Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The blood pressure effect and related plasma levels of flavan-3-ols in spontaneously hypertensive rats
Mar
Quiñones†
*a,
Maria
Margalef
b,
Anna
Arola-Arnal
b,
Begoña
Muguerza
*bc,
Marta
Miguel
d and
Amaya
Aleixandre
a
aDpto. Farmacología, Fac. Medicina, U. Complutense, Avda. Complutense s/n, 28040 Madrid, Spain. E-mail: mar.quinones@usc.es; Fax: +34-91-3941463; Tel: +34-91-3941475
bNutrigenomic Group, Department of Biochemistry and Biotechnology, Rovira i Virgili University, Tarragona, Spain. E-mail: begona.muguerza@urv.cat; Fax: +34 977 55 82 32; Tel: +34 977 55 95 66
cCentre Tecnològic de Nutrició i Salut (CTNS), TECNIO, CEICS, Reus, Spain
dInstituto de Investigación en Ciencias de Alimentación (CIAL, CSIC-UAM, CEI UAM+CSIC), Madrid, Spain
First published on 7th August 2015
Abstract
We studied the short-term antihypertensive effect of flavan-3-ols (−)-epicatechin, (+)-catechin and (−)-catechin, in spontaneously hypertensive rats (SHR). Plasma metabolites and the corresponding plasma antioxidant capacity were determined. All the assayed flavan-3-ols decreased systolic blood pressure (SBP) in SHR. Their antihypertensive effects were less pronounced than that of Captopril (50 mg kg−1) and were not shown in normotensive Wistar-Kyoto rats. 6 mg kg−1 (−)-epicatechin caused the maximum decrease in SBP. The maximum effects of the catechin monomers were observed post-administration of 0.5 mg kg−1 of flavan-3-ols, (−)-catechin being the least effective among the three assayed compounds. Glucuronide and methyl glucuronide metabolites were obtained in the flavan-3-ol treated SHR, but it was not possible to relate the antihypertensive effect of the assayed flavan-3-ols with a concrete plasma metabolite or with their antioxidant effect. In conclusion, the studied flavan-3-ols could be responsible for the antihypertensive effect of cocoa products.
1. Introduction
In recent years, numerous studies have demonstrated the health benefits of polyphenols, and special attention has been paid to their beneficial effect on hypertension and cardiovascular diseases.1–3 In particular, flavan-3-ols, also known as flavanols, are the most structurally complex subclass of flavonoids, and have been recognized as antihypertensive agents.1,4 These compounds are present in different foods such as cocoa, grape, tea, apple and berries.5 Cocoa, in particular, has a high content in flavan-3-ols.6,7 Elegant reports have shown the beneficial effect of cocoa on blood pressure.6,8 Moreover, our research group demonstrated that a polyphenol enriched cocoa powder showed antihypertensive properties,9 improved endothelial function,10 and exhibited an antioxidant capacity.11Cocoa flavan-3-ols consist of a complex mixture of the monomeric (−)-epicatechin and (+)-catechin and the oligomers of these monomeric base units known as procyanidins.5 It is nevertheless worth nothing that the preservation of flavan-3-ols during cocoa manufacturing is important to exhibit the health effects associated with cocoa consumption, and it has been recently described that roasted cocoa beans and cocoa products additionally contained (−)-catechin. This atypical flavan-3-ol is generally formed during the cocoa manufacturing process by an epimerization which converts (−)-epicatechin to its epimer (−)-catechin. High temperatures during the cocoa bean roasting process, and particularly the alkalization of the cocoa powder, are the main factors inducing the epimerization reaction.12
The bioavailability of flavan-3-ols is higher than the bioavailability of other flavonoids, even if it is also relatively poor.13 Moreover, flavan-3-ols, as other kinds of flavonoids, occur in plasma in more diverse forms than in food.14,15 In fact, the uptake and metabolism of polyphenols are usually associated with their methylation, sulphation or glucuronidation, afforded by phase-II enzymes.16 In addition, considerable quantities of ingested flavonoids are degraded by colonic microbiota upon reaching the large intestine, where they yield other smaller molecules that are also absorbed into the body.17 Therefore, the identification of the specific flavan-3-ol metabolites present in the body is crucial for understanding their biological activities.
Different studies have demonstrated that (−)-epicatechin, (+)-catechin and their oligomers, have important cardiovascular beneficial effects, such as the ability to inhibit LDL oxidation18 and the capacity to promote endothelium-dependent relaxation.19 Moreover these compounds can also modulate the production of inflammatory cytokines20 and can inhibit pro-inflammatory response in in vitro systems.21 Nevertheless, scarce research exists for evaluating the short-term antihypertensive effect of the main cocoa flavan-3-ols. The aim of this study was to characterize the dose dependent short-term antihypertensive effect of (−)-epicatechin, (+)-catechin and the atypical flavan-3-ol (−)-catechin, in spontaneously hypertensive rats (SHR). The corresponding plasma flavan-3-ol compound and its main metabolites in this fluid were quantified. Moreover, since the antioxidant effect of polyphenols enables us to explain many of their health benefits, the corresponding plasma antioxidant effect was also determined.
2. Materials and methods
2.1. Chemicals and reagents
Captopril, (−)-epicatechin, (+)-catechin and (−)-catechin were used for the experiments, and all these compounds were purchased from Sigma Chemical (Fluka/Sigma Aldrich, Madrid, Spain). Captopril was dissolved in water to be administered to the rats. (+)-Catechin, (−)-catechin and (−)-epicatechin were prepared with 10% DMSO in water and sonicated for 45 minutes before administration to the animals.For the chromatographic analysis, methanol (Scharlab S.L., Barcelona, Spain), acetone (Sigma-Aldrich, Madrid, Spain) and glacial acetic acid (Panreac, Barcelona, Spain) of HPLC analytical grade, were used. Ultrapure water was obtained from a Milli-Q advantage A10 system (Madrid, Spain). The 2000 mg l−1 standard stock solutions of (+)-catechin, (−)-epicatechin and pyrocatechol (Sigma Aldrich, Madrid, Spain) in methanol as the internal standard (IS), were stored in dark-glass flasks at −20 °C. A 200 mg l−1 stock standard mixture of (+)-catechin and (−)-epicatechin was prepared weekly and stored at −20 °C. The stock standard solution was diluted daily to the desired concentration using an acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (70
acetic acid (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.5
29.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, v
0.5, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v
v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) solution.
v) solution.
2.2. Experimental procedure in rats
The assayed flavan-3-ols were orally administered by gastric intubation to the rats and tentative trials were formerly carried out in order to estimate the doses of each monomer that could be efficient in the SHR. In accordance with these initial trials, we evaluated the effect of four different doses of (−)-epicatechin (1 mg kg−1, 2 mg kg−1, 6 mg kg−1 and 12 mg kg−1), four different doses of (+)-catechin (0.25 mg kg−1, 0.5 mg kg−1, 1.5 mg kg−1 and 3 mg kg−1) and four different doses of (−)-catechin (0.25 mg kg−1, 0.5 mg kg−1, 1.5 mg kg−1 and 3 mg kg−1) in the SHR. Each flavan-3-ol was evaluated by using a minimum of 8 rats and we have always administered increasing doses in the same animal by waiting for at least 4 days between the administrations of two different doses. The most effective dose to lower the arterial blood pressure in the SHR of each flavan-3-ols [6 mg kg−1 (−)-epicatechin, 0.5 mg kg−1 (+)-catechin and 0.5 mg kg−1 (−)-catechin], was also administered to WKY rats. In all cases, 1 ml of the corresponding solution was orally administered by gastric intubation, between 9 and 10 a.m. to the rats. Captopril (50 mg kg−1), a known antihypertensive drug, served as the positive control, and 1 ml 10% DMSO water solution served as the negative control. We measured the SBP of the rats by the tail cuff method22 before administration and also 2, 4, 6, 8, 24, 48 and 72 hours post-administration. Before the measurement, the rats were maintained at 30 °C for 10 minutes to make the pulsations of the tail artery detectable. The person who measured the arterial blood pressure in the animals did not know either the compound or the dose that had been administered.
2.3. Plasma determination
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29.5
29.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, v
0.5, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v
v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) solution. The eluted solutions were then directly injected into the HPLC tandem triple quadrupole mass spectrometer (HPLC-MS/MS) for chromatographic analysis.
v) solution. The eluted solutions were then directly injected into the HPLC tandem triple quadrupole mass spectrometer (HPLC-MS/MS) for chromatographic analysis.
The chromatographic analysis was performed using a 1290 Infinity UHPLC coupled with a 6490 QqQ/MS (Agilent Technologies, Palo Alto, CA, USA). The separations were achieved by using a Zorbax SB-Aq (150 mm × 2.1 mm i.d., 3.5 μm of particle size) as a chromatographic column from Agilent Technologies. The mobile phase consisted of 0.2% acetic acid (solvent A) and acetonitrile (solvent B) at a flow rate of 0.4 ml min−1. The elution gradient was 0–10 min, 5–55% B; 10–12 min, 55–80% B; 12–15 min, 80% B isocratic; 15–16 min 80.5% B. A post run of 10 min was applied. The sample volume injected was 2.5 μl. The electrospray ionisation (ESI) conditions were: 150 °C and 14 l min−1 of drying gas temperature and flow, respectively, a nebulizer gas pressure of 30 psi, and 3000 V of capillary voltage. MS/MS was operated in a negative mode. MS/MS acquisition was performed in a multiple reaction monitoring (MRM) mode for flavan-3-ols and their metabolites, using the same quantification previously reported by Serra et al. (2009).24 Data acquisition was conducted by using the MassHunter software (Agilent Technologies, Palo Alto, CA, USA).
Spiked blank plasmas with standard compounds at 8 different concentrations were used to obtain calibration curves for quantification. Standard compounds in the samples were quantified by interpolating the analyte/IS peak abundance ratio in these curves. (−)-Epicatechin, (+)-catechin and (−)-catechin metabolites were tentatively quantified by using the standard (−)-epicatechin and (+)-catechin calibration curves respectively. The sensitivity was evaluated by determining the limit of detection (LOD), which is defined as the concentration that corresponds to three times the signal-to-noise ratio, and the limit of quantification (LOQ), which is defined as the concentration that corresponds to 10 times the signal-to-noise ratio. The method detection and quantification limits (MDL and MQL, respectively) were calculated in the analysis of 250 μl of a sample. Table 1 shows the values that were obtained for each quality parameter.
| Compound | Calibration curve | R 2 | LOD (μM) | LOQ (μM) | MDL (μM) | MQL (μM) |
|---|---|---|---|---|---|---|
| Abbreviations: LOD (limit of detection); LOQ (limit of quantification); MDL (method detection limit); MQL (method quantification limit). The (+)-catechin calibration curve was used to quantify catechin and its metabolites and the (−)-epicatechin calibration curve was used to quantify (−)-epicatechin and its metabolites. | ||||||
| (+)-Catechin | y = 0.0159x | 0.992 | 0.003 | 0.009 | 0.001 | 0.003 |
| (−)-Epicatechin | y = 0.0179x | 0.993 | 0.002 | 0.008 | 0.001 | 0.003 |
All the above-mentioned experiments were designed and performed in accordance with the European and Spanish legislation on care and use of experimental animals (2010/63/UE; Real Decreto 53/2013), and were approved by the Ethics Committees at Universidad Complutense de Madrid (UCM).
2.4. Statistical analysis
SBP results are expressed as mean values ± standard error of the mean (SEM) for a minimum of 8 rats and the plasma results are expressed as mean values ± SEM for a minimum of 6 rats. They were analyzed by a one- or two-way analysis of variance (ANOVA), using the GraphPad Prism 4 software in the case of SBP values, and using the SPSS software (Version 20.0.0) in the case of plasma values. The differences between the groups were assessed by the Bonferroni test and the differences were always considered to be significant when p < 0.05.3. Results
Before administration of the different products, the SHR showed SBP values of 236 ± 2.5 mmHg; n = 30. The values of SBP obtained after the oral administration of 1 ml of 10% DMSO solution (used as the negative control), were very similar to those obtained before its administration. On the contrary, the dose of 50 mg kg−1 of Captopril (used as the positive control) caused a clear decrease in SBP in the SHR. The maximum decrease in SBP caused by this drug (60.5 ± 2.7 mmHg) was observed 4 hours post-administration, and this variable returned to the baseline 48 hours post-administration. The oral administration of (−)-epicatechin also resulted in a significant decrease of the SBP in the SHR. The maximum effect was attained 6–8 hours post-administration of 6 mg kg−1 of this flavan-3-ol. The decreases of SBP at that moment were, respectively, 34.0 ± 4.8 mmHg and 34.3 ± 5.6 mmHg, and SBP remained still lower than the basal SBP value when measurements were made 48 hours post-administration of (−)-epicatechin. Nevertheless, the values of SBP obtained 72 hours post-administration of this flavan-3-ol were similar to those obtained before the administration (Fig. 1A). The administration of (+)-catechin also caused a significant decrease in the SBP of the SHR, and the decrease was maximum 4 hours post-administration of 0.5 mg kg−1 of this flavan-3-ol (29.9 ± 0.8 mmHg). SBP returned to the baseline 48 hours post-administration of this dose of (+)-catechin (Fig. 2A). The administration of (−)-catechin also caused a significant decrease in the SBP of the SHR. Nevertheless, the effect of this flavan-3-ol on this variable was less accentuated than the effect of (−)-epicatechin or (+)-catechin. The more effective dose of (−)-catechin was 0.5 mg kg−1, and the maximum decrease in SBP caused by this dose of this flavan-3-ol was observed 6–8 hours post-administration. At that moment, the decreases in SBP were, respectively, 23.6 ± 5.9 mmHg and 24.5 ± 5.7 mmHg. In addition, we could observe that, 72 hours post-administration of 0.5 mg kg−1 of (−)-catechin, the values of SBP were already similar to those obtained before administration (Fig. 3A).None of the assayed flavan-3-ols modified SBP in the WKY rats. This variable was similar in the WKY rats that were treated with these products and in the WKY rats that were treated with the 10% DMSO solution (Fig. 1B, 2B and 3B).
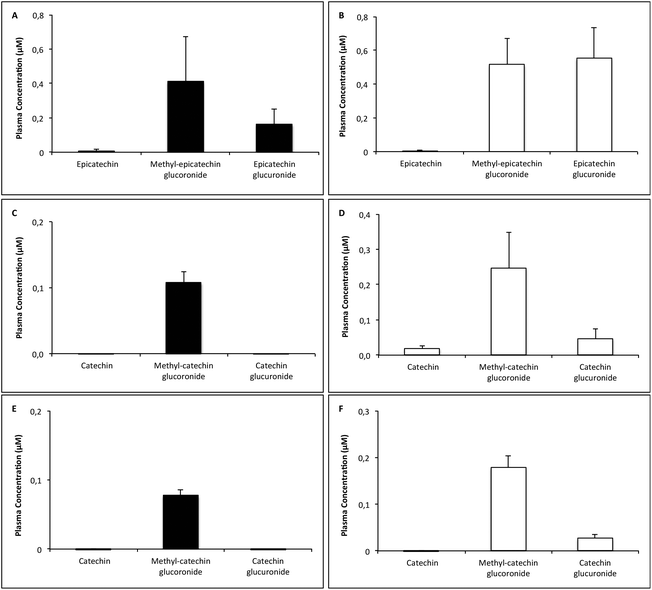
Fig. 4 shows the different flavan-3-ol signals obtained in the chromatographic analysis of plasma samples. This analysis revealed only glucuronide metabolites and methyl-glucuronide metabolites, apart from the original flavan-3-ols, as all other potential phase II metabolites, as sulfated and methylated derivatives, were not detected. In detail, the following concrete metabolites were detected: epicatechin glucuronide, methyl-epicatechin glucuronide, catechin glucuronide and methyl-catechin glucuronide. Fig. 5 shows the concentration of the all these flavan-3-ol compounds, that had been detected in the plasma after the different administrations. As this figure shows, scarce concentrations of unconjugated flavan-3-ols appeared in the plasma of the treated rats, regardless of the administered dose and the time elapsed after the treatment. On the contrary, methyl-glucuronide metabolites were always obtained in this biological fluid after different treatments. Significant concentrations of the glucuronide metabolite were appreciated after 6 mg kg−1 (−)-epicatechin administration, but these conjugated products did not appear in the plasma when the most effective doses of the other flavan-3-ols were administered (0.5 mg kg−1 (+)-catechin or 0.5 mg kg−1 (−)-catechin). However, glucuronide metabolites were obtained when the highest doses of the different flavan-3-ols (12 mg kg−1 (−)-epicatechin, 3 mg kg−1 (+)-catechin or 3 mg kg−1 (−)-catechin) were administered, being the concentration of the glucuronide metabolite particularly high after the administration of 12 mg kg−1 (−)-epicatechin. In any case, no correlation could be established between the plasma concentration of glucuronide and/or methyl-glucuronide metabolites, and the antihypertensive effect caused in the animals by (−)-epicatechin or the catechin monomers. 72 hours post-flavan-3-ol administration, neither flavan-3-ol compounds nor flavan-3-ol metabolites could be detected in plasma (data not shown).
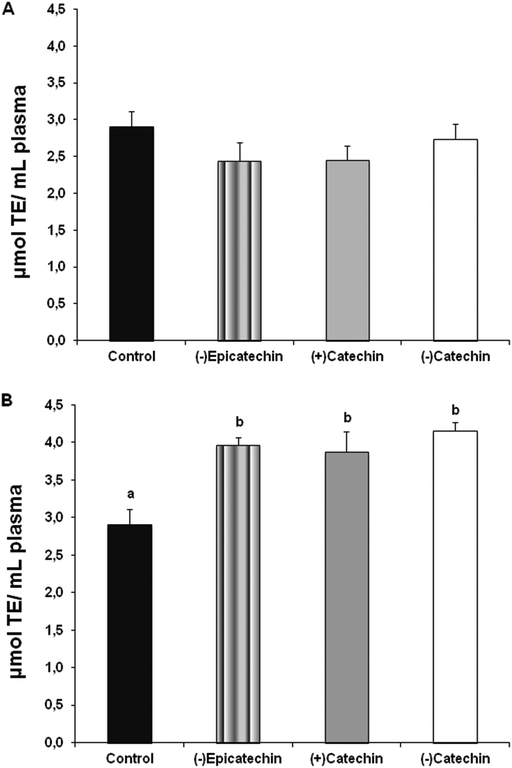
As panel A in Fig. 6 shows, no differences were observed in the plasma antioxidant capacity between the rats administered with 10% DMSO solution and the rats administered with more effective antihypertensive doses of the different flavan-3-ols (6 mg kg−1 (−)-epicatechin, 0.5 mg kg−1 (+)-catechin or 0.5 mg kg−1 (−)-catechin). Nevertheless, as panel B in Fig. 6 shows, the administration of the highest doses of the different flavan-3-ols (12 mg kg−1 (−)-epicatechin, 3 mg kg−1 (+)-catechin or 3 mg kg−1 (−)-catechin) caused always an increase in the plasma antioxidant capacity in the rats.
4. Discussion
SHR are frequently used to carry out initial studies with antihypertensive functional food ingredients because these animals represent nowadays the best experimental model for essential hypertension in humans.26 In the present study, we have demonstrated that the short-term administration of (−)-epicatechin and (+)-catechin, two flavan-3-ols present in cocoa and other different foods, decreased SBP in SHR. (−)-Catechin, an atypical flavan-3-ol produced by changes in the chiral nature of (−)-epicatechin during the cocoa manufacturing process,12 also decreased the arterial blood pressure in this strain. On the contrary, none of these flavan-3-ols decreased SBP in the normotensive WKY rats. The arterial blood pressure effects of the assayed flavan-3-ols are therefore specific for the hypertensive condition.The healthy properties of cocoa seem to be related to its high content in monomeric, dimeric and potentially polymers of flavan-3-ols.27 (−)-Epicatechin is the most abundant monomer in cocoa seeds and cocoa derived foods. In accordance with the results that we have obtained in SHR, a recent study has demonstrated that the long-term administration of 10 mg kg−1 (−)-epicatechin prevented deoxycorticosterone acetate-salt induced hypertension in rats.28 The high amount of (−)-epicatechin in cocoa seems actually to be important, because, in previous studies an increased plasma level of this flavan-3-ol was accompanied by a dose-dependent increase in the plasma antioxidant capacity,29,30 and also with a dose-dependent decrease in plasma lipid oxidation29 and a beneficial effect on the vascular function.31 Moreover, (−)-epicatechin decreases serum oxidative stress8 and restores the NO bioavailability.32 In addition, a study of our research group has also demonstrated the participation of NO in the antihypertensive effect of a polyphenol-rich cocoa in SHR.33
(+)-Catechin predominates in cocoa beans and (−)-catechin in chocolate, and it has been postulated that (+)-catechin is almost 10 times more absorbed than (−)-catechin.12 In this study, the administration of (+)-catechin, and also the administration of (−)-catechin, caused a significant decrease in the SBP of the SHR. Nevertheless, the effect of (−)-catechin on this variable was less accentuated than the effect of (−)-epicatechin or (+)-catechin. In the last few years, an important role has been attributed to the chiral nature of polyphenols and the effects of chirality on bioavailability. Ottaviani et al. have demonstrated the significance of the stereochemical configuration of flavan-3-ols for their biological activity.34 These facts may explain why catechin from processed cocoa [mainly (−)-catechin], is not as well absorbed as (+)-catechin.35 In any case, in this study, the effects of (+)-catechin in SHR, and the effects of (−)-catechin in these animals were not very different, and the metabolites of both catechin monomers in the plasma of the treated rats were also very similar, indicating a similar oral absorption of these two isomers. The effect seems however to last somewhat more when (−)-catechin was administered and we shall comment on this later.
It is therefore clear that all the flavan-3-ols assayed showed a blood pressure lowering effect in the SHR, and, this study enables us to characterize and to compare their antihypertensive effect in these animals. Among the three used flavan-3-ols, (−)-epicatechin was the most effective one, even if (+)-catechin and (−)-catechin were more potent than (−)-epicatechin to decrease the arterial blood pressure in the SHR. In fact, maximum decreases in SBP were obtained when (−)-epicatechin was administered, but lower doses of (+)-catechin and (−)-catechin were needed for the antihypertensive effect. It is also important to highlight that in this study we failed to demonstrate a dose-dependent antihypertensive effect for the three monomers that we have used. In fact, the maximum effect was attained always with a dose different form the highest one (6 mg kg−1 of (−)-epicatechin, 0.5 mg kg−1 of (+)-catechin and 0.5 mg kg−1 of (−)-catechin). A similar paradox was observed when we had administered a polyphenol-rich cocoa powder in SHR, since the maximum antihypertensive effect in these animals was neither obtained when we administered the highest dose of this cocoa powder.9 The results obtained by our research group using both the cocoa monomers and the cocoa powder are somewhat difficult to understand. They might be explained by keeping in mind different studies that demonstrated that a high quantity of polyphenols could exhibit pro-oxidant properties instead of antioxidant properties.36,37 It is true that, in the present study, an increase in the dose of the assayed flavan-3-ols was related to an increased plasma antioxidant capacity, but the plasma antioxidant capacity cannot totally define the endothelium redox status. An improved vascular oxidative stress in the SHR treated with the most effective doses of the assayed flavan-3-ols, or a pro-oxidant effect on arterial tissue in the rats previously treated with the highest doses of these compounds, cannot be ruled out. In addition, it is also important to bear in mind that other properties of the assayed flavan-3-ols could explain their blood pressure effects. In this context, activation of the deacetylase sirtuin 1 (SIRT1) and up-regulation of endothelial nitric oxide synthase38,39 have also been proposed to explain the cardiovascular effects of polyphenols. Moreover, the effects of polyphenols have also been attributed to the induction of antioxidant enzymes in cardiovascular tissues40,41 and also to the inhibition of the angiotensin converting enzyme.42
On seeing the period of time elapsed to recover the baseline values of SBP from administration, we can also assume that the effects of (−)-epicatechin and (−)-catechin (Fig. 1A and 3A) were longer than the antihypertensive effect of (+)-catechin (Fig. 2A). The in vivo bioactivity of the flavan-3-ols depends on their process of absorption and metabolism after ingestion, and the reducing properties of resulting metabolites. The highest plasma peak concentrations of flavan-3-ols in humans are obtained 2–3 hours after ingestion of these compounds29,30 and they are still measurable after 8 hours.43 Nevertheless, it is important to note that in this study the metabolite profile has been performed for 4 and 6 h in order to evaluate the bioavailable plasma metabolites at the maximum blood pressure decrease time point. In this study, the effect of (−)-epicatechin, (+)-catechin and (−)-catechin could also be appreciated 8 hours post-administration. It should be noted that (−)-epicatechin and (+)-catechin in particular, showed a good bioavailability in humans.13 Flavan-3-ols are conjugated after ingestion by phase-II enzymes in the small intestine and liver, and it has been demonstrated that some of the beneficial effects of these compounds are due to the metabolized forms.14 Even if (−)-epicatechin and catechin monomers are usually conjugated to produce sulphate, glucuronide and methyl-glucuronide metabolites,14,44 in this study, only glucuronide and methyl-glucuronide metabolites were quantified in the plasma from the treated rats. These metabolites, but not sulphate metabolites, could therefore be responsible for the antihypertensive effects of the assayed flavan-3-ols. At first sight, the products enriched in these monomers might represent a good strategy in biomedicine, but it is nevertheless true that no correlation could be established in our study between the plasma concentration of the glucuronide and methyl-glucuronide metabolites, and the antihypertensive effect caused by the (−)-epicatechin or the catechin monomers. Thus, when the most effective doses of these flavan-3-ols were administered, we did not obtain the highest concentration of these metabolites. Moreover, the administration of the most effective doses of the catechin monomers (0.5 mg kg−1 for both catechin monomers) were not accompanied with glucuronide metabolites in plasma, and this suggests that methylglucuronide metabolites could actually be responsible for the effect of the catechin monomers. Glucuronide metabolites appeared nevertheless in the plasma obtained from the rats that were treated with the highest dose of these monomers, and also in all the plasma samples from the (−)-epicatechin treated rats, but the concentration of the (−)-epicatechin glucuronide metabolite was also lower in the plasma of the rats treated with the most effective antihypertensive dose of (−)-epicatechin (6 mg kg−1) than in the plasma obtained from the rats that had been treated with the highest dose of this flavan-3-ol (12 mg kg−1). It seems in addition that according to our results, the effect of the assayed flavan-3-ols was always elapsed 72 hours after their administration and that at this time no flavan-3-ol metabolites were present in the plasma of the rats.
In conclusion, we have demonstrated the antihypertensive properties of the main cocoa flavan-3-ols in SHR and we have also demonstrated that the effect of the evaluated monomers is specific to the hypertensive conditions. Therefore, the flavan-3-ols (−)-epicatechin and (+)-catechin, and also the atypical epimer (−)-catechin, would be beneficial for controlling arterial blood pressure. They could be responsible for the anti-hypertensive effect of different cocoa powders and functional foods. The present study also represents a good contribution to clarify the metabolites generated in the SHR when these flavan-3-ols are administered to these animals. Nevertheless, our results neither enable us to relate the antihypertensive effect of the assayed flavan-3-ols with the presence of a concrete flavan-3-ol metabolite in plasma, nor to consider the antioxidant effect of the used flavan-3-ols as their main antihypertensive mechanism. The concentration of flavan-3-ol metabolites in arterial tissues may provide interesting information in the future to elucidate their cardiovascular role, and further research should be interesting to go deep into the mechanisms that could explain the antihypertensive effects of the assayed flavan-3-ols.
Acknowledgements
This study was supported by Natraceutical Group (36/2007 U.C.M. Project) and for the Spanish Government (AGL2013-40707-R). We also thank Manuel Bas Caro, Technician in Pharmacology, for his excellent care of the rats. We are grateful to PhD student Zara Pons (Nutrigenomic group, Department of Biochemistry and Biotechnology, Rovira i Virgili University) for her excellent collaboration. M.Q. is a recipient of a Postdoctoral fellowship from Galician Government (Xunta de Galicia ED481B2014/039-0).References
- M. Galleano, O. Pechanova and C. G. Fraga, Curr. Pharm. Biotechnol., 2010, 11, 837–848 CAS.
- A. Lynn, H. Hamadeh, W. C. Leung, J. M. Russell and M. E. Barker, Plant Foods Hum. Nutr., 2012, 67, 309–314 CrossRef CAS PubMed.
- A. S. Mathew, G. M. Capel-Williams, S. E. E. Berry and W. L. Hall, Plant Foods Hum. Nutr., 2012, 67, 351–357 CrossRef CAS PubMed.
- N. Mihailovic-Stanojevic, A. Belščak-Cvitanović, J. Grujić-Milanović, M. Ivanov, D. Jovović, D. Bugarski and Z. Miloradović, Plant Foods Hum. Nutr., 2013, 68, 235–240 CrossRef CAS PubMed.
- S. Ellinger, A. Reusch, P. Stehle and H.-P. Helfrich, Am. J. Clin. Nutr., 2012, 95, 1365–1377 CrossRef CAS PubMed.
- R. Corti, A. J. Flammer, N. K. Hollenberg and T. F. Lüscher, Circulation, 2009, 119, 1433–1441 CrossRef PubMed.
- K. W. Lee, Y. J. Kim, H. J. Lee and C. Y. Lee, J. Agric. Food Chem., 2003, 51, 7292–7295 CrossRef CAS PubMed.
- H. Schroeter, C. Heiss, J. Balzer, P. Kleinbongard, C. L. Keen, N. K. Hollenberg, H. Sies, C. Kwik-Uribe, H. H. Schmitz and M. Kelm, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 1024–1029 CrossRef CAS PubMed.
- E. Cienfuegos-Jovellanos, M. Quiñones, B. Muguerza, L. Moulay, M. Miguel and A. Aleixandre, J. Agric. Food Chem., 2009, 57, 6156–6162 CrossRef CAS PubMed.
- M. Quiñones, D. Sánchez, B. Muguerza, L. Moulay, S. Laghi, M. Miguel and A. Aleixandre, Food Chem., 2010, 122, 1013–1019 CrossRef PubMed.
- M. Quiñones, D. Sánchez, B. Muguerza, M. Miguel and A. Aleixandre, Food Res. Int., 2011, 44, 1203–1208 CrossRef PubMed.
- M. Kofink, M. Papagiannopoulos and R. Galensa, Molecules, 2007, 12, 1274–1288 CrossRef CAS.
- F. A. Tomas-Barberan, E. Cienfuegos-Jovellanos, A. Marín, B. Muguerza, A. Gil-Izquierdo, B. Cerda, P. Zafrilla, J. Morillas, J. Mulero, A. Ibarra, M. A. Pasamar, D. Ramón and J. C. Espín, J. Agric. Food Chem., 2007, 55, 3926–3935 CrossRef CAS PubMed.
- L. Guerrero, M. Margalef, Z. Pons, M. Quiñones, L. Arola, A. Arola-Arnal and B. Muguerza, J. Nutr. Biochem., 2013, 24, 2092–2099 CrossRef CAS PubMed.
- M. Margalef, L. Guerrero, Z. Pons, F. I. Bravo, L. Arola, B. Muguerza and A. Arola-Arnal, Food Res. Int., 2014, 64, 500–507 CrossRef CAS PubMed.
- M. Monagas, M. Urpi-Sarda, F. Sánchez-Patán, R. Llorach, I. Garrido, C. Gómez-Cordovés, C. Andres-Lacueva and B. Bartolomé, Food Funct., 2010, 1, 233–253 CAS.
- D. Del Rio, A. Rodriguez-Mateos, J. P. E. Spencer, M. Tognolini, G. Borges and A. Crozier, Antioxid. Redox Signaling, 2013, 18, 1818–1892 CrossRef CAS PubMed.
- A. L. Waterhouse, J. R. Shirley and J. L. Donovan, Lancet, 1996, 348, 834 CrossRef CAS.
- M. Karim, K. McCormick and C. T. Kappagoda, J. Nutr., 2000, 130, 2105S–22108 CAS.
- E. Ramiro, A. Franch, C. Castellote, F. Pérez-Cano, J. Permanyer, M. Izquierdo-Pulido and M. Castell, J. Agric. Food Chem., 2005, 53, 8506–8511 CrossRef CAS PubMed.
- E. O. Cuevas-Rodríguez, V. P. Dia, G. G. Yousef, P. A. García-Saucedo, J. López-Medina, O. Paredes-López, E. Gonzalez de Mejia and M. A. Lila, J. Agric. Food Chem., 2010, 58, 9542–9548 CrossRef PubMed.
- R. D. Buñag, J. Appl. Physiol., 1973, 34, 279–282 Search PubMed.
- M. Margalef, Z. Pons, B. Muguerza and A. Arola-Arnal, J. Agric. Food Chem., 2014, 62, 7698–7706 CrossRef CAS PubMed.
- A. Serra, A. Macià, M. P. Romero, M. J. Salvadó, M. Bustos, J. Fernández-Larrea and M. J. Motilva, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2009, 877, 1169–1176 CrossRef CAS PubMed.
- D. Huang, B. Ou, M. Hampsch-Woodill, J. A. Flanagan and R. L. Prior, J. Agric. Food Chem., 2002, 50, 4437–4444 CrossRef CAS PubMed.
- K. Okamoto and K. Aoki, Jpn. Circ. J., 1963, 27, 282–293 CrossRef CAS.
- K. A. Cooper, J. L. Donovan, A. L. Waterhouse and G. Williamson, Br. J. Nutr., 2008, 99, 1–11 CrossRef CAS PubMed.
- M. Gómez-Guzmán, R. Jiménez, M. Sánchez, M. J. Zarzuelo, P. Galindo, A. M. Quintela, R. López-Sepúlveda, M. Romero, J. Tamargo, F. Vargas, F. Pérez-Vizcaíno and J. Duarte, Free Radical Biol. Med., 2012, 52, 70–79 CrossRef PubMed.
- D. Rein, S. Lotito, R. R. Holt, C. L. Keen, H. H. Schmitz and C. G. Fraga, J. Nutr., 2000, 130, 2109S–2114S CAS.
- M. Serafini, R. Bugianesi, G. Maiani, S. Valtuena, S. De Santis and A. Crozier, Nature, 2003, 424, 1013 CrossRef CAS PubMed.
- C. Heiss, A. Dejam, P. Kleinbongard, T. Schewe, H. Sies and M. Kelm, JAMA, 2003, 290, 1030–1031 CrossRef PubMed.
- M. C. Litterio, G. Jaggers, G. Sagdicoglu Celep, A. M. Adamo, M. A. Costa, P. I. Oteiza, C. G. Fraga and M. Galleano, Free Radical Biol. Med., 2012, 53, 1894–1902 CrossRef CAS PubMed.
- M. Quiñones, B. Muguerza, M. Miguel and A. Aleixandre, Pharmacol. Res., 2011, 64, 478–481 CrossRef PubMed.
- J. I. Ottaviani, T. Y. Momma, C. Heiss, C. Kwik-Uribe, H. Schroeter and C. L. Keen, Free Radical Biol. Med., 2011, 50, 237–244 CrossRef CAS PubMed.
- J. L. Donovan, C. Manach, R. M. Faulks and P. A. Kroon, in Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet, ed. A. Crozier, M. N. Clifford and H. Ashihara, Blackwell Publishing, Oxford, 2006, pp. 303–351 Search PubMed.
- B. S. P. Nicole Cotelle, Curr. Top. Med. Chem., 2001, 1, 569–590 CrossRef.
- M. Lahouel, S. Amedah, A. Zellagui, A. Touil, S. Rhouati, F. Benyache, E. Leghouchi and H. Bousseboua, Therapie, 2007, 61, 347–355 CrossRef.
- I. Mattagajasingh, C.-S. Kim, A. Naqvi, T. Yamamori, T. A. Hoffman, S.-B. Jung, J. DeRicco, K. Kasuno and K. Irani, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 14855–14860 CrossRef CAS PubMed.
- T. Wallerath, Circulation, 2002, 106, 1652–1658 CrossRef CAS PubMed.
- Z. Cao and Y. Li, Cardiovasc. Toxicol., 2004, 4, 339–354 CrossRef CAS PubMed.
- Y. Li, Z. Cao and H. Zhu, Pharmacol. Res., 2006, 53, 6–15 CrossRef CAS PubMed.
- L. Guerrero, J. Castillo, M. Quiñones, S. Garcia-Vallvé, L. Arola, G. Pujadas and B. Muguerza, PLoS One, 2012, 7, e49493 CAS.
- M. Richelle, I. Tavazzi, M. Enslen and E. A. Offord, Eur. J. Clin. Nutr., 1999, 53, 22–26 CAS.
- A. Arola-Arnal, G. Oms-Oliu, A. Crescenti, J. M. Del Bas, M. R. Ras, L. Arola and A. Caimari, Mol. Nutr. Food Res., 2013, 57, 1741–1752 CAS.
Footnote |
| † Present address: Department of Physiology, CIMUS, University of Santiago de Compostela-Instituto de Investigación Sanitaria, Santiago de Compostela, 15782, Spain. CIBER Fisiopatología de la Obesidad y Nutrición (CIBERobn), 15706, Spain. |
| This journal is © The Royal Society of Chemistry 2015 |