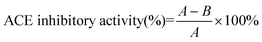Anxiolytic effects of ACE inhibitory peptides on the behavior of rats in an elevated plus-maze
Zhipeng
Yu
a,
Wenzhu
Zhao
*a,
Long
Ding
b,
Yiding
Yu
b and
Jingbo
Liu
*b
aCollege of Food Science and Engineering, Bohai University, Jinzhou 121013, P.R. China. E-mail: zhaowenzhu777@163.com
bLab of Nutrition and Functional Food, Jilin University, Changchun 130062, P.R. China. E-mail: ljb168@sohu.com
First published on 23rd October 2015
Abstract
Three novel egg white-derived peptides were demonstrated to display in vitro activities against the angiotensin converting enzyme (ACE). A further study was conducted to assess their anxiolytic-like effects upon spontaneously hypertensive rats (SHRs) after an oral administration of the peptides, which showed the same behavioral performance as that using an elevated plus maze (EPM). In the EPM experiment, the behavioral effects of peptides TNGIIR, RVPSL, and QIGLF were investigated at doses ranging from 5 to 50 mg kg−1, with records of the number of entries by the rats into the open arms, the time the rats spent in the open arms, and the total amount of entries into the open + closed arms. The results showed that the peptides TNGIIR and RVPSL, in a range of 5–50 mg kg−1, exerted an anxiolytic effect on the SHRs. These results suggested that the egg white derived peptides TNGIIR and RVPSL could be considered as possible functional food or food ingredients due to their in vivo anti-stress and anti-anxiety effects on the SHRs.
1. Introduction
The angiotensin-converting enzyme (ACE) is a peptidyl-dipeptidase (E.C. 3.4.15.1) which plays a vital physiological role in regulating blood pressure in the renin–angiotensin system (RAS).1,2 ACE converts inactive Ang I into the potent vasoconstrictor Ang II and also catalyses the degradation of bradykinin, a vasodilator peptide.1,2 Inhibition of ACE activity leads to a decrease in the concentration of angiotensin II with a concomitant reduction of the blood pressure.1 Since the discovery of an ACE inhibitor in snake venom, ACE inhibitors have shown a pivotal influence in hypertension therapy, particularly, in understanding cardiovascular pathophysiology. Up to now, some bioactive peptides with ACE inhibitory activity have been isolated and purified from various food sources, such as cod,2 sweet potato,3 milk,4,5 corn6 and egg.7 Many foods are being increasingly considered as not only a source of nutrients but also a potential source of bioactive components, such as biological active peptides. On the other hand, bioactive peptides from food proteins are considered to be milder and safer without undesirable effects compared to synthetic compounds.8Many bioactive compounds were considered to have the effects of provoking anxiety and/or calmness.9 Although people have applied the ACE inhibitory peptides for treatment of hypertension for decades worldwide, scientific information of the ACE inhibitory peptides on rats’ behavior is very limited. Thus, such a kind of study is very important, and also indispensable in concerns of the safety of peptides applied to the hypertension treatment. In this context, the elevated plus maze (EPM) is the most commonly employed models to test animal behavior.10 The EPM, which consists of a plus-shaped apparatus with two open and two closed arms, is designed based on rodents’ aversion of open spaces. This aversion leads to the animal behavior termed thigmotaxis, which involves avoidance of open areas by confining movements to enclosed spaces or to the edges of a bounded space.10 Moreover, the EPM is used to measure, both behaviorally and physiologically, the clinical effects of drugs. Therefore, it was also a desirable model to measure the effects of peptides on the behavior of rats.
Previously, we have found three novel ACE inhibitory peptides (i.e., RVPSL,1 QIGLF11 and TNGIIR12) from egg white protein. The present study intends to further investigate the antidepressant activity of these ACE inhibitory peptides. In this context, the anxiety-related behavioral effects of these peptides on the spontaneously hypertensive rats (SHRs) were investigated by the elevated plus-maze and the autonomic activation, respectively.
2. Materials and methods
2.1 Preparation and purification of egg white peptides
Egg white protein which was dissolved in distilled water to obtain 5% protein slurry (w/v) was heated to 90 °C for 10 min aimed to denature the egg white protein. Subsequently, it was cooled down to 50 °C to be hydrolyzed in a 250 mL reactor at the controlled temperature and pH. The pH value was adjusted to 10.0 using 1 M NaOH before the alkaline proteinase Alcalase was added to 4.0% of protein (w/w). Samples were withdrawn after the reaction for 180 min, and placed in a boiling water bath for 10 min to inactivate the enzyme and be centrifuged (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 min, 4 °C). The supernatant was then spray dried, and the powder of the spray dried hydrolysate was added to a column (1.6 × 100 cm) packed with Sephadex G 25 gel, which had been pre-equilibrated with distilled water. The hydrolysate was eluted with distilled water at a speed of 60 mL h−1. The elution peaks were monitored at 220 nm.1 These fractions were vacuum freeze-dried and the highest active fraction was purified for a further study.
000g, 10 min, 4 °C). The supernatant was then spray dried, and the powder of the spray dried hydrolysate was added to a column (1.6 × 100 cm) packed with Sephadex G 25 gel, which had been pre-equilibrated with distilled water. The hydrolysate was eluted with distilled water at a speed of 60 mL h−1. The elution peaks were monitored at 220 nm.1 These fractions were vacuum freeze-dried and the highest active fraction was purified for a further study.
2.2 Identification of the purified peptides by LC/MS/MS
The LC/MS/MS system consisted of an Agilent 1200 HPLC system and a 4000 Q-Trap mass spectrometer (Applied Biosystems) with a Turbo spray. The chromatography was performed on a reverse phase C18 column (column size 250 × 4.6 mm, serial no. 6032551). An isocratic mobile phase consisted of 70% acetonitrile (B) and deionizer water with 0.1% TFA (A). The gradient elution program was conducted as follows: the elution started from 10% B (70% acetonitrile) initially followed by a linear gradient to 100% B in 10 min. The total mobile phase flow was set at 1 mL min−1. The acquisition method consisted of an information-dependent data acquisition (IDA) scan cycle, including the enhanced mass scan (EMS) as the survey scan, enhanced resolution scan (ER) to confirm the charge state, and two dependent enhanced product ion (EPI) scans. With the threshold of the ion intensity at 5500 counts per second (cps), the IDA criteria were set to allow the most abundant ions in the EMS scan to trigger EPI scans.11 To minimize possible fragmentation in the EMS, the collision energy was set at 5 eV and the collision-induced dissociation (CID) gas was set at ‘Low’. The scan range of the EMS was set at m/z 275.00–1000.00. The dependent EPIs scanned the range of m/z 275–1000, including the setting for CID gas, 55 eV (GS 1) and 60 eV (GS 2) for the collision energy.13 The speed of the EMS and EPI scans were recorded at a speed of 4000 Da s−1.2.3 Synthetic peptides
The peptide sequences derived from ovotransferrin in egg white were synthesized by the solid phase procedure using the FMOC protected amino acid synthesis methods with an AAPPTEC 396 Automated Peptide Synthesizer (Advanced Automated Peptide Protein Technologies, USA). These peptides were synthesized on Fmoc-Met-Wang Resin and elongated using the standard Fmoc solid-phase peptide conditions on an AAPPTEC Apex 396 peptide synthesizer as previously described.14 These peptides were provided by Shanghai Science Peptide Biological Technology Corporation. The synthesized peptides were purified by HPLC on an ODS column.11 Their purity was verified by HPLC in our laboratory. The molecular masses of these synthesized peptides were determined by mass spectrometry (Dionex, MSQ). The synthetic peptides were used for a further study.2.4 ACE-inhibitory activity
ACE-inhibitory activity was measured by high-performance liquid chromatography15 with some modifications as explained below. A reaction mixture in a volume of 60 μL contained 100 mM borate buffer (pH 8.3), 4 mM hippuryl-L-histidyl-L-leucine (HHL), 300 mM NaCl, and 10 milliunit ACE. All solutions were incubated at 37 °C for 30 min in a thermostatically controlled water bath prior to mixing, subsequently for an additional 30 min to react at the same temperature after mixing. The reaction was stopped by adding 60 μL of 1 M HCL before injecting the sample into the HPLC sample loop to quantify the hippuric acid produced by the enzymatic hydrolysis of the substrate HHL.12 The isocratic mobile phase consisted of 25% acetonitrile in deionizer water (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) with 0.5% TFA. It was filtered through 0.45 μm cellulose filters and degassed by ultrasound for 30 min. An aliquot of 10 μL of the reaction mixture was analyzed on a Shimadzu C18 column. HHL and hippuric acid (HA) were detected at 228 nm, as the mobile phase was controlled at 0.5 mL min−1.
v) with 0.5% TFA. It was filtered through 0.45 μm cellulose filters and degassed by ultrasound for 30 min. An aliquot of 10 μL of the reaction mixture was analyzed on a Shimadzu C18 column. HHL and hippuric acid (HA) were detected at 228 nm, as the mobile phase was controlled at 0.5 mL min−1.
The inhibitory activity degree was calculated as follows:
where A is the peak area of the reaction blank. The reaction blank mixture contained the same volume of the buffer solution instead of the sample; B is the peak area of the reaction in the presence of both ACE and the enzymatic peptide sample, and the IC50 value was defined as the concentration of the inhibitor inhibiting 50% of the ACE activity under the assayed conditions.
2.5 Animal welfare
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Jilin University, IACUC (Approval number: 20140405). The SHRs were housed in temperature-controlled rooms (22 ± 2 °C) with 12 h light/dark cycles, where the SHRs could consume tap water and standard diets ad libitum. After 4 weeks of oral administration with different products, the elevated plus maze apparatus testing and autonomic activation procedures were performed.2.6 A single test in the elevated plus maze
The EPM consisted of two open arms (50 cm long × 10 cm wide), two closed arms (50 cm long × 10 cm wide × 40 cm high), and a middle compartment (10 cm long × 10 cm wide) forming the shape of a plus. The EPM was elevated 50 cm above the ground. The floor of the EPM and the walls of the enclosed room were made of black acrylic. The rats were habituated to the testing room for 30 min before testing and the room was under dim light, with no experimenter present in the room.10 A total of 66 male SHRs (purchased from Vital River Laboratories), aged 9–10 weeks and weighing 220–270 g, were used in the course of this study. The body weight was measured once a day. The feed intake was measured from one day after starting the experiment and calculated as the mean daily value in each cage by deducting the remaining feed amount from the feed amount filled into the feed container on the day before and dividing the difference by the number of animals in the cage.16 Tap water consumption was measured by deducting the remaining water volume from the initial volume. The total 66 rats were divided into 11 groups, i.e., the saline group, the captopril group, and 9 treatment groups (n = 6). Each rat was placed in the middle compartment (head facing an open arm) and allowed to freely explore the apparatus for 5 min. The apparatus was cleaned with a disinfectant after each 5 min run. The movements of each rat were recorded by using a digital video camera mounted at a height of 130 cm and connected to a computer. Data were analyzed by using an Apciexp software program installed on the computer.10 The number of entries into as well as the time spent in the open arms was recorded. We measured the time the rats spent in the open and closed arms of the EPM, and that spent in the center zone and the total distance traveled. Thus, anti-anxiety behavior (increased time in the open arm and/or open arm entries) of the animals can be easily accessed and quantified, whereas, in the EPM, a decrease of anxiety corresponds to a behavioural disinhibition (the rat exits more often in the open arms). The EPM test was conducted seven times for each rat.2.7 Statistical analysis
Origin 8.0 was used for major data processing throughout this work. All the results were expressed as the mean ± SD of 6 rats per group. The differences were considered significantly at P < 0.05.3. Results and discussion
3.1 Behavior of rats with administered peptides in an elevated plus-maze
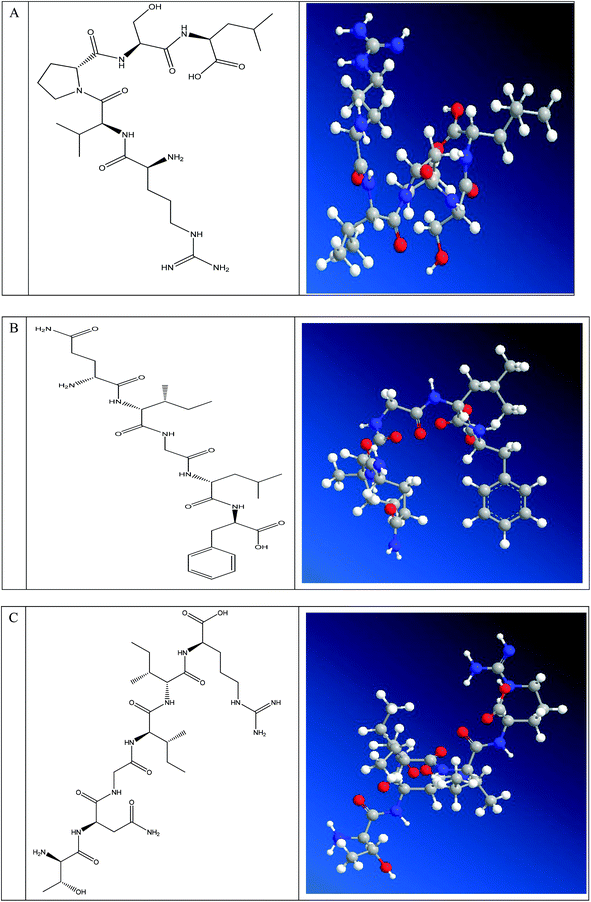
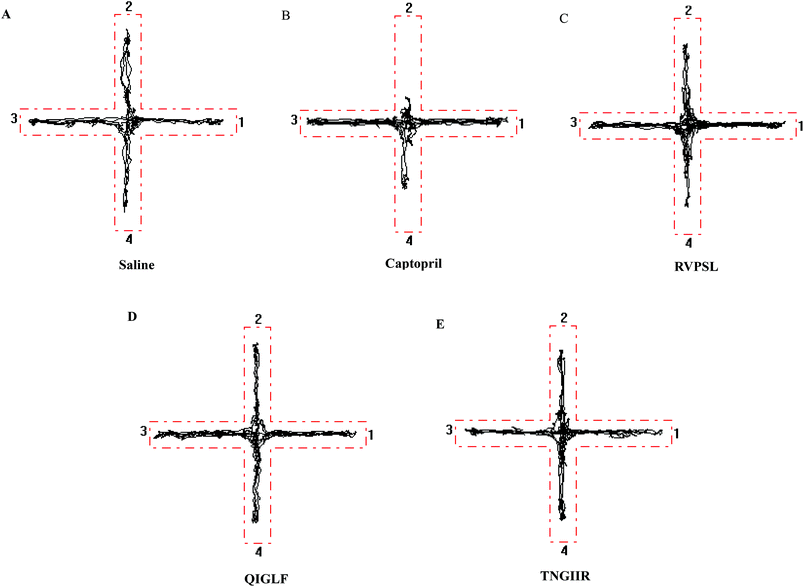
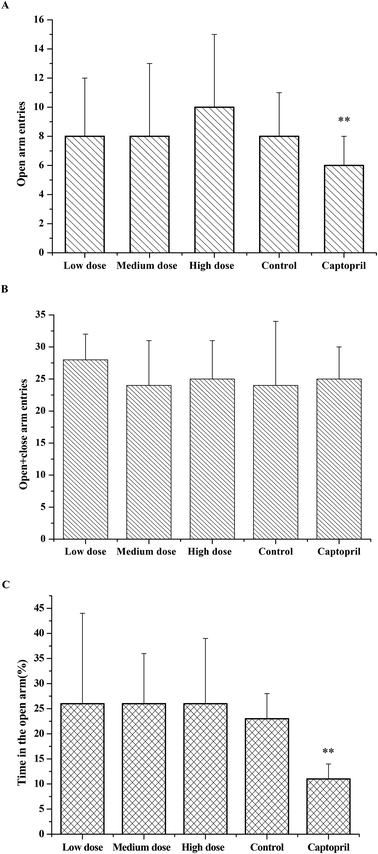
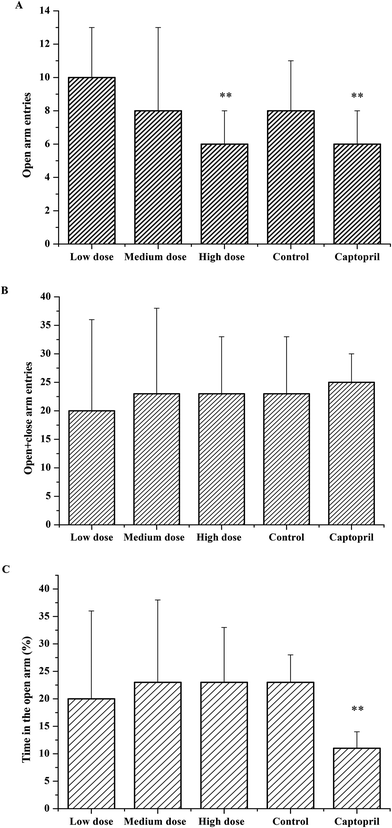
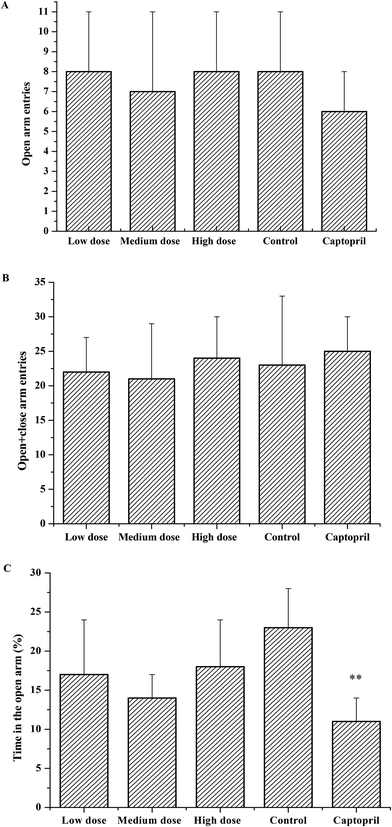
As the effects of ACE inhibitory peptides on the behavior of rats have not been reported, this research was the first to study the behavioral effects of the bioactive peptides with ACE inhibitory activity on the SHRs by using the EPM method for evaluating animal's anxiety. Our previous work has demonstrated that the peptides (i.e., RVPSL, QIGLF and TNGIIR) from egg white protein possessed an activity against the angiotensin converting enzyme. The chemical structures of these peptides are presented in Fig. 1. In the current work, the number of entries by rats into the open and closed arms, as well as the time spent in the open arm during the EPM test were recorded for the SHRs, which were treated with saline, captopril, and different concentrations of TNGIIR (low, medium, and high doses), respectively. Fig. 2(A–E) shows the movements of rats after oral administration with saline, captopril, a high dose of RVPSL, a high dose of QIGLF, and a high dose of TNGIIR, respectively, which were recorded by using a digital video camera mounted at a height of 130 cm and connected to a computer. In the EPM, the total number of arm entries was generally considered as a reflection of the locomotor activity of the rats.17 The time that the rats spent in the open arms of the EPM increased by TNGIIR treatment compared to the negative control (shown in Fig. 3). However, the result also showed that the group of rats that were treated by captopril spent less time in the open arms compared to the negative control group. In general, normal rats without treatment had significantly fewer entries into the open arms than those into the closed arms, and spent significantly less time in the open arms. In comparison, some clinically effective anxiolytics were reported to have a significant increase in time that was spent by the rats in the open arms, and remarkably increased amounts of entries of the rats into the open arms.18 The result suggested that the rats under peptide TNGIIR treatment spent an increased time in the open arms compared to the negative control group. Thus, the present investigation demonstrated that the egg white-derived peptide TNGIIR possessed the activity of reducing anxiety. Nevertheless, the tested SHRs in the open arms were associated with an observation of significantly more anxiety-related behaviors than those in the closed arms, which was consistent with a previous report.19In addition, Fig. 4 shows the effect of the egg white-derived peptide QIGLF on the rats under the EPM method. Although the total number of entries of the rats into the open + closed arms leveled off, the number of entries into the open arms significantly decreased after the QIGLF treatment compared to that of the control, which had a similar effect to the drug captopril (Fig. 4A). However, unlike the captopril, the peptide QIGLF did not cause the SHRs to stay for a longer time in the open arm.
As shown in Fig. 5, the rats treated with the egg white-derived peptide RVPSL spent the same length of time in the open arms as the negative control did. At the same time, the number of entries of the rats into the open arms and the number of entries into the open + closed arms were also close to those of the negative control group without a significant difference. Recently, it has been reported that bioactive peptides of 6 to 15 amino acids were able to cross through the blood–brain barrier and act on the central nervous system in mice.20 It was suggested that the peptides TNGIIR and RVPSL with anxiolytic-like activity on the SHRs after oral administration would also possibly cross through the biological barriers and modify the central nervous system of the SHRs.
4. Conclusion
In summary, egg white-derived peptides TNGIIR and RVPSL exerted an anxiolytic effect on the SHRs in the EPM test, under oral administration with 50 mg kg−1. Although the peptide QIGLF did not show anxiolytic activity, the results demonstrated that the other two egg white-derived peptides, i.e., TNGIIR and RVPSL, had not only ACE inhibitory activity, but also anxiolytic activity, which make them the potential candidates as a safer functional food suitable for further investigation. Further pharmacological and biochemical investigations are in progress.Acknowledgements
This paper was supported by The National Natural Science Funds of China (no. 31271907) and the key scientific and technical problems of Liaoning province (no. 2015103020).References
- J. B. Liu, Z. P. Yu, W. Z. Zhao, S. Y. Lin, E. L. Wang, Y. Zhang, H. Hao, Z. Z. Wang and F. Chen, Isolation and identification of angiotensin-converting enzyme inhibitory peptides from egg white protein hydrolysates, Food Chem., 2010, 122, 1159–1163 CrossRef CAS.
- S. W. A. Himaya, D.-H. Ngo, B. Ryu and S.-K. Kim, An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress, Food Chem., 2012, 132, 1872–1882 CrossRef CAS.
- K. Ishiguro, Y. Sameshima, T. Kume, K.-i. Ikeda, J. Matsumoto and M. Yoshimoto, Hypotensive effect of a sweetpotato protein digest in spontaneously hypertensive rats and purification of angiotensin I-converting enzyme inhibitory peptides, Food Chem., 2012, 131, 774–779 CrossRef CAS.
- P. Ruiz-Gimenez, J. B. Salom, J. F. Marcos, S. Valles, D. Martinez-Maqueda, I. Recio, G. Torregrosa, E. Alborch and P. Manzanares, Antihypertensive effect of a bovine lactoferrin pepsin hydrolysate: Identification of novel active peptides, Food Chem., 2012, 131, 266–273 CrossRef CAS.
- D. Pan, J. Cao, H. Guo and B. Zhao, Studies on purification and the molecular mechanism of a novel ACE inhibitory peptide from whey protein hydrolysate, Food Chem., 2012, 130, 121–126 CrossRef CAS.
- W.-H. Huang, J. Sun, H. He, H.-W. Dong and J.-T. Li, Antihypertensive effect of corn peptides, produced by a continuous production in enzymatic membrane reactor, in spontaneously hypertensive rats, Food Chem., 2011, 128, 968–973 CrossRef CAS.
- H. Fujita, R. Sasaki and M. Yoshikawa, Potentiation of the antihypertensive activity of orally administered ovokinin, a vasorelaxing peptide derived from ovalbumin, by emulsification in egg phosphatidylcholine, Biosci., Biotechnol., Biochem., 1995, 59, 2344–2345 CrossRef CAS PubMed.
- J. P. Wang, J. E. Hu, J. Z. Cui, X. F. Bai, Y. G. Du, Y. Miyaguchi and B. C. Lin, Purification and identification of a ACE inhibitory peptide from oyster proteins hydrolysate and the anti hypertensive effect of hydrolysate in spontaneously hypertensive rats, Food Chem., 2008, 111, 302–308 CrossRef CAS PubMed.
- A. M. Becerra Garcia, F. P. Cardenas and S. Morato, The effects of pentylenetetrazol, chlordiazepoxide and caffeine in rats tested in the elevated plus-maze depend on the experimental illumination, Behav. Brain Res., 2011, 217, 171–177 CrossRef PubMed.
- X. Xiang, W. Huang, C. N. Haile and T. A. Kosten, Hippocampal GluR1 associates with behavior in the elevated plus maze and shows sex differences, Behav. Brain Res., 2011, 222, 326–331 CrossRef CAS PubMed.
- Z. P. Yu, W. Z. Zhao, J. B. Liu, J. Lu and F. Chen, QIGLF, a novel angiotensin I-converting enzyme-inhibitory peptide from egg white protein, J. Sci. Food Agric., 2011, 91, 921–926 CrossRef CAS PubMed.
- Z. P. Yu, B. Q. Liu, W. Z. Zhao, Y. G. Yin, J. B. Liu and F. Chen, Primary and secondary structure of novel ACE-inhibitory peptides from egg white protein, Food Chem., 2012, 133, 315–322 CrossRef CAS PubMed.
- Z. P. Yu, Y. G. Yin, W. Z. Zhao, Y. D. Yu, B. Q. Liu, J. B. Liu and F. Chen, Novel peptides derived from egg white protein inhibiting alpha-glucosidase, Food Chem., 2011, 129, 1376–1382 CrossRef CAS.
- S. Aggarwal, P. Singh, O. Topaloglu, J. T. Isaacs and S. R. Denmeade, A dimeric peptide that binds selectively to prostate-specific membrane antigen and inhibits its enzymatic activity, Cancer Res., 2006, 66, 9171–9177 CrossRef CAS PubMed.
- J. P. Wu, R. E. Aluko and A. D. Muir, Improved method for direct high-performance liquid chromatography assay of angiotensin-converting enzyme-catalyzed reactions, J. Chromatogr. A, 2002, 950, 125–130 CrossRef CAS PubMed.
- W. Zhao, Y. Yin, Z. Yu, J. Liu and F. Chen, Comparison of anti-diabetic effects of polysaccharides from corn silk on normal and hyperglycemia rats, Int. J. Biol. Macromol., 2012, 50, 1133–1137 CrossRef CAS PubMed.
- N. Violle, M. Messaoudi, C. Lefranc-Millot, D. Desor, A. Nejdi, B. Demagny and H. Schroeder, Ethological comparison of the effects of a bovine alpha(s1)-casein tryptic hydrolysate and diazepam on the behaviour of rats in two models of anxiety, Pharmacol., Biochem. Behav., 2006, 84, 517–523 CrossRef CAS PubMed.
- S. Pellow, P. Chopin, S. E. File and M. Briley, Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat, J. Neurosci. Methods, 1985, 14, 149–167 CrossRef CAS PubMed.
- A. A. Walf and C. A. Frye, The use of the elevated plus maze as an assay of anxiety-related behavior in rodents, Nat. Protoc., 2007, 2, 322–328 CrossRef CAS PubMed.
- R. D. Egleton, E. J. Bilsky, G. Tollin, M. Dhanasekaran, J. Lowery, I. Alves, P. Davis, F. Porreca, H. I. Yamamura, L. Yeomans, C. M. Keyari and R. Polt, Biousian glycopeptides penetrate the blood-brain barrier, Tetrahedron: Asymmetry, 2005, 16, 65–75 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |