Supercritical fluid rectification of lignin microwave-pyrolysis oil
B. P.
Mudraboyina
,
D.
Fu
and
P. G.
Jessop
*
Department of Chemistry, Queen's University, 90 Bader Lane, Kingston, Ontario, Canada K7L 3N6. E-mail: jessop@chem.queensu.ca; Fax: +1-613-533-6669; Tel: +1-613-533-3212
First published on 10th September 2014
Abstract
A supercritical fluid rectification method (SFR) is described for selective extraction of single ring phenolic components from lignin microwave-pyrolysis oil. Supercritical CO2 at 35 °C and 8 MPa was used to extract phenols from the oil, after which the CO2 solution was passed up a column with a temperature gradient of 55–95 °C. SFR yielded up to 73% single ring phenolic components.
Lignocellulose is the most abundant biomass, representing close to 70% of the total plant biomass with an annual production of 200 billion metric tons.1–4 Pyrolysis of lignin, either by conventional or microwave heating, produces a pyrolysis oil that is a promising liquid fuel and a source of valuable single-ring phenolic compounds such as guaiacols, creosols and catechols that are not readily obtainable from other biomass sources. Guaiacols are precursors to various flavorants such as eugenol and vanillin.5,6 Creosols and catechols are widely known for their use in production of pesticides and are used as precursors to fine chemicals such as perfumes and pharmaceuticals.7,8 However, the extraction and purification of these desirable phenols from the heavier pyrolysis components has been a challenge to researchers. Numerous methods such as extraction through acids, alkalis, steam explosions and liquid hot water treatments have been reported which often are associated with disadvantages of high alkali catalyst costs, need for recovery of acids, difficult recovery methods, partial degradation of hemicellulose structures to yield undesirable heavier fractions, need for high cost corrosion resistant equipment, need for neutralization steps and formation of unwanted side products. Alternatively, extractions with green solvents such as switchable CO2 containing tertiary amine solutions which are more known as switchable hydrophilicity solvents (SHSs) have been reported to give better separations with no unwanted side products formed.9–13 However, cross contaminations, the low extractability of phenolic components and the many necessary process steps make them commercially less appealing for this application. Supercritical fluid extraction (SFE) using supercritical CO2 is a simpler, cleaner, and likely greener approach. In this communication, we present a supercritical fluid rectification (SFR) method, which displays high selectivity for the separation of single-ring phenols from lignin microwave-pyrolysis oil components.
Supercritical carbon dioxide is advantageous as a mobile phase in extractions because of its low cost, non-flammability, and ease of removal from products. A published variation of supercritical fluid extraction (SFE) is supercritical rectification (SFR), in which a rectifier column having a temperature gradient is placed after the extractor. Because of the increasing temperature up the column, an internal reflux of compounds is established, increasing the selectivity for some compounds over others. Though we could find no prior report of the use of the SFR method to extract lignin pyrolysis oil, this process has been reported for separations or purifications of food products, perfumes, medicinal compounds, natural products and biodiesel.14–17 The process was reported for separations of omega-3-fatty acids such as eicosapentaenoic acid and docosahexaenoic acid from fish oil fatty acid ethyl esters.18
Microwave-pyrolysis of a softwood Kraft lignin† was performed using the following conditions: microwave nominal power setting of 1.5 kW; heating time 800 s; initial mass of lignin and char (a microwave thermal catalyst) 180 g and 120 g respectively.19 The yields of non-condensable gas, heavy bio-oil, light bio-oil and solid charcoal were 21.4%, 19.4%, 16.7% and 42.5%. The pyrolysis oil automatically formed two phases. The upper layer (light bio-oil) is mostly water (78% determined by Karl–Fischer titration), while the bottom layer (heavy bio-oil) contains a lot of phenols and much less water (14% determined by Karl–Fischer titration). The heavy bio-oil was used in this work for extraction of the phenols. The bio-oil was kept in a refrigerator at 3 °C until needed. Even though we used lignin microwave pyrolysis oil, we anticipate that our SFR method should be general to all pyrolysis methods such as thermal or fluidized-bed pyrolysis.
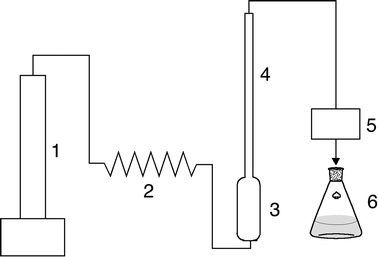
The SFR equipment is shown schematically in Fig. 1. It consists of an ISCO syringe pump (model 500D), a coiled length of tubing immersed in the water bath to preheat the CO2, a 160 mL stainless steel pressure vessel fitted with a dip tube so that the CO2 bubbles through the oil, a temperature graduated rectification column filled with Dixon rings and directly attached to the extractor, and a JASCO back pressure regulator (model 880-81). Both the coiled length of tubing and the vessel are kept in a 35 °C water bath. The extractor and the column (length: 14½ inches and inner diameter: 5/16 inch) were modified from a line filter and a micro reactor tube (High Pressure Equipment Company, Cat. # 20-51LF9, MS-12 and 20-LM9-18). The rectifier column was packed with Dixon rings (2 × 2 mm) SS304 (Pingxiang Naike Chemical Industry Equipment Packing Company). A linear temperature gradient up the column was established using three sets of temperature controls, thermocouples and heating tapes (Cole-Parmer, Cat. # A-36225-63, A-08469-24 and A-36110-00). Weighed bio-oil in the extractor was equilibrated with CO2 at the desired pressure and temperature for 90 min before the start of the extraction. Supercritical fluid extraction (SFE) experiments were performed using the same procedure and the same equipment but without the rectifier column. The SFR or SFE extracts vented from the back-pressure regulator were collected in methanol in an Erlenmeyer flask, which was changed periodically and weighed after rotary evaporation to remove the solvent.
The key single ring phenols in the bio-oil and its SFR or SFE extracts were identified by GC-MS and quantified by GC-FID (Table 1). The GC-MS and GC-FID methods have been described previously (SHS paper).12 The microwave pyrolysis oil samples consisted of desirable single ring phenols and undesirable multi-ring phenols. The primary aim of the study was to determine whether an SFR method would be more capable than an SFE method to selectively extract the key single ring phenol components.
| Compound | Concentration before rectification (wt%) | Concentration after rectificationa (wt%) |
|---|---|---|
| a Concentration in the cumulative extract collected from 0 to 30 minutes, 8 MPa, 35 °C water bath, 55–95 °C up the column, and 10 mL min−1 flow rate. (Same experiment as that shown in Fig. 2, green). | ||
| Creosol | 4.9 | 18.5 |
| Guaiacol | 3.9 | 17.0 |
| 4-Ethylguaiacol | 2.7 | 9.6 |
| 4-Methylcatechol | 2.7 | 0.0 |
| 2,3-Dimethylphenol | 1.3 | 4.1 |
| p-Cresol | 1.3 | 3.6 |
| 3-Methylcatechol | 1.2 | 0.4 |
| Phenol | 1.1 | 3.4 |
| o-Cresol | 0.9 | 3.4 |
| 4-Propylguaiacol | 0.7 | 0.4 |
| Eugenol | 0.7 | 0.8 |
| 3-Allylguaiacol | 0.6 | 1.2 |
| 4-Ethylcatechol | 0.5 | 1.3 |
| 6-Methylguaiacol | 0.5 | 2.2 |
| 2,4-Dimethylphenol | 0.5 | 1.3 |
| 4-Ethylphenol | 0.3 | 0.5 |
| Total | 24 | 68 |
In our initial tests, a pressure of 13 MPa, a temperature gradient of 40–70 °C and a flow rate of 0.3–1.2 mL min−1 were chosen, but we soon found that lower pressure, higher temperature and higher flow rates were more favorable to extract the identified single ring phenols. Improved conditions (8 MPa, 35 °C water bath, a temperature gradient of 55, 75, 95 °C up the column, and 10 mL min−1 flow rate) lead to up to 73% phenols concentration in the extract (Fig. 2, incremental concentration of single ring phenols (wt%) using 200 mL of CO2).
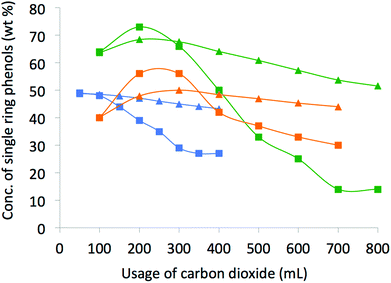 | ||
| Fig. 2 Incremental and cumulative concentration of the identified single ring phenols in the bio-oil extracts. ■: incremental, ▲: cumulative; Green: SFR, blue: SFE at 35 °C, orange: SFE at 95 °C. | ||
Although the results from SFE and SFR at different temperature and pressure conditions are both promising, the distinctive advantage of using SFR is seen upon comparison. Both incremental (concentration of rectified fractions) and cumulative (from mixture of the accumulated fractions) concentrations (Fig. 2) are higher than SFE carried out at either 35 or 95 °C with the usage of 100–300 mL of scCO2. Yields of the single ring phenols (Fig. 3) show significant improvement with higher usage of CO2, typically over 200 mL of scCO2 in the case of SFE at 35 °C or over 300 mL of scCO2 in the case of SFR or of SFE carried out at 95 °C. However, catechols present in the lignin pyrolysis oil were not selectively extracted.
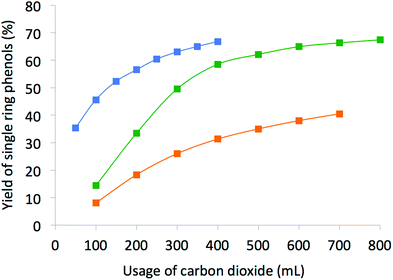 | ||
| Fig. 3 Yield of the identified single ring phenols in the extracts. Green: SFR, blue: SFE at 35 °C, orange: SFE at 95 °C. | ||
In a comparison of the cumulative concentration of the identified phenols in the extracts versus yields (Fig. 4), it is evident that the SFR method displayed greater concentrations of phenols (wt%), even when taken to fairly high yields, than SFE at either of the two temperatures tested.
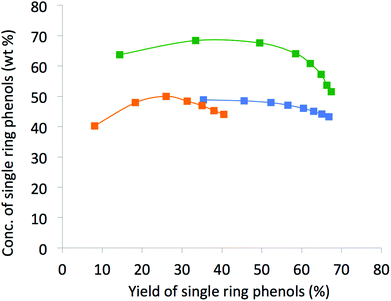 | ||
| Fig. 4 A plot of accumulated concentration of the identified single ring phenols in the extracts vs. yield of desirable single ring phenols. Green: SFR, blue: SFE at 35 °C, orange: SFE at 95 °C. | ||
The effect of flow rate, pressure of the scCO2 and temperature of extraction and rectification on the yield and composition of the extracts was measured in a series of experiments (Table 2). The scCO2 volume used was constant for all conditions: 200 mL liquid CO2 volume measured at −5 °C. Conditions A–E were employed to obtain different concentrations of the identified single ring phenols. Condition A: 5 g bio-oil, 10 MPa and 10 mL min−1 of scCO2, a temperature of 35 °C at the extractor and a gradient of 45–65 °C in the column; Condition B: 2 g bio-oil, 10 MPa and 2 mL min−1 of scCO2, a temperature of 35 °C at the extractor and a gradient of 45–65 °C in the column; Condition C: 5 g bio-oil, 10 MPa and 10 mL min−1 of scCO2, a temperature of 35 °C at the extractor and a gradient of 55–95 °C in the column; Condition D: 4 g bio-oil, 8 MPa and 10 mL min−1 of scCO2, a temperature of 35 °C at the extractor and a gradient of 45–65 °C in the column; Condition E: 4 g bio-oil, 8 MPa and 10 mL min−1 of scCO2, a temperature of 35 °C at the extractor and a gradient of 55–95 °C in the column. Application of these conditions resulted in progressive increase of the concentrated fractions from A–E yielding 55 to 68 wt% of identified single ring phenols. The preliminary results are promising and optimization by tuning the conditions should lead to increased yields and higher concentrations of identified single ring phenols in the rectified fractions.
| Various SFR conditions | A | B | C | D | E |
|---|---|---|---|---|---|
| Mass of the extract (g) | 1.01 | 0.62 | 0.98 | 0.44 | 0.47 |
| Conc. in the extract (wt%) | 55 | 40 | 57 | 55 | 68 |
Conclusions
Supercritical fluid rectification, with a temperature profile of 35 to 95 °C, extracts single-ring phenols from lignin microwave pyrolysis oil with superior selectivity compared to conventional supercritical fluid extraction at comparable conditions. The SFR method also allows facile removal and potentially recycling of the green solvent. Future work will include optimization of the method and study of the temperature and pressure dependence of solubility of the key phenols to help explain the selectivity observed.We are grateful to Mr Sherif Farag and Prof. Jamal Chaouki of the École Polytechnique de Montréal for the pyrolysis oil samples. This work was financially supported by the Natural Sciences and Engineering Research Council (NSERC), Lignoworks – the NSERC Biomaterials and Chemicals Strategic Network, which is a member of FIBRE – Forest Innovation by Research & Education. The authors thank Françoise Sauriol for technical support on NMR measurements, FPInnovations for lignin samples and their support of Lignoworks, and the Canada Research Chairs Program.
Notes and references
- Y.-H. P. Zhang, S.-Y. Ding, J. R. Mielenz, J. B. Cui, R. T. Elander, M. Laser, M. E. Himmel, J. R. McMillan and L. R. Lynd, Biotechnol. Bioeng., 2007, 97, 214–223 CrossRef CAS PubMed.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick Jr., J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Meilenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- Y.-H. P. Zhang, M. E. Himmel and J. R. Mielenz, Biotechnol. Adv., 2006, 24, 452–481 CrossRef CAS PubMed.
- Y.-H. P. Zhang, J. Ind. Microbiol. Biotechnol., 2008, 35, 367–375 CrossRef CAS PubMed.
- C. F. H. Allen and J. W. Gates Jr., Org. Synth., 1945, 25, 49 CrossRef CAS.
- H. O. Mottern, J. Am. Chem. Soc., 1934, 56, 2107–2108 CrossRef CAS.
- J. C. Spain and D. T. Gibson, Appl. Environ. Microbiol., 1988, 54, 1399–1404 CAS.
- L. C. Nolan and K. E. O'Connor, Biotechnol. Lett., 2008, 30, 1879–1891 CrossRef CAS PubMed.
- P. G. Jessop, L. Phan, A. Carrier, S. Robinson, C. J. Dürr and J. R. Harjani, Green Chem., 2010, 12, 809–814 RSC.
- P. G. Jessop, L. Kozycz, Z. G. Rahami, D. Scheonmakers, A. R. Boyd, D. Wechsler and A. M. Holland, Green Chem., 2011, 13, 619–623 RSC.
- P. G. Jessop, L. N. Phan, A. J. Carrier, R. Resendes and D. Wechsler, PCT Intl, WO2011050469, 2011 Search PubMed.
- D. Fu, S. Farag, J. Chaouki and P. G. Jessop, Bioresour. Technol., 2014, 154, 101–108 CrossRef CAS PubMed.
- J. R. Vanderveen, J. Durelle and P. G. Jessop, Green Chem., 2014, 16, 1187–1197 RSC.
- M. Sato, M. Goto and T. Hirose, Ind. Eng. Chem. Res., 1996, 35, 1906–1911 CrossRef CAS.
- Y. Shibuya, H. Ohinata, Y. Yonei and T. Ono, Proc. Int. Solv. Extr. Conf, ed. D. H. Logsdail and M. J. Slater, Elsevier Applied Science, London, 1993, 2, 684–691 Search PubMed.
- X. Liu and H. Zhang, Liaoning Zhongyiyao Daxue Xuebao, 2013, 15, 182–184 CAS.
- X. Hou, Y. Qi and X. Qiao, PCT Intl, CN2006112548 20060330, 2006 Search PubMed.
- J. D. Kim, Y. W. Lee and J. S. Lim, J. Korean Oil Chem. Soc., 1997, 14, 49–55 Search PubMed.
- S. Farag, D. Fu, P. G. Jessop and J. Chaouki, J. Anal. Appl. Pyrolysis, 2014, 109, 249–257 CrossRef CAS PubMed.
Footnote |
| † The raw material for pyrolysis was a softwood Kraft lignin, obtained from FPInnovations. Guaiacol, methanol and propylbenzene were purchased from Sigma-Aldrich. SFC grade CO2 was purchased from Praxair Inc. |
| This journal is © The Royal Society of Chemistry 2015 |