polysaccharides | Sulfolobus solfataricus endoglucanase | [DMIM]DMP, [EMIM]AcO | Hydrolysis | Thermostable endoglucanase shows high activity in 80% IL at 90 °C | Klose et al. (2012)164 |
| CMC, MCC, straw, cotton, filter paper | Cellulase powder | [E(OH)MIM]AcO | Hydrolysis, stability | Enzyme-compatible and cellulose-dissolving IL was developed | Li et al. (2012)134 |
| IL-pretreated switchgrass | Cellulases from a thermophilic bacterial consortia | [EMIM]AcO | Hydrolysis | Thermophilic cellulase cocktail optimized for switchgrass hydrolysis in up to 20% IL. | Park et al. (2012)106 |
| Delignified IL-pretreated bagasse | Accellerase 1500 cellulase | [EMIM]DEP | Hydrolysis | High glucose yields were obtained in enzymatic hydrolysis in the presence of IL | Su et al. (2012)92 |
| CMC | Commercial cellulase cocktail | [DMIM]DMP | Activity | Cellulase covalently immobilized on chitosan showed increased cellulolytic action on CMC in aqueous IL solution | Su et al. (2012)180 |
| MCC | Trichoderma reesei Cel5A and Cel7B | [DMIM]DMP, [EMIM]AcO | Hydrolysis | Enzymatic cellulose chain length scission observed in treatments in 90% [DMIM]DMP | Wahlström et al. (2012)131 |
| Filter paper regenerated from IL | Liquid cellulase from Imperial Jade Bio-technology | [DMIM]DMP, [EMIM]DEP, [BMIM]DBP | Activity, hydrolysis, stability | IL-pretreated filter paper was enzymatically hydrolyzed in the presence of IL with increased kinetics | Zhi et al. (2012)147 |
| Cellulose | Sodium alginate-immobilized cellulase | [DMIM]DMP | Hydrolysis | Immobilized cellulase was successfully used for hydrolysis in IL solution | Fei et al. (2013)178 |
| CMC | Trichoderma reesei, T. viride, Aspergillus sp. and Aspergillus niger cellulases | 5 imidazolium-based ILs | Stability | Cellulase from Aspergillus sp. showed remarkable IL-tolerance in long-term incubation in IL solution | Ilmberger et al. (2013)149 |
| Xylan | Trichoderma longibrachiatum GH11 xylanase | [EMIM]AcO, [EMIM]EtSO4 | Activity, stability | Molecular dynamics simulations suggested that IL cations become trapped in the active site of the enzyme causing competitive inhibition | Jaeger and Pfaendtner (2013)141 |
| MCC, CMC | Cellulase powder | [BMIM]Cl | Activity, hydrolysis, stability | Cellulase was stabilized by PEGylation of the N-terminal and used for hydrolysis in IL solution | Li et al. (2013)181 |
| Xylan | Dictyoglomus thermophilum GH11 xylanase | [EMIM]AcO | Activity, stability | An N-terminal disulphide bridge was introduced for better IL-tolerance; IL appeared to interfere with enzyme–substrate interactions | Li et al. (2013)155 |
| CMC | Paenibacillus tarimensis endoglucanases | [BMIM]Cl, [EMIM]AcO | Activity | The studied halo-alkali-tolerant endoglucanases showed good activity in IL solutions | Raddadi et al. (2013)169 |
| CMC, regenerated cellulose, cotton linters, algal biomass | Pseudoalteromonas sp. cellulase | 6 different ILs | Activity, stability | Thermo-, halo- and alkali-tolerant cellulase exhibited good IL-tolerance | Trivedi et al. (2013)167 |
| MCC, cello-oligomers | Trichoderma reesei Cel5A, β-glucosidase (Novozym 188) | [DMIM]DMP, [EMIM]AcO | Hydrolysis | Different modes of endoglucanase inactivation observed in [EMIM]AcO and [DMIM]DMP | Wahlström et al. (2013)185 |
| MCC, pulp | Trichoderma reesei Cel5A, IndiAGE® ONE, Puradax® HA1200 E, Thermotoga maritima cellulase | [EMIM]AcO, TMG- and DBN-based distillable ILs | Hydrolysis | TMG- and DBN-based ILs were at least as inactivating as [EMIM]AcO, enzyme thermostability was linked to increased action in IL solution | Wahlström et al. (2013)133 |
| Cellulose powder | Trichoderma viride cellulase | [BMIM]Cl | Hydrolysis | Cellulase stabilized in liposomes showed higher IL-tolerance than free cellulases in hydrolysis in IL solution | Yoshimoto et al. (2013)182 |
| MCC, CMC | Trichoderma reesei Cel12A (IndiAge® Super GX Plus) | [BMIM]Cl | Activity, hydrolysis, stability | The studied cellulase exhibited good stability and activity in IL, cellulose hydrolysis happened in almost pure IL | D'Arrigo et al. (2014)151 |
| Cotton linters, pulp, CMC | Trichoderma reesei cellulase | [EMIM]AcO | Activity, stability | Enzyme inactivation was measured in 90–100% [EMIM]AcO | Ebner et al. (2014)145 |
| CMC, 4-PN-glycosides | Cellulases derived from thermophilic bacterial consortia | [EMIM]AcO | Activity | Good IL-tolerances were found for cellulases with high temperature optima | Gladden et al. (2014)172 |
| Filter paper | Aspergillus terreus cellulase | [BMIM]AcO, [BMIM]Cl, [EMIM]AcO | Activity | The studied halophilic and thermostable cellulase showed good IL-tolerance | Gunny et al. (2014)170 |
| Cellobiose | Thermotoga maritima β-glucosidase | [BMIM]Cl, [BMIM]AcO, [EMIM]Cl, [EMIM]AcO | Activity, stability | [BMIM]AcO increased the activity of the β-glucosidase due to increased structural flexibility, and structural changes in IL were monitored by fluorescence and circular dichroism spectroscopy | Kudou et al. (2014)175 |
| 4-NP-β-cellobioside, 4-MUC | CelA2 endoglucanase | [BMIM]Cl | Activity | Endoglucanase mutants with ionic strength activity switch reported | Lehmann et al. (2014)176 |
| MCC | Trichoderma reesei cellulase cocktail | [BMIM]Cl | Hydrolysis | Charge engineering of the cellulase surface significantly improved hydrolysis performance in IL solution | Nordwald et al. (2014)183 |
| MCC | Trichoderma reesei Cel5A and Cel7A | [EMIM]AcO, [DMIM]DMP | Hydrolysis, substrate binding | The CBM of cellulases were shown to be sensitive to IL, and reduced substrate binding in IL solution | Wahlström et al. (2014)154 |
| CMC, rice straw | Aspergillus fumigatus cellulase | Various ILs screened | Stability, hydrolysis | A cellulase from chemically polluted microhabitats showed good stability in 30% IL and IL-pretreated rice straw was hydrolysed in this solution | Xu et al. (2014)173 |
Turner et al. were the first to report cellulase activity in cellulose-dissolving ILs.33Trichoderma reesei cellulase was found to be much inactivated in [BMIM]Cl, which was proposed to be due to the high concentration of Cl− ions. A high degree of dilution of the IL solution with water led to refolding of the cellulase, suggesting the inactivation to be reversible in [BMIM]Cl for the studied enzyme. In another early study, a Humicola insolens cellulase was found to be stable in [BMIM]PF6 and [BMIM]BF4, which do not dissolve cellulose, but the cellulase was inactivated in cellulose-dissolving [BMIM]Cl.142
Salvador et al. found the activity of A. niger cellulase to correlate linearly with the water activity in [BMIM]Cl solutions.143 The cellulase was noticed to regain its activity upon dilution with buffer after incubation in 10% [BMIM]Cl, supporting the earlier observations by Turner et al. about reversible inactivation in this IL.33 Cellulase inactivation in ILs has been shown to be temperature dependent, as a cellulase mixture had only minor activity losses during incubation in 30% [EMIM]AcO at 4 °C, but more rapid inactivation at 50 °C.144
Engel et al. made activity measurements on α-cellulose, which, being a solid substrate, represents a more practical substrate for activity measurements than the usual soluble substrates.132 Interestingly, cellulases have been found to show increased thermal stability in the presence of regenerated cellulose, indicating that inactivation kinetics measured in IL solutions without substrate may not give the complete picture of cellulase inactivation in practice.73 In a study by Ebner et al. residual endoglucanase activity measurements were done on both soluble CMC and regenerated pulp after incubation in 90–100% (w/w) [EMIM]AcO, whereby it was noticed that the endoglucanase retained residual activity for significantly longer times in the presence of (dissolved) pulp, although the different inactivation kinetics measured may have also been due to different sensitivities and substrates of the two activity assays used.145 Engel et al. found that cellulases retained their activity towards soluble substrates to a higher degree than towards α-cellulose in aqueous [DMIM]DMP, demonstrating that the outcome of activity measurements in IL solutions may also be dependent on the substrate used.132
Wolski et al. have reported a screening method using green fluorescent protein as a probe for determining protein stability in ILs with fluorescence measurements.146 Preserved protein fluorescence was found to correlate with a well-retained activity of cellulase in IL solutions. [DMIM]DMP and [EMIM]lactate were by this method identified as potentially enzyme-compatible ILs for one-pot cellulose hydrolysis and T. reesei cellulases were found to retain their activity in up to 40% [DMIM]DMP or [EMIM]lactate and A. niger β-glucosidase in up to 60% [DMIM]DMP in validation experiments. Bose et al. studied the stability of a T. reesei cellulase cocktail in eight different ILs and found tris-(2-hydroxyethyl)methylammonium methylsulphate ([HEMA]MeSO4) to stabilize this enzyme mixture at temperatures up to 115 °C.136 Although [HEMA]MeSO4 showed great promise in view of its cellulase compatibility, this IL is only capable of dissolving ∼1% of cellulose,140 which may limit its applicability to biomass pretreatment. Zhi et al. studied the cellulase stability in a series of dialkylphosphate ILs with increasing alkyl substituent size.147 [DMIM]DMP, with the smallest alkyl substituents, had the highest cellulase compatibility. In a comparison of the cellulase compatibility of the most common cellulose-dissolving ILs ([DMIM]DMP, [BMIM]Cl, [EMIM]AcO and [AMIM]Cl), cellulase activity was observed to generally decrease with 70–85% in the presence of 10% (v/v) IL as compared to buffer systems.132 In some cases larger differences in relative cellulase activity were noticed for the same IL from different manufacturers than between different ILs. [DMIM]DMP could be concluded to be the least cellulase-inactivating of the studied ILs. Likewise, Yang et al. identified [DMIM]DMP as the most cellulase-compatible IL out of a set of six phosphate ILs and optimized different hydrolysis parameters in this IL.70 Substituted imidazoles (i.e. uncharged imidazole derivatives) have been shown to cause considerable inhibition of glycosyl hydrolases and the inhibition efficiency was very dependent on the substituent types and positions on the imidazole ring.148 The results suggest that some imidazole derivatives interact in a very precise manner with glycosyl hydrolases, thus inhibiting them.
In a comparison of four commercial cellulase preparations derived from Aspergillius sp., A. niger, Trichoderma viride and T. reesei, the cellulase extract from Aspergillus sp. was found to retain a remarkable activity in 30% (v/v) of five different ILs, among which was the cellulose-dissolving [BMIM]Cl earlier reported to be severely inactivating.149 The other cellulases became almost completely inactivated under the corresponding conditions. Also the long term stability of this cellulase was found to be extraordinarily high in 60% (v/v) of IL. This finding shows that IL-tolerant cellulases can be found in already commercialised enzyme products.
T. reesei Cel7A (cellobiohydrolase) and Cel7B (endoglucanase) were in one study found to be inactivated in a similar manner in the presence of [DMIM]DMP, with some residual activity in 30% (v/v) of this IL.150 The β-glucosidase of A. niger was more IL sensitive and lost its activity already in 15% (v/v) of [DMIM]DMP.150 Cellulase inactivation in aqueous [DMIM]DMP has been found to be reversible, and also the storage stability has been found to be sufficiently steady for long hydrolyses in 10% (v/v) [DMIM]DMP, after a first initial rapid decrease in the activity.132,150 Wahlström et al. measured the residual endoglucanase activity of T. reesei Cel5A and Cel7B after incubation in 90% (v/v) [DMIM]DMP at 45 °C; a short incubation time of 15 min led to an activity decrease of ca. 50%, but the activity then stayed steady at this level for days as verified for Cel5A.131T. reesei Cel12A (EGIII) has been found to show both good activity and stability in [BMIM]Cl, in which the enzyme was even stabilised at 75 °C as compared to incubation in buffer.151
There appear to be great differences between the inactivation rates (stability) of cellulases in different cellulose-dissolving imidazolium-based ILs. When the inactivation of endoglucanase activity on CMC was studied in pure [EMIM]AcO and [DMIM]DMP, the endoglucanase was noticed to be completely inactivated in 4 h in [EMIM]AcO at 40 °C, whereas the activity only declined slowly in [DMIM]DMP and over 60% of the initial activity was retained even after 3 days.145,152 Furthermore, the inactivation appeared to be irreversible in [EMIM]AcO, as no regain of activity could be measured when the incubated cellulase mixture was diluted to below 4% of IL,145,152 contrasting with the earlier reported reversibility of cellulase inactivation reported in aqueous [BMIM]Cl and [DMIM]DMP.33,132,143
Substrate binding of cellulases and other glycosyl hydrolases and the role of the carbohydrate-binding module (CBM) in ionic liquids
Pottkämper et al. proposed the carbohydrate-binding module (CBM) of cellulases to be particularly sensitive to ILs, based on comparing the activity of different cellulase mutants on CMC in several ILs.153 In a comparison of T. reesei Cel5A and its core domain (CD, i.e. the cellulase lacking its CBM), the CBM was found important for MCC hydrolysis in buffer, but not in the studied IL solution.131 In the presence of 20% (v/v) [DMIM]DMP the hydrolysis yield of the intact cellulase decreased by over 80%, whereas the action of the CD cellulase was not significantly affected by adding IL to the hydrolysis mixture, which was interpreted as a strong IL-sensitivity of the CBM. This was confirmed with hydrolysis experiments in a follow-up study in which the action of the cellobiohydrolase T. reesei Cel7A, the endoglucanase T. reesei Cel5A and their CDs was compared in IL solutions.154 Binding isotherms measured using tritium-labeled cellulases confirmed that the presence of [DMIM]DMP and [EMIM]AcO seriously decreased the cellulase binding to MCC even at 4 °C. The cellulase binding is temperature-dependent and so it can be anticipated that at hydrolysis temperatures of 40–50 °C cellulase binding is even lower in the presence of ILs. The reasons for the reduced cellulase binding were not elucidated in this study, but the authors suggested several possible explanations: solvent effects interfering with the hydrophobic interactions governing the binding to cellulose, conformational changes in the CBM or even complete unfolding of the CBM. Another interesting result from this study was that the T. reesei cellobiohydrolase, with the catalytically active site in a tunnel, was able to bind to cellulose to a much higher degree in IL matrices in comparison with the endoglucanase with the active site in a surface cleft.Competitive inhibition by ILs with subsequent reduced substrate binding as the reason for the observed glycosyl hydrolase inactivation has been discussed in some articles. Molecular dynamics simulations by Jaeger and Pfaendtner suggested that imidazolium cations are trapped in the active site cleft of a xylanase (xylanase II from Trichoderma longibrachiatum, a GH11 enzyme) and that the imidazoliums may cause competitive inhibition of the substrate binding.141 Kinetic measurements also suggested competitive inhibition of the active site in xylanases as the main reason for the observed low hydrolysis efficiency in IL solution.155
Hemicellulases and other glycosyl hydrolases in ionic liquids
Hemicellulases are often close in structure and catalytic mechanism to the cellulases, which is why it is of interest to compare the action of hemicellulases and other related enzymes, e.g. glycosyl transferases, to cellulases in IL matrices. Hemicellulases also play an important role in the total enzymatic hydrolysis of lignocellulosic biomass, which typically contains significant amounts of hemicelluloses. Different glycosyl hydrolases have been studied in the synthesis of small oligosaccharides in aqueous systems with ILs added as co-solvents.156–158 Thomas et al. studied the activity of two β-glucosidases, a xylanase and two arabinofuranosidases in the presence of three dialkylphosphate ILs and [EMIM]AcO and found the dialkylphosphates to be generally more compatible with these enzymes.159 When the activity of two α-galactosidases and a β-glucosidase was studied in imidazolium-based ILs, a strict correlation between the thermostability and IL-tolerance of these glycosyl hydrolases could be established.160Introducing a new disulphide bridge into the N-terminus of an extremophilic Dictyoglomus thermophilum GH11 xylanase increased its activity at elevated temperatures and to some extent in the presence of [EMIM]AcO.155 This xylanase was inactivated linearly to the [EMIM]AcO concentration and the enzyme was completely inactivated at 25% of this IL. The main IL effect was not in changing the protein structure, but the IL was proposed to cause severe effects on the substrate binding capability of the xylanase. Similar results were obtained in a molecular dynamics simulation of the effects of [EMIM]AcO on Trichoderma longibrachiatum xylanase II (a GH11 enzyme).141 This simulation study also demonstrated the strong influence of IL on the protein structure, e.g. there are fluctuations in the structure close to the substrate binding site of this xylanase.
Discovery and development of ionic liquid-tolerant cellulases
Metagenomic approaches and mutation experiments have led to encouraging results in finding more IL-tolerant cellulases. Adsul et al. reported several glycosyl hydrolases from Penicillium janthinellum mutants which exhibited an improved IL-tolerance.161 In a screening study by Pottkämper et al., the IL-tolerance of 24 bacterial cellulases from metagenomic libraries was elucidated in a number of different ILs.153 Most of the cellulases had very low IL-tolerance, but it was found that most cellulases displaying increased IL-tolerance also showed high halotolerance, suggesting the correlation between halotolerance and IL-tolerance. Also, Ilmberger et al. reported that several cellulases with good IL-tolerances were found when screening metagenomic libraries.162Cellulases have been screened from different extremophilic and halophilic sources for better enzyme performance in cellulose-dissolving ILs.162–166 Generally, there appears to be a clear correlation between thermostability and IL-tolerance. Increased IL-tolerance has been reported for enzymes active at high pH originating from solvent-tolerant bacteria.167,168 Extremotolerant (high alkali-, thermo- and salt tolerance) cellulases derived from Paenibacillus tarimensis, found in some very saline environments, have been reported to have a high tolerance towards the ILs [BMIM]Cl and [EMIM]AcO.169 The activity and stability of two α-glucosidases and a β-glucosidase in imidazolium-based ILs were found to correlate well with the enzymes’ thermostability in another study.160 The cellulase inactivation was found to take place through a slow irreversible denaturation which was dependent on the enzyme properties and the specific IL, but the reduced activity was also suggested to be due to non-competitive inhibition by the imidazolium ions and low water activity at high IL concentrations.
When screening cellulases from micro-organisms living in highly saline environments for IL-tolerance, several cellulases with high salt (NaCl) tolerance were found. The high IL-tolerance apparently also correlated with thermostability.170 These cellulases showed good activity in [BMIM]AcO and [EMIM]AcO in the studied IL concentration range of 0–20% (v/v). The IL-tolerance was explained by a high number of acidic amino acid residues on the cellulases’ surfaces, which are suggested to prevent protein aggregation in highly ionic environments. Similarly, a haloalkaliphilic Halorhabdus utahensis cellulase had good IL-tolerance, which was linked to the presence of a large number of negatively charged amino acid residues on the protein surface, a low content of hydrophobic amino acids and a compact packing of the protein structure.171 A negative charge on the protein surface is anticipated to interact well with water and high ion concentrations,130 and this hypothesis is supported by many of the reviewed studies.
Gladden et al. screened for IL-tolerant cellulases from a library of thermophilic cellulases derived from a switchgrass-adapted microbial community, and found many cellulases active in 10% (v/v) [EMIM]AcO, and some displaying significant activity even in 40% (v/v) [EMIM]AcO.172 Both endoglucanase, cellobiohydrolase and β-glucosidase activities were studied in the presence of [EMIM]AcO. The correlation between thermotolerance and IL-tolerance could also be strengthened as those enzymes with the highest optimum temperatures also showed the best IL-tolerance. Some of the IL-tolerant enzymes showed good activity under slightly alkaline conditions, but it was generally concluded that alkali tolerance and IL-tolerance were not strongly correlated for the studied enzymes. Similar results were obtained when the hydrolytic performances of an alkali-tolerant and a thermostable endoglucanase were compared in MCC hydrolysis in [EMIM]AcO and tetramethylguanidinium acetate ([TMGH]AcO).133 A surprising result of the study by Gladden et al. was that several of the studied cellulases showed increased activity in low (5–10%) concentrations of [EMIM]AcO, and in some cases up to a several fold increase in activity could be measured, compared to the activity in buffer.172 The isolation of an IL-tolerant cellulase-producing fungus (Aspergillus fumigatus) from chemically polluted habitats was recently reported.173 The thus derived cellulase was used in the hydrolysis of rice straw in the presence of 25% (v/v) [EMIM]DMP with approximately two-fold yields as compared to the buffer reference system.
A β-glucosidase from the hyperthermostable Pyrococcus furiosus showed high IL-tolerance and retained full activity even in the presence of 50% [DMIM]MeSO4 at 80 °C, and was inactivated but not irreversibly denatured in 70% of this IL.174 When Kudou et al. studied the activity of a β-glucosidase from Thermotoga maritima in aqueous solutions of [EMIM]AcO, [EMIM]Cl, [BMIM]Cl and [BMIM]AcO during 15 min activity measurements, [BMIM]AcO was found to increase the activity of this cellulase, whereas the other ILs slightly decreased the activity.175 The authors proposed the increased activity to be due to increased flexibility of the cellulase caused by the presence of [BMIM]AcO. The introduction of an ionic strength activation switch into an endoglucanase by directed evolution was recently reported.176 The endoglucanase mutant had low activity in buffer but was highly activated when 7.5% (v/v) of [BMIM]Cl was added to the reaction medium. The most IL-tolerant cellulase to date is a hyperthermophilic and halophilic GH12 endoglucanase from Sulfolobus solfataricus, which was reported by Klose et al. to hydrolyse dissolved or regenerated MCC well in 80% (v/v) [DMIM]DMP and [EMIM]AcO at 90 °C.164 In all, very impressive progress has been made in discovering cellulases with high IL-tolerances as compared to the earlier studied mesophilic cellulases.
Enzyme-compatible cellulose-dissolving ionic liquids
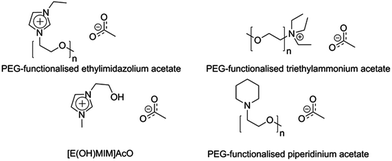
The attempts to design IL- and enzyme-compatible systems have concentrated mainly on discovering or developing more suited enzymes for this purpose, whereas the reverse approach, to develop enzyme-friendly ILs, has not been explored to a comparable extent. Some examples of specially designed enzyme-compatible ILs are, however, available in the literature (Fig. 6). A common approach has been to introduce polyethyleneglycol (PEG) chains into the cation; e.g. PEG-substituted imidazolium-, piperidinium- and ammonium-based ILs have been reported.50–52 The PEG substituent has been assigned three different functions: (1) it interacts with cellulose through its oxygen atoms in a manner beneficial for cellulose dissolution, (2) it interacts with the enzyme in a stabilizing manner, and (3) the cation becomes larger, which means that the overall ion concentration of the neat IL decreases.50–52 The enzyme inactivating effect has been assigned mainly to the anions, and so having a lower net concentration of anions in the medium has been proposed to favour a highly retained enzyme activity.50 The enzyme-compatibility of these PEG-substituted ILs did not compromise their cellulose solubilisation capacity, as the PEGylated ILs were reported to dissolve more than 10% (w/w) of cellulose.50 Li et al. reported another cellulose-dissolving IL with increased enzyme-compatibility, 1-hydroxyethyl-3-methylimidazolium acetate ([E(OH)MIM]AcO).134 This IL was able to dissolve more than 10% (w/w) of MCC and a commercial cellulase showed good stability and action in MCC hydrolysis in aqueous solutions of it. Also in this design, the hydroxyl group on the cation was claimed to interact well with cellulose and enzymes. Many of the specially designed cellulose-dissolving and enzyme-compatible ILs have a high structural complexity compared to the more commonly used ILs and they are produced in multistep synthetic procedures. Thus, their use in large-scale applications may be very sensitive to their production economics.Stabilization techniques for cellulases in ionic liquids
Cellulases may be stabilised by employing different enzyme stabilization techniques. Several reviews dealing with various stabilisation procedures for enzymes in IL systems are available.120,128 Jones and Vasudevan reported the cross-linking of cellulase with glutaraldehyde and its use in media containing low concentrations (2% v/v) of 1-ethyl-3-methylimidazolium diethylphosphate ([EMIM]DEP).177 Cellulase immobilization onto sodium alginate has also been reported for saccharification in [DMIM]DMP solutions.178 In a study by Lozano et al. a commercial cellulase was immobilized on a polymeric support, Amberlite XAD4, and subsequently coated with a hydrophobic IL, which stabilized the cellulase against thermal inactivation.179 The immobilized and IL-coated enzyme showed a better stability in [BMIM]Cl, known to be a strongly inactivating IL. Cellulase immobilization on chitosan has also been reported to increase cellulase action on CMC in the presence of [DMIM]DMP.180Several successful stabilization techniques for cellulases employ the use of PEG. Turner et al. reported a better IL-tolerance of cellulase after lyophilizing it with PEG.33 Another successful approach was to attach PEG chains to the N-terminal end of a cellulase.181 The PEG chain was suggested to form a hydrophilic region around the cellulase, which protects the cellulase from the IL and also increases the interactions between the modified enzyme and cellulose. Trichoderma viride cellulase was stabilised as liposomes, which successfully increased the IL-tolerance of the enzyme.182 Nordwald et al. have reported surface charge modification of cellulases by chemical succinylation as an efficient means of increasing the enzyme stability in IL.183 MCC hydrolysis in 15% (v/v) [BMIM]Cl showed significantly higher yields with succinylated T. reesei cellulase as compared to the native enzyme. Furthermore, cellulase succinylation led to lower cellulase binding to lignin.
Hydrolysis experiments in ionic liquid solutions
Activity and stability measurements in IL solutions are commonly made with soluble substrates and short hydrolysis times (from 5 min to some hours). The use of soluble substrates may not correspond well to total hydrolysis experiments with solid lignocellulosic substrates. For process development and research, it is necessary to study how the ILs affect the hydrolysis of real substrates during longer times. In the original work by Kamiya et al., cellulose regeneration from IL was observed to greatly increase hydrolysis rates, but the presence of the IL used, [EMIM]DEP, halted the subsequent enzymatic cellulose hydrolysis when the IL concentration was >40% (v/v).32 After this initial work, enzymatic hydrolysis of IL-pretreated substrates in the presence of various amounts of different ILs (one-pot procedure) has been studied with both cellulase cocktails and monocomponent cellulases.In the total enzymatic hydrolysis of regenerated cellulose and yellow poplar in 15% (v/v) [EMIM]AcO, an almost complete conversion of the regenerated cellulose was achieved, whereas IL-pretreated yellow poplar had lower hydrolysis yields (33%).144 The hydrolysis of regenerated filter paper in aqueous 10% [DMIM]DMP had doubled yields compared to the corresponding hydrolysis of untreated filter paper.147 When comparing untreated and from [DMIM]DMP regenerated α-cellulose hydrolysis in buffer and in 10, 20 and 30% (v/v) [DMIM]DMP, it was found that the initial hydrolysis rates were greater for regenerated cellulose even in 30% (v/v) [DMIM]DMP compared to untreated α-cellulose in buffer.132 However, in prolonged hydrolyses the increasing presence of IL lowered the hydrolysis yields. In the hydrolysis of IL-pretreated Miscanthus with T. reesei cellulase in aqueous [DMIM]DMP and [EMIM]lactate, [DMIM]DMP was found to be significantly more enzyme-compatible than [EMIM]lactate.146 Hydrolysis still took place in 50% (w/w) [DMIM]DMP. High hydrolysis yields have been reported in the hydrolysis of delignified and IL-treated bagasse in the presence of [EMIM]DEP.92 Li et al. reported the successful hydrolysis of straw, cotton and filter paper with a cellulase mixture in 15% (w/v) of [E(OH)MIM]AcO, an IL specially designed for combined cellulose-solubility and enzyme-compatibility.134 Auxenfans et al. showed that cellulose could be successfully hydrolysed by a T. reesei cellulase cocktail in a one-pot procedure with various amounts of 1-ethyl-3-methylimidazolium methylphosphonate ([EMIM]MeO(H)PO2).184 The highest hydrolysis yields were obtained in 10% (v/v) IL and comparably good yields were obtained in up to 40% (v/v) IL, but at higher IL concentrations hydrolysis yields were very low, in agreement with the results obtained previously by Kamiya et al.32
Studying the action of monocomponent cellulases on hydrolysis in IL solutions is necessary to gain in-depth knowledge on how the individual cellulases respond to the presence of IL. The effect of [EMIM]AcO and [DMIM]DMP on the hydrolytic action of T. reesei endoglucanases Cel5A and Cel7B has been studied in long (72 h) hydrolyses on untreated MCC and both ILs were found to be severely harmful to the hydrolysis.131 In 40% (v/v) of ILs there was almost no formation of soluble hydrolysis products. On the other hand, in 90% (v/v) [DMIM]DMP both endoglucanases were able to reduce the molecular weight of MCC, which was not observed in other hydrolysis matrices, including buffer. [DMIM]DMP did not appear to inactivate the endoglucanases irreversibly, and the treatment of MCC in 90% IL partly dissolved the substrate, rendering it accessible for intrachain scission. In a follow-up study it was found that [DMIM]DMP did not completely inactivate the endoglucanase Cel5A but rather slowed it down, whereas the enzymatic hydrolysis stopped completely in a matter of hours in aqueous [EMIM]AcO.185 In another study, T. reesei Cel12A hydrolysed MCC in low and high concentrations of [BMIM]Cl, but in media containing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [BMIM]Cl–water, this enzyme was virtually non-active in hydrolysis.151 In addition to imidazolium-based ILs enzymatic cellulose hydrolysis has also been studied in ILs consisting of [TMGH]+ and [DBNH]+ carboxylates.133 These ILs are interesting in combining the cellulose dissolution capability with being distillable. They were, however, found to be at least as harmful to the action of T. reesei Cel5A as the [EMIM]AcO used as the reference IL in this study.
1 [BMIM]Cl–water, this enzyme was virtually non-active in hydrolysis.151 In addition to imidazolium-based ILs enzymatic cellulose hydrolysis has also been studied in ILs consisting of [TMGH]+ and [DBNH]+ carboxylates.133 These ILs are interesting in combining the cellulose dissolution capability with being distillable. They were, however, found to be at least as harmful to the action of T. reesei Cel5A as the [EMIM]AcO used as the reference IL in this study.
The action of A. niger β-glucosidase (Novozyme 188) on cello-oligomer hydrolysis in aqueous [DMIM]DMP and [EMIM]AcO has been studied in long hydrolysis experiments.185 [EMIM]AcO was significantly more harmful to enzyme performance than [DMIM]DMP. The β-glucosidase was sensitive to the increase of pH but the different basicity of the studied ILs could not alone explain the difference in their impact on the enzyme. Long-time hydrolysis (72 h) of MCC with T. reesei cellobiohydrolase Cel7A and endoglucanase Cel5A has also been studied in aqueous [DMIM]DMP and [EMIM]AcO.154 In comparison, the cellobiohydrolase was somewhat more tolerant towards the presence of IL than the endoglucanase Cel5A.
New innovative concepts for enzymatic lignocellulose hydrolysis in IL matrices have recently been proposed. Klose et al. added a hyperthermophilic endoglucanase gene to induce cellulase production into a plant.164 The enzyme is not active under normal plant growth conditions, whereas under pretreatment conditions with high temperatures (90 °C) it becomes active and starts degrading the plant cell wall from the inside. This cellulase also had an extraordinary IL-tolerance, meaning that ILs can be used for pretreatment. The combination of IL pretreatment (with [EMIM]AcO, [EMIM]DEP and [EMIM]Cl), enzymatic hydrolysis and fermentation to ethanol in a one-pot procedure has been demonstrated by Nakashima et al.186 The employed yeast displayed cellulases on the cell surface, but additional free cellulases needed to be added for an efficient cellulose hydrolysis. The yeast was capable of fermenting the liberated saccharides to ethanol in a maximum IL concentration of 200 mM.
The one-pot or in situ hydrolysis is still a new concept and suffers from problems with enzyme activity in the presence of ILs. Cellulases with high IL-tolerance have, as has been described in this review, recently been reported and potentially offer a solution to this problem. Another challenge needing further research efforts is the separation of the released glucose from the IL-containing hydrolysate in an economical manner. For this purpose, the use of alumina column chromatography,187 liquid–liquid extraction using organic boronate carriers,188 and combined filtration and electrodialysis procedures189 has been proposed. In spite of many remaining challenges, one-pot hydrolysis appears as an interesting and relevant alternative to the previously suggested regeneration pathway for using ILs in lignocellulose saccharification.
Optimization of cellulase cocktails for use in ionic liquid solutions and on ionic liquid-pretreated substrates
Most commercial cellulase cocktails for total enzymatic hydrolysis of lignocellulose have fairly low tolerance towards biomass-dissolving ILs. Therefore, some research groups have optimized new cellulase mixtures for better performance in IL matrices. In the work of Park et al., an optimized cellulase cocktail (“JTherm”) consisting of thermophilic enzymes retained over 50% of its activity in 20% (w/v) [EMIM]AcO at 70 °C, as compared to aqueous conditions.106 Engel et al. optimized a cellulase cocktail for the hydrolysis of regenerated α-cellulose in the presence of 10% (v/v) [DMIM]DMP based on inactivation data acquired for the cellulases T. reesei Cel7A and Cel7B and A. niger β-glucosidase in this IL.150 In several articles whole cell cultures have been studied under conditions similar to those in the hydrolysis of IL-pretreated biomass for developing IL-tolerant enzyme systems.190–192 In addition to the need for IL-tolerant cellulase cocktails, the increased amorphous character of regenerated cellulosic substrates emphasizes the role of endoglucanases in total hydrolysis, as they apparently have much larger target regions in the substrate.150 Specific cellulases, such as the thermophilic Cel5A from Thermotoga maritima, have been subjected to random mutation experiments aimed at increasing their activity on especially IL-pretreated switchgrass.193 Also more complex cocktails with various cellulase and hemicellulase activities have been optimized for the hydrolysis of IL-regenerated lignocellulose.82Analysis of hydrolysis products in ionic liquid solutions
The presence of IL in enzymatic hydrolysates has in several studies been found challenging for saccharide analytics.185,194,195 Accurate and high-throughput analytics are, however, needed for following hydrolysis kinetics and formed saccharide product distributions. Typically, the yields of released saccharides are quantified by spectrophotometric assays such as the 3,5-dinitrosalicylic acid (DNS)196 or para-hydroxybenzoic acid hydrazide (PAHBAH)197 assays, which have the benefit of allowing rapid analysis of reducing saccharides, but suffer from drawbacks such as not being able to discriminate between oligomers of different lengths and types. For more accurate analyses of saccharide compositions in liquid samples, high performance liquid chromatography (HPLC), gas chromatography (GC) and capillary electrophoresis (CE) techniques are used. In chromatography, the presence of IL has been reported to be problematic as the columns only tolerate low amounts of salts.194 ILs may also give rise to broad peaks which overlap with the saccharide peaks in the chromatograms and the retention times varied with sample composition.194 ILs have been used as components in the background electrolyte in CE when analysing saccharides, but with IL concentrations higher than 20–30 mM, baseline fluctuations were observed.198The presence of IL appears to interfere with the DNS assay at least in high concentrations (over 40%, v/v),185 although it has been shown by measuring the saccharide standard curves in IL solutions that the IL does not affect analysis results when the samples contain 0–20% (v/v) [EMIM]AcO.144 ILs can cause background absorption interference due to its own colour and apparently also interfere with the colour forming reaction by reacting with the DNS reagent, although it is not clear whether it is the IL itself or some impurity in it that causes the additional colour.152 ILs have also been reported to interfere with other spectroscopic techniques, such as circular dichroism (CD), used for studying conformational changes in the enzyme structure.158
Sugar derivatization with aromatic amines has been used in several studies as a means to increase the saccharide detectability in the presence of ILs in HPLC or CE.32,47,185 The increased selectivity of the detection is highly beneficial in reducing IL interference. Both the spectrophotometric assays and many of the derivatization methods are based on reactions with the reducing end of the saccharides. It has, however, been shown that the imidazolium cations in some ILs form carbenes which react with the reducing ends of the saccharides.199 If this reaction takes place to a significant extent, it will alter the analysis results. This reaction has not been studied in further detail in IL-containing hydrolysates. It is surprising how little the influence of ILs on saccharide analytics has been commented on in the literature, taking into account the large number of articles in which IL-containing hydrolysates have been analysed.
Summary and outlook
It is just over a decade since cellulose dissolution in ILs was first reported rapidly followed by the observation that cellulases are inactivated in ILs. In the recent literature, a more detailed picture has been drawn about the mechanisms and reasons for the apparent cellulase inactivation, but several pieces are still missing from the puzzle. Deeper insights into the molecular scale mechanisms of cellulase inactivation in cellulose-dissolving ILs are still needed in order to be able to design fully compatible systems in which cellulases are truly active in cellulose hydrolysis in the presence of significant amounts of IL. Several groups have reported impressive results on the discovery and development of IL-tolerant cellulases. It should be mentioned that cellulase activity has been studied in other cellulose solvents, such as NMMO200 and deep eutectic solvents (DES),201 which are currently gaining considerable interest as an extension to the IL family. It appears that some of these alternative cellulose solvents display higher enzyme-compatibility than the cellulose-dissolving ILs.The most important challenges in applying ILs in lignocellulose pretreatment for the biotechnical production of biofuels and -chemicals from lignocellulose are now in finding economical solutions to IL recycling and studying their effects on health and the environment, which so far has been much overlooked. Techno-economic evaluations have shown that co-product streams need to be obtained from the hydrolysis step in addition to the glucose main product for an IL-based biorefinery to be economically viable.59
Many lignocellulose-hydrolysing enzymes have recently been reported to have high IL-tolerance, but so far the general cellulolytic activity of these enzymes has scarcely been compared. Having high IL-tolerance is not sufficient for application in industrial processes, if the enzyme itself has low catalytic activity, or the enzymes are difficult to produce with a similar cost efficiency as the traditional cellulase preparations. A logical next step in the development of IL-tolerant glycosyl hydrolases is to increase their general activity while maintaining their tolerance to IL, and in the long run, to find good production hosts. In addition to the impressive advances made in finding IL-tolerant cellulases for the one-pot hydrolysis, advances have also been made in developing the IL pretreatment technology, e.g. in introducing pretreatment methods with very high biomass loadings and using aqueous ILs instead of dry ILs. The recent advances in this field of biomass processing are highly promising for the future design of biorefinery processes with IL technology.
Acknowledgements
The authors gratefully acknowledge VTT Graduate School and the Finnish Bioeconomy Cluster's (FIBIC) Future Biorefinery (FuBio) programme for financial support for this article.Notes and references
- R. D. Perlack, L. L. Wright, A. F. Turhollow, R. L. Graham, J. B. Stokes and D. C. Erbach, Biomass as feedstock for a bioenergy and bioproducts industry: the technical feasibility of a billion-ton annual supply, 2005, DOE/GO-102995-2135.
- A. Stark, Energy Environ. Sci., 2011, 4, 19–32 CAS.
- J. Larsen, M. T. Haven and L. Thirup, Biomass Bioenergy, 2012, 46, 36–45 CrossRef CAS PubMed.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS PubMed.
- A. C. O'Sullivan, Cellulose, 1997, 4, 173–207 CrossRef.
- R. Alén, in Forest Products Chemistry, ed. P. Stenius, Fapet OY, Helsinki, Finland, 2000, pp. 12–57 Search PubMed.
- Y. P. Zhang and L. R. Lynd, Biotechnol. Bioeng., 2004, 88, 797–824 CrossRef CAS PubMed.
- M. M. Campbell and R. R. Sederoff, Plant Physiol., 1996, 110, 3–13 CAS.
- A. Björkman, Svensk Papperstidn., 1957, 60, 243–251 Search PubMed.
- A. V. Bridgwater, Biomass Bioenergy, 2012, 38, 68–94 CrossRef CAS PubMed.
- R. E. H. Sims, W. Mabee, J. N. Saddler and M. Taylor, Bioresour. Technol., 2010, 101, 1570–1580 CrossRef CAS PubMed.
- A. P. Dadi, S. Varanasi and C. A. Schall, Biotechnol. Bioeng., 2006, 95, 904–910 CrossRef CAS PubMed.
- C. E. Wyman, B. E. Dale, R. T. Elander, M. Holtzapple, M. R. Ladisch and Y. Y. Lee, Bioresour. Technol., 2005, 96, 1959–1966 CrossRef CAS PubMed.
- J. B. Binder and R. T. Raines, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4516–4521 CrossRef CAS PubMed.
- H. B. Klinke, A. B. Thomsen and B. K. Ahring, Appl. Microbiol. Biotechnol., 2004, 66, 10–26 CrossRef CAS PubMed.
- S. J. Horn, G. Vaaje-Kolstad, B. Westereng and V. G. H. Eijsink, Biotechnol. Biofuels, 2012, 5, 45 CrossRef CAS PubMed.
- T. W. Jeffries, Curr. Opin. Biotechnol., 2006, 17, 320–326 CrossRef CAS PubMed.
- J. Park, S. Kim, H. Park, J. S. Kim and J. Yoon, Renewable Energy, 2014, 65, 213–218 CrossRef CAS PubMed.
- E. Margolles-clark, M. Ihnen and M. Penttilä, J. Biotechnol., 1997, 57, 167–179 CrossRef CAS.
- H. van Tilbeurgh, P. Tomme, M. Claeyssens, R. Bhikhabhai and G. Pettersson, FEBS Lett., 1986, 204, 223–227 CrossRef CAS.
- P. Tomme, H. van Tilbeurgh, G. Petterson, J. van Damme, J. Vandekerckhove, J. Knowles, T. Teeri and M. Clayessens, Eur. J. Biochem., 1988, 170, 575–581 CrossRef CAS PubMed.
- J. Gorke, F. Srienc and R. Kazlauskas, Biotechnol. Bioprocess Eng., 2010, 15, 40–53 CrossRef CAS.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC.
- F. van Rantwijk and R. A. Sheldon, Chem. Rev., 2007, 107, 2757–2785 CrossRef CAS PubMed.
- Z. Yang and W. Pan, Enzyme Microb. Technol., 2005, 37, 19–28 CrossRef CAS PubMed.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- M. Zavrel, D. Bross, M. Funke, J. Büchs and A. C. Spiess, Bioresour. Technol., 2009, 100, 2580–2587 CrossRef CAS PubMed.
- D. A. Fort, R. C. Remsing, R. P. Swatloski, P. Moyna, G. Moyna and R. D. Rogers, Green Chem., 2007, 9, 63–69 RSC.
- I. Kilpeläinen, H. Xie, A. King, M. Granström, S. Heikkinen and D. S. Argyropoulos, J. Agric. Food Chem., 2007, 55, 9142–9148 CrossRef PubMed.
- X. Honglu and S. Tiejun, Holzforschung, 2006, 60, 509–512 CrossRef CAS.
- S. Singh, B. A. Simmons and K. P. Vogel, Biotechnol. Bioeng., 2009, 104, 68–75 CrossRef CAS PubMed.
- N. Kamiya, Y. Matsushita, M. Hanaki, K. Nakashima, M. Narita, M. Goto and H. Takahashi, Biotechnol. Lett., 2008, 30, 1037–1040 CrossRef CAS PubMed.
- M. B. Turner, S. K. Spear, J. G. Huddleston, J. D. Holbrey and R. D. Rogers, Green Chem., 2003, 5, 443–447 RSC.
- H.-P. Fink, P. Weigel, H. J. Purz and J. Ganster, Prog. Polym. Sci., 2001, 26, 1473–1524 CrossRef CAS.
- T. Heinze and T. Liebert, Prog. Polym. Sci., 2001, 26, 1689–1762 CrossRef CAS.
- C. Graenacher, Cellulose solution, US Pat, 1943176, 1934 Search PubMed.
- H. Zhang, J. Wu, J. Zhang and J. He, Macromolecules, 2005, 38, 8272–8277 CrossRef CAS.
- J. Wu, J. Zhang, H. Zhang, J. He, Q. Ren and M. Guo, Biomacromolecules, 2004, 5, 266–268 CrossRef CAS PubMed.
- M. Mazza, D. Catana, C. Vaca-Garcia and C. Cecutti, Cellulose, 2009, 16, 207–215 CrossRef CAS.
- P. Mäki-Arvela, I. Anugwom, P. Virtanen, R. Sjöholm and J. P. Mikkola, Ind. Crops Prod., 2010, 32, 175–201 CrossRef PubMed.
- S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Energy Fuels, 2010, 24, 737–745 CrossRef CAS.
- Z. Z. Chowdhury, S. Mohd. Zain, S. B. Abd Hamid and K. Khalid, Bioresources, 2014, 9, 1787–1823 CrossRef PubMed.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- A. W. T. King, J. Asikkala, I. Mutikainen, P. Järvi and I. Kilpeläinen, Angew. Chem., Int. Ed., 2011, 50, 6301–6305 CrossRef CAS PubMed.
- A. Parviainen, A. W. T. King, I. Mutikainen, M. Hummel, C. Selg, L. K. J. Hauru, H. Sixta and I. Kilpeläinen, ChemSusChem, 2013, 6, 2161–2169 CrossRef CAS PubMed.
- Uju, A. Nakamoto, Y. Shoda, M. Goto, W. Tokuhara, Y. Noritake, S. Katahira, N. Ishida, C. Ogino and N. Kamiya, Bioresour. Technol., 2013, 135, 103–108 CrossRef CAS PubMed.
- K. Ohira, Y. Abe, M. Kawatsura, K. Suzuki, M. A. Mizuno, Y. Amano and T. Itoh, ChemSusChem, 2012, 5, 388–391 CrossRef CAS PubMed.
- A. J. Holding, M. Heikkilä, I. Kilpeläinen and A. W. T. King, ChemSusChem, 2014, 7, 1422–1434 CrossRef CAS PubMed.
- H. Zhao, G. A. Baker, Z. Song, O. Olubajo, T. Crittle and D. Peters, Green Chem., 2008, 10, 696–705 RSC.
- H. Zhao, C. L. Jones and J. V. Cowins, Green Chem., 2009, 11, 1128–1138 RSC.
- S. Tang, G. A. Baker, S. Ravula, J. E. Jones and H. Zhao, Green Chem., 2012, 14, 2922–2932 RSC.
- T. V. Doherty, M. Mora-Pale, S. E. Foley, R. J. Linhardt and J. S. Dordick, Green Chem., 2010, 12, 1967–1975 RSC.
- M. Mora-Pale, L. Meli, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2011, 108, 1229–1245 CrossRef CAS PubMed.
- H. Liu, K. L. Sale, B. M. Holmes, B. A. Simmons and S. Singh, J. Phys. Chem. B, 2010, 114, 4293–4301 CrossRef CAS PubMed.
- T. G. A. Youngs, C. Hardacre and J. D. Holbrey, J. Phys. Chem. B, 2007, 111, 13765–13774 CrossRef CAS PubMed.
- R. C. Remsing, G. Hernandez, R. P. Swatloski, W. W. Massefski, R. D. Rogers and G. Moyna, J. Phys. Chem. B, 2008, 112, 11071–11078 CrossRef CAS PubMed.
- J. L. Anderson, J. Ding, T. Welton and D. W. Armstrong, J. Am. Chem. Soc., 2002, 124, 14247–14254 CrossRef CAS PubMed.
- D. Klein-Marcuschamer, B. A. Simmons and H. W. Blanch, Biofuels, Bioprod. Biorefin., 2011, 5, 562–569 CrossRef CAS.
- R. P. Chandra, R. Bura, W. E. Mabee, A. Berlin, X. Pan and J. N. Saddler, Adv. Biochem. Eng. Biotechnol., 2007, 108, 67–93 CrossRef CAS.
- H. E. Grethlein, Nat. Biotechnol., 1985, 3, 155–160 CrossRef CAS PubMed.
- C. A. Mooney, S. D. Mansfield, M. G. Touhy and J. N. Saddler, Bioresour. Technol., 1998, 64, 113–119 CrossRef CAS.
- R. Sutcliffe and J. N. Saddler, Biotechnol. Bioeng. Symp., 1986, 17, 749–762 CAS.
- T. M. Wood, Biotechnol. Bioeng. Symp., 1975, 5, 111–137 CAS.
- K. Shill, S. Padmanabhan, Q. Xin, J. M. Prausnitz, D. S. Clark and H. W. Blanch, Biotechnol. Bioeng., 2011, 108, 511–520 CrossRef CAS PubMed.
- H. W. Blanch, B. A. Simmons and D. Klein-Marcuschamer, Biotechnol. J., 2011, 6, 1086–1102 CrossRef CAS PubMed.
- J. Kudakasseril Kurian, G. Raveendran Nair, A. Hussain and G. S. Vijaya Raghavan, Renewable Sustainable Energy Rev., 2013, 25, 205–219 CrossRef CAS PubMed.
- N. Mosier, C. Wyman, B. Dale, R. Elander, Y. Y. Lee, M. Holtzapple and M. Ladisch, Bioresour. Technol., 2005, 96, 673–686 CrossRef CAS PubMed.
- A. P. Dadi, C. A. Schall and S. Varanasi, Appl. Biochem. Biotechnol., 2007, 137, 407–421 CrossRef PubMed.
- F. Yang, L. Li, Q. Li, W. Tan, W. Liu and M. Xian, Carbohydr. Polym., 2010, 81, 311–316 CrossRef CAS PubMed.
- R. Q. Xie, X. Y. Li and Y. F. Zhang, Cellul. Chem. Technol., 2012, 46, 349–356 CAS.
- P. Lozano, B. Bernal, I. Recio and M. Belleville, Green Chem., 2012, 14, 2631–2637 RSC.
- H. Zhao, C. L. Jones, G. A. Baker, S. Xia, O. Olubajo and V. N. Person, J. Biotechnol., 2009, 139, 47–54 CrossRef CAS PubMed.
- P. Engel, L. Hein and A. C. Spiess, Biotechnol. Biofuels, 2012, 5, 77 CrossRef CAS PubMed.
- Q. Li, Y. He, M. Xian, G. Jun, X. Xu, J. Yang and L. Li, Bioresour. Technol., 2009, 100, 3570–3575 CrossRef CAS PubMed.
- L. Liu and H. Chen, Chin. Sci. Bull., 2006, 51, 2432–2436 CrossRef CAS PubMed.
- Q. Liu, X. Hou, N. Li and M. Zong, Green Chem., 2012, 14, 304–307 RSC.
- X. Hou, T. J. Smith, N. Li and M. Zong, Biotechnol. Bioeng., 2012, 109, 2484–2493 CrossRef CAS PubMed.
- X. Hou, N. Li and M. Zong, Bioresour. Technol., 2013, 136, 469–474 CrossRef CAS PubMed.
- X. Hou, N. Li and M. Zong, Biotechnol. Bioeng., 2013, 110, 1895–1902 CrossRef CAS PubMed.
- G. Cheng, P. Varanasi, C. Li, H. Liu, Y. B. Melnichenko, B. A. Simmons, M. S. Kent and S. Singh, Biomacromolecules, 2011, 12, 933–941 CrossRef CAS PubMed.
- C. J. Barr, J. A. Mertens and C. A. Schall, Bioresour. Technol., 2012, 104, 480–485 CrossRef CAS PubMed.
- A. Goshadrou, K. Karimi and M. Lefsrud, Carbohydr. Polym., 2013, 96, 440–449 CrossRef CAS PubMed.
- S. H. Lee, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2009, 102, 1368–1376 CrossRef CAS PubMed.
- Y. Sun, J. Xu, F. Xu and R. Sun, Process Biochem., 2013, 48, 844–852 CrossRef CAS PubMed.
- N. Sathitsuksanoh, Z. Zhu and Y.-H. P. Zhang, Cellulose, 2012, 19, 1161–1172 CrossRef CAS.
- X. Geng and W. A. Henderson, Biotechnol. Bioeng., 2012, 109, 84–91 CrossRef CAS PubMed.
- H. Wu, M. Mora-Pale, J. Miao, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2011, 108, 2865–2875 CrossRef CAS PubMed.
- D. Groff, A. George, N. Sun, N. Sathitsuksanoh, G. Bokinsky, B. A. Simmons, B. M. Holmes and J. D. Keasling, Green Chem., 2013, 15, 1264–1267 RSC.
- Z. Qiu and G. Aita, Bioresour. Technol., 2013, 129, 532–537 CrossRef CAS PubMed.
- J. Bian, F. Peng, X. Peng, X. Xiao, P. Peng, F. Xu and R. Sun, Carbohydr. Polym., 2014, 100, 211–217 CrossRef CAS PubMed.
- C. Su, M. Chung, H. Hsieh, Y. Chang, J. Ding, H. Wu and J. Taiwan, Inst. Chem. Eng. Symp. Ser., 2012, 43, 573–577 CAS.
- F. Hong, X. Guo, S. Zhang, S. Han, G. Yang and L. J. Jönsson, Bioresour. Technol., 2012, 104, 503–508 CrossRef CAS PubMed.
- J. Holm, U. Lassi, H. Romar, R. Lahti, J. Kärkkäinen and M. Lajunen, Catal. Today, 2012, 196, 11–15 CrossRef CAS PubMed.
- N. Sathitsuksanoh, A. George and Y. P. Zhang, J. Chem. Technol. Biotechnol., 2013, 88, 169–180 CrossRef CAS.
- H. Tadesse and R. Luque, Energy Environ. Sci., 2011, 4, 3913–3929 CAS.
- T. Vancov, A. Alston, T. Brown and S. McIntosh, Renewable Energy, 2012, 45, 1–6 CrossRef CAS PubMed.
- K. Ninomiya, T. Yamauchi, M. Kobayashi, C. Ogino, N. Shimizu and K. Takahashi, Biochem. Eng. J., 2013, 71, 25–29 CrossRef CAS PubMed.
- C. Kuo and C. Lee, Carbohydr. Polym., 2009, 77, 41–46 CrossRef CAS PubMed.
- S. H. Ha, N. L. Mai and Y. M. Koo, Bioresour. Technol., 2011, 102, 1214–1219 CrossRef CAS PubMed.
- M. Wada, M. Ike and K. Tokuyasu, Polym. Degrad. Stab., 2010, 95, 543–548 CrossRef CAS PubMed.
- L. Li, S.-T. Yu, F.-S. Liu, C.-X. Xie and C.-Z. Xu, Bioresources, 2011, 6, 4494–4504 CAS.
- C. Sievers, M. Valenzuela-Olarte, T. Marzialetti, I. Musin, P. K. Agrawal and C. W. Jones, Ind. Eng. Chem. Res., 2009, 48, 1277–1286 CrossRef CAS.
- N. M. Konda, J. Shi, S. Singh, H. W. Blanch, B. A. Simmons and D. Klein-Marcuschamer, Biotechnol. Biofuels, 2014, 7, 86 CrossRef PubMed.
- P. Oleskowicz-Popiel, D. Klein-Marcuschamer, B. A. Simmons and H. W. Blanch, Bioresour. Technol., 2014, 158, 294–299 CrossRef CAS PubMed.
- J. I. Park, E. J. Steen, H. Burd, S. S. Evans, A. M. Redding-Johnson, T. Batth, P. I. Benke, P. D'haeseleer, N. Sun, K. L. Sale, J. D. Keasling, T. S. Lee, C. J. Petzold, A. Mukhopadhyay, S. W. Singer, B. A. Simmons and J. M. Gladden, PLoS One, 2012, 7, e37010 CAS.
- C. He, S. Li, H. Liu, K. Li and F. Liu, J. Chromatogr., A, 2005, 1082, 143–149 CrossRef CAS PubMed.
- S. Li, C. He, H. Liu, K. Li and F. Liu, J. Chromatogr., B: Biomed. Appl., 2005, 826, 58–62 CrossRef CAS PubMed.
- K. E. Gutowski, G. A. Broker, H. D. Willauer, J. G. Huddleston, R. P. Swatloski, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2003, 125, 6632–6633 CrossRef CAS PubMed.
- N. Sun, H. Liu, N. Sathitsuksanoh, V. Stavila, M. Sawant, A. Bonito, K. Tran, A. George, K. Sale, S. Singh, B. Simmons and B. Holmes, Biotechnol. Biofuels, 2013, 6, 39 CrossRef CAS PubMed.
- S. Xia, G. A. Baker, H. Li, S. Ravula and H. Zhao, RSC Adv., 2014, 4, 10586–10596 RSC.
- A. Zaks and A. M. Klibanov, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 3192–3196 CrossRef CAS.
- D. Magnuson, J. Bodley and D. F. Evans, J. Solution Chem., 1984, 13, 583–587 CrossRef CAS.
- U. Kragl, M. Eckstein and N. Kaftzik, Curr. Opin. Biotechnol., 2002, 13, 565–571 CrossRef CAS.
- M. Naushad, Z. A. ALOthman, A. B. Khan and M. Ali, Int. J. Biol. Macromol., 2012, 51, 555–560 CrossRef CAS PubMed.
- M. Moniruzzaman, K. Nakashima, N. Kamiya and M. Goto, Biochem. Eng. J., 2010, 48, 295–314 CrossRef CAS PubMed.
- R. Jaenicke, Naturwissenschaften, 1996, 83, 544–554 CrossRef CAS.
- H. Zhao, O. Olubajo, Z. Song, A. L. Sims, T. E. Person, R. A. Lawal and L. A. Holley, Bioorg. Chem., 2006, 34, 15–25 CrossRef CAS PubMed.
- J. L. Kaar, A. M. Jesionowski, J. A. Berberich, R. Moulton and A. J. Russell, J. Am. Chem. Soc., 2003, 125, 4125–4131 CrossRef CAS PubMed.
- H. Zhao, J. Chem. Technol. Biotechnol., 2010, 85, 891–907 CrossRef CAS.
- K. Fujita, D. R. MacFarlane and M. Forsyth, Chem. Commun., 2005, 4804–4806 RSC.
- C. A. Summers and R. A. Flowers 2nd, Protein Sci., 2000, 9, 2001–2008 CrossRef CAS PubMed.
- S. H. Lee, S. H. Ha, S. B. Lee and Y. Koo, Biotechnol. Lett., 2006, 28, 1335–1339 CrossRef CAS PubMed.
- S. Park and R. J. Kazlauskas, J. Org. Chem., 2001, 66, 8395–8401 CrossRef CAS PubMed.
- F. Hofmeister, Arch. Exp. Pathol. Pharmakol., 1888, 24, 247–260 Search PubMed.
- D. Constantinescu, H. Weingärtner and C. Herrmann, Angew. Chem., Int. Ed., 2007, 46, 8887–8889 CrossRef CAS PubMed.
- J. Lai, Z. Li, Y. Lu and Z. Yang, Green Chem., 2011, 13, 1860–1868 RSC.
- M. Moniruzzaman, N. Kamiya and M. Goto, Org. Biomol. Chem., 2010, 8, 2887–2899 CAS.
- R. A. Sheldon, R. M. Lau, M. J. Sorgedrager, F. van Rantwijk and K. R. Seddon, Green Chem., 2002, 4, 147–151 RSC.
- H. Karbalaei-Heidari, M. Shahbazi and G. Absalan, Appl. Biochem. Biotechnol., 2013, 170, 573–586 CrossRef CAS PubMed.
- R. Wahlström, S. Rovio and A. Suurnäkki, RSC Adv., 2012, 2, 4472–4480 RSC.
- P. Engel, R. Mladenov, H. Wulfhorst, G. Jäger and A. C. Spiess, Green Chem., 2010, 12, 1959–1966 RSC.
- R. Wahlström, A. King, A. Parviainen, K. Kruus and A. Suurnäkki, RSC Adv., 2013, 3, 20001–20009 RSC.
- L. Li, J. Xie, S. Yu, Z. Su, S. Liu, F. Liu, C. Xie and B. Zhang, RSC Adv., 2012, 2, 11712–11718 RSC.
- H. Zhao, L. Jackson, Z. Song and O. Olubajo, Tetrahedron: Asymmetry, 2006, 17, 2491–2498 CrossRef CAS PubMed.
- S. Bose, D. W. Armstrong and J. W. Petrich, J. Phys. Chem. B, 2010, 114, 8221–8227 CrossRef CAS PubMed.
- Z.-G. Chen, M.-H. Zong and G.-J. Li, J. Chem. Technol. Biotechnol., 2006, 81, 1225–1231 CrossRef CAS.
- R. Rinaldi, Chem. Commun., 2011, 47, 511–513 RSC.
- K. Ohira, K. Yoshida, S. Hayase and T. Itoh, Chem. Lett., 2012, 41, 987–989 CrossRef CAS.
- S. Bose, C. A. Barnes and J. W. Petrich, Biotechnol. Bioeng., 2012, 109, 434–443 CrossRef CAS PubMed.
- V. W. Jaeger and J. Pfaendtner, ACS Chem. Biol., 2013, 8, 1179–1186 CrossRef CAS PubMed.
- M. Paljevac, M. Habulin and Z. Knez, Chem. Ind. Chem. Eng. Q., 2006, 12, 181–186 CrossRef CAS.
- A. C. Salvador, M. d. C. Santos and J. A. Saraiva, Green Chem., 2010, 12, 632–635 RSC.
- Y. Wang, M. Radosevich, D. Hayes and N. Labbé, Biotechnol. Bioeng., 2011, 108, 1042–1048 CrossRef CAS PubMed.
- G. Ebner, P. Vejdovszky, R. Wahlström, A. Suurnäkki, M. Schrems, P. Kosma, T. Rosenau and A. Potthast, J. Mol. Catal. B: Enzym., 2014, 99, 121–129 CrossRef CAS PubMed.
- P. W. Wolski, D. S. Clark and H. W. Blanch, Green Chem., 2011, 13, 3107–3110 RSC.
- S. Zhi, Y. Liu, X. Yu, X. Wang and X. Lu, Energy Procedia, 2012, 14, 1741–1747 CrossRef PubMed.
- P. Magdolen and A. Vasella, Helv. Chim. Acta, 2005, 88, 2454–2469 CrossRef CAS.
- N. Ilmberger, J. Pottkämper and W. R. Streit, Catalysts, 2013, 3, 584–587 CrossRef CAS PubMed.
- P. Engel, S. Krull, B. Seiferheld and A. C. Spiess, Bioresour. Technol., 2012, 115, 27–34 CrossRef CAS PubMed.
- P. D'Arrigo, C. Allegretti, S. Tamborini, C. Formantici, Y. Galante, L. Pollegioni and A. Mele, J. Mol. Catal. B: Enzym., 2014, 106, 76–80 CrossRef PubMed.
- R. Wahlström, PhD Thesis, VTT – Technical Research Centre of Finland, 2014.
- J. Pottkämper, P. Barthen, N. Ilmberger, U. Schwaneberg, A. Schenk, M. Schulte, N. Ignatiev and W. R. Streit, Green Chem., 2009, 11, 957–965 RSC.
- R. Wahlström, J. Rahikainen, K. Kruus and A. Suurnäkki, Biotechnol. Bioeng., 2014, 111, 726–733 CrossRef PubMed.
- H. Li, A. Kankaanpää, H. Xiong, M. Hummel, H. Sixta, H. Ojamo and O. Turunen, Enzyme Microb. Technol., 2013, 53, 414–419 CrossRef CAS PubMed.
- N. Kaftzik, P. Wasserscheid and U. Kragl, Org. Process Res. Dev., 2002, 6, 553–557 CrossRef CAS.
- M. Lang, T. Kamrat and B. Nidetzky, Biotechnol. Bioeng., 2006, 95, 1093–1100 CrossRef CAS PubMed.
- M. Sandoval, A. Cortes, C. Civera, J. Trevino, E. Ferreras, M. Vaultier, J. Berenguer, P. Lozano and M. J. Hernaiz, RSC Adv., 2012, 2, 6306–6314 RSC.
- M. F. Thomas, L. Li, J. M. Handley-Pendleton, D. van der Lelie, J. J. Dunn and J. F. Wishart, Bioresour. Technol., 2011, 102, 11200–11203 CrossRef CAS PubMed.
- S. Ferdjani, M. Ionita, B. Roy, M. Dion, Z. Djeghaba, C. Rabiller and C. Tellier, Biotechnol. Lett., 2011, 33, 1215–1219 CrossRef CAS PubMed.
- M. G. Adsul, A. P. Terwadkar, A. J. Varma and D. V. Gokhale, Bioresources, 2009, 4, 1670–1681 CAS.
- N. Ilmberger, D. Meske, J. Juergensen, M. Schulte, P. Barthen, U. Rabausch, A. Angelov, M. Mientus, W. Liebl, R. A. Schmitz and W. R. Streit, Appl. Microbiol. Biotechnol., 2012, 95, 135–146 CrossRef CAS PubMed.
- S. Datta, B. Holmes, J. I. Park, Z. Chen, D. C. Dibble, M. Hadi, H. W. Blanch, B. A. Simmons and R. Sapra, Green Chem., 2010, 12, 338–345 RSC.
- H. Klose, J. Roder, M. Girfoglio, R. Fischer and U. Commandeur, Biotechnol. Biofuels, 2012, 5, 63 CrossRef CAS PubMed.
- C. Liang, Y. Xue, M. Fioroni, F. Rodrìguez-Ropero, C. Zhou, U. Schwaneberg and Y. Ma, Appl. Microbiol. Biotechnol., 2011, 89, 315–326 CrossRef CAS PubMed.
- J. M. Gladden, M. Allgaier, C. S. Miller, T. C. Hazen, J. S. VanderGheynst, P. Hugenholtz, B. A. Simmons and S. W. Singer, Appl. Environ. Microbiol., 2011, 77, 5804–5812 CrossRef CAS PubMed.
- N. Trivedi, V. Gupta, C. R. K. Reddy and B. Jha, Bioresour. Technol., 2013, 132, 313–319 CrossRef CAS PubMed.
- N. Trivedi, V. Gupta, M. Kumar, P. Kumari, C. R. K. Reddy and B. Jha, Chemosphere, 2011, 83, 706–712 CrossRef CAS PubMed.
- N. Raddadi, A. Cherif, D. Daffonchio and F. Fava, Bioresour. Technol., 2013, 150, 121–128 CrossRef CAS PubMed.
- A. A. N. Gunny, D. Arbain, R. Edwin Gumba, B. C. Jong and P. Jamal, Bioresour. Technol., 2014, 155, 177–181 CrossRef CAS PubMed.
- T. Zhang, S. Datta, J. Eichler, N. Ivanova, S. D. Axen, C. A. Kerfeld, F. Chen, N. Kyrpides, P. Hugenholtz, J. Cheng, K. L. Sale, B. Simmons and E. Rubin, Green Chem., 2011, 13, 2083–2090 RSC.
- J. Gladden, J. Park, J. Bergmann, V. Reyes-Ortiz, P. D'haeseleer, B. Quirino, K. Sale, B. Simmons and S. Singer, Biotechnol. Biofuels, 2014, 7, 15 CrossRef PubMed.
- J. Xu, B. He, B. Wu, B. Wang, C. Wang and L. Hu, Bioresour. Technol., 2014, 157, 166–173 CrossRef CAS PubMed.
- M. Lang, T. Kamrat and B. Nidetzky, Biotechnol. Bioeng., 2006, 95, 1093–1100 CrossRef CAS PubMed.
- M. Kudou, Y. Kubota, N. Nakashima, F. Okazaki, K. Nakashima, C. Ogino and A. Kondo, J. Mol. Catal. B: Enzym., 2014, 104, 17–22 CrossRef CAS PubMed.
- C. Lehmann, M. Bocola, W. R. Streit, R. Martinez and U. Schwaneberg, Appl. Microbiol. Biotechnol., 2014, 98, 5775–5785 CrossRef CAS PubMed.
- P. O. Jones and P. T. Vasudevan, Biotechnol. Lett., 2010, 32, 103–106 CrossRef CAS PubMed.
- J. J. Fei, Q. Li, Y. Y. Feng, G. S. Ji, X. D. Gu, T. C. Li and Y. Liu, Appl. Mech. Mater., 2013, 361–363, 339–342 CrossRef.
- P. Lozano, B. Bernal, J. M. Bernal, M. Pucheault and M. Vaultier, Green Chem., 2011, 13, 1406–1410 RSC.
- Z. Su, X. Yang, Luli, H. Shao and S. Yu, Afr. J. Microbiol. Res., 2012, 6, 64–70 CAS.
- L. Li, J. Xie, S. Yu, Z. Su, S. Liu, F. Liu, C. Xie, B. Zhang and C. Zhang, Green Chem., 2013, 15, 1624–1630 RSC.
- M. Yoshimoto, K. Tanimura, K. Tokunaga and A. Kamimura, Biotechnol. Prog., 2013, 29, 1190–1196 CrossRef CAS PubMed.
- E. M. Nordwald, R. Brunecky, M. E. Himmel, G. T. Beckham and J. L. Kaar, Biotechnol. Bioeng., 2014, 111, 1541–1549 CrossRef CAS PubMed.
- T. Auxenfans, S. Buchoux, K. Djellab, C. Avondo, E. Husson and C. Sarazin, Carbohydr. Polym., 2012, 90, 805–813 CrossRef CAS PubMed.
- R. Wahlström, S. Rovio and A. Suurnäkki, Carbohydr. Res., 2013, 373, 42–51 CrossRef PubMed.
- K. Nakashima, K. Yamaguchi, N. Taniguchi, S. Arai, R. Yamada, S. Katahira, N. Ishida, H. Takahashi, C. Ogino and A. Kondo, Green Chem., 2011, 13, 2948–2953 RSC.
- D. Feng, L. Li, F. Yang, W. Tan, G. Zhao, H. Zou, M. Xian and Y. Zhang, Appl. Microbiol. Biotechnol., 2011, 91, 399–405 CrossRef CAS PubMed.
- T. R. Brennan, S. Datta, H. Blanch, B. Simmons and B. Holmes, Bioenergy Res., 2010, 3, 123–133 CrossRef.
- C. Abels, K. Thimm, H. Wulfhorst, A. C. Spiess and M. Wessling, Bioresour. Technol., 2013, 149, 58–64 CrossRef CAS PubMed.
- C. W. Simmons, A. P. Reddy, J. S. VanderGheynst, B. A. Simmons and S. W. Singer, Biotechnol. Prog., 2014, 30, 311–316 CrossRef CAS PubMed.
- A. P. Reddy, C. W. Simmons, J. Claypool, L. Jabusch, H. Burd, M. Z. Hadi, B. A. Simmons, S. W. Singer and J. S. VanderGheynst, J. Appl. Microbiol., 2012, 113, 1362–1370 CrossRef CAS PubMed.
- S. W. Singer, A. P. Reddy, J. M. Gladden, H. Guo, T. C. Hazen, B. A. Simmons and J. S. VanderGheynst, J. Appl. Microbiol., 2011, 110, 1023–1031 CrossRef CAS PubMed.
- Z. Chen, J. H. Pereira, H. Liu, H. M. Tran, N. S. Y. Hsu, D. Dibble, S. Singh, P. D. Adams, R. Sapra, M. Z. Hadi, B. A. Simmons and K. L. Sale, PLoS One, 2013, 8, e79725 Search PubMed.
- S. Hyvärinen, P. Damlin, J. Gräsvik, D. Y. Murzin and J. Mikkola, Cellul. Chem. Technol., 2011, 45, 483–486 Search PubMed.
- S. Klembt, S. Dreyer, M. Eckstein and U. Kragl, in Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH Verlag GmbH & Co, Weinheim, 2nd edn, 2008, pp. 641–661 Search PubMed.
- J. B. Sumner, J. Biol. Chem., 1924, 62, 287–290 CAS.
- M. Lever, Biochem. Med., 1973, 7, 274–281 CrossRef CAS.
- M. Vaher, M. Koel, J. Kazarjan and M. Kaljurand, Electrophoresis, 2011, 32, 1068–1073 CrossRef CAS PubMed.
- G. Ebner, S. Schiehser, A. Potthast and T. Rosenau, Tetrahedron Lett., 2008, 49, 7322–7324 CrossRef CAS PubMed.
- S. Ramakrishnan, J. Collier, R. Oyetunji, B. Stutts and R. Burnett, Bioresour. Technol., 2010, 101, 4965–4970 CrossRef CAS PubMed.
- C. Lehmann, F. Sibilla, Z. Maugeri, W. R. Streit, d. M. Dominguez, R. Martinez and U. Schwaneberg, Green Chem., 2012, 14, 2719–2726 RSC.
| This journal is © The Royal Society of Chemistry 2015 |