Comparative study of tissue deposition of omega-3 fatty acids from polar-lipid rich oil of the microalgae Nannochloropsis oculata with krill oil in rats
Michael L.
Kagan
*a,
Aharon
Levy
b and
Alicia
Leikin-Frenkel
c
aQualitas Health Ltd, 19 Hartom Street, P.O. Box 45423, Jerusalem 91450, Israel. E-mail: mkagan@qualitas-health.com
bPharmaseed Ltd, 9 Hamazmera St., Ness Ziona 74047, Israel
cSackler School of Medicine, Tel Aviv University and The Bert W. Strassburger Lipid Center, Sheba Medical Center, Tel Hashomer, 52621, Israel
First published on 20th October 2014
Abstract
Long-chain omega-3 polyunsaturated fatty acids (LC n-3 PUFA) exert health benefits which are dependent upon their incorporation into blood, cells and tissues. Plasma and tissue deposition of LC n-3 PUFA from oils extracted from the micro-algae Nannochloropsis oculata and from krill were compared in rats. The algal oil provides eicosapentaenoic acid (EPA) partly conjugated (15%) to phospholipids and glycolipids but no docosahexaenoic acid (DHA), whereas krill oil provides both EPA and DHA conjugated in part (40%) to phospholipids. Rats fed a standard diet received either krill oil or polar-lipid rich algal oil by gavage daily for 7 days (5 ml oil per kg body weight each day). Fatty acid concentrations were analyzed in plasma, brain and liver, and two adipose depots since these represent transport, functional and storage pools of fatty acids, respectively. When measuring total LC n-3 PUFA (sum of EPA, docosapentaenoic acid (DPA) and DHA), there was no statistically significant difference between the algal oil and krill oil for plasma, brain, liver and gonadal adipose tissue. Concentrations of LC n-3 PUFA were higher in the retroperitoneal adipose tissue from the algal oil group. Tissue uptake of LC n-3 PUFA from an algal oil containing 15% polar lipids (glycolipids and phospholipids) was found to be equivalent to krill oil containing 40% phospholipids. This may be due to glycolipids forming smaller micelles during ingestive hydrolysis than phospholipids. Ingestion of fatty acids with glycolipids may improve bioavailability, but this needs to be further explored.
Introduction
The two long-chain omega-3 polyunsaturated fatty acids (LC n-3 PUFA) of most importance to human health are eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3).1,2 EPA and DHA exert a wide range of physiological effects impacting on brain and visual development,3,4 cardiovascular morbidity and mortality,5,6 inflammatory conditions,7,8 cognitive decline9 and cancer risk.10,11 The effects of LC n-3 PUFA on human health outcomes rely upon the incorporation of those fatty acids into bloods, cells and tissues.1,2 Due to their beneficial effects on human health, particularly the cardio-protective effects, there have been recommendations that individuals should increase their daily intake of LC n-3 PUFA.12,13 Seafood, especially fatty fish, is a good source of EPA and DHA. However, advice to increase fish consumption has had limited effect. Supplements in the form of oil capsules containing purified or processed fish oil offer an opportunity for consumers to increase their LC n-3 PUFA intake without changing their diet. However, fish oil presents issues of sustainability and therefore alternative sources of EPA and DHA are being sought. These include krill oil,14 algal oils15 and other non-fish oils.16–18 These oils contain different amounts and relative proportions of EPA and DHA and present the LC n-3 PUFA in different chemical forms. For example, in fish oils the LC n-3 PUFA are primarily conjugated to a triglyceride (TAG) backbone, whereas in krill oil the fatty acids are largely conjugated to phospholipids.19 This phospholipid structure in krill oil has been shown to promote improved absorption of LC n-3 PUFA into blood plasma compared to TAG structures found in fish oil.20 It is important to identify whether other chemical forms of LC n-3 PUFA also show similar or even better absorption and incorporation of LC n-3 PUFA.Various species of the algal genus Nannochloropsis have been found to contain high concentrations of EPA with no DHA21 and to present the LC n-3 PUFA as a mixture of phospholipids and glycoplipids (polar-lipids).22 We recently compared the appearance of EPA and DHA in plasma of healthy humans taking krill oil or polar-lipid rich oil from Nannochloropsis oculata over 10 hours following the oil consumption as part of a high fat meal.23 We found that when the subjects consumed the polar-rich algal oil they had higher post-prandial EPA concentrations in their plasma than when they consumed the krill oil. When comparing the content of phospholipids in krill oil (∼40%) to the polar-lipids in algal oil (∼15%), where the main difference is the presence of glycolipids, it may be inferred from the results of this study that LC n-3 PUFA, and EPA specifically, when conjugated to glycolipids, may be more efficiently handled in the gastrointestinal tract; this may relate to enhanced digestion or absorption. This suggests that the glycolipids in algal oil may offer an advantage in delivering EPA to blood plasma and thus in influencing those biological functions where EPA is important.
Thus far, the appearance of LC n-3 PUFA from the novel algal oil has only been examined acutely (i.e. over 10 hours following consumption by healthy human volunteers).23 In the current study, we examined the incorporation of LC n-3 PUFA not only into plasma but further into several tissues in the rat. Thus this rat study represents a natural extension of our earlier human study. We set out to compare krill oil and polar-lipid rich oil from Nannochloropsis oculata by providing these two oils to rats daily for seven days. We analyzed the EPA, docosapentaenoic acid (DPA; 22:5n-3) and DHA concentrations of plasma, brain and liver, and two adipose depots. These sites were selected because they represent transport, functional and storage pools of fatty acids,24 because liver and brain represent key targets for functional activity of LC n-3 PUFA,25–29 and because these sites have all been studied in earlier research evaluating incorporation patterns of LC n-3 PUFA in rats.30–32
Results
Body weight
Body weight did not differ between groups at study entry and was not different between groups after 3 or 7 days of oil treatment (data not shown). Two animals, one female from the krill oil group and one male from the algae oil group were found dead in their cages. Postmortem analysis for the cause of death was not possible since the animals died overnight.LC n-3 PUFA in plasma
Table 1 shows the LC n-3 PUFA concentrations in plasma in rats receiving either algal oil or krill oil for 7 days. There was no statistically significant difference between total LC n-3 PUFA in the plasma, although EPA was higher and DHA lower in the plasma of rats receiving polar-rich algal oil compared with those receiving krill oil.| Algal oil | Krill oil | |
|---|---|---|
| Plasma | ||
| EPA | 9.8** (6.25, 13.34) | 5.22 (3.89, 6.55) |
| DPA | 0.86 (0.02, 1.7) | 1.24 (−0.19, 2.67) |
| DHA | 1.31** (0.81, 1.81) | 3.48 (2.46, 7.99) |
| Total EPA + DPA | 10.66* (6.52, 14.79) | 6.46 (4.94, 7.99) |
| Total LC n-3 PUFA (EPA + DPA + DHA) | 11.97 (7.42, 16.51) | 9.95 (8.12, 11.78) |
LC n-3 PUFA in tissues
Table 2 shows the LC n-3 PUFA concentrations in liver, brain and two adipose depots in rats receiving either algal oil or krill oil for 7 days. There was no difference between the two groups in total LC n-3 PUFA in the brain, liver, or gonadal adipose tissue, but there was a higher total LC n-3 PUFA content in retroperitoneal adipose tissue with the polar-lipid rich algal oil. Looking at the specific LC n-3 PUFA, there was no difference in the brain, while DPA was higher and DHA lower in the liver of rats receiving algal oil compared with those receiving krill oil. Retroperitoneal adipose tissue had a higher LC n-3 PUFA content than gonadal adipose tissue and EPA and DPA concentrations were higher in retroperitoneal adipose tissue of rats receiving algal oil, while DHA concentration was higher in gonadal adipose tissue from rats receiving krill oil.| Algal oil | Krill oil | |
|---|---|---|
| Liver | ||
| EPA | 116.1 (90.53, 141.7) | 95.79 (64.3, 127.3) |
| DPA | 116.2** (88.79, 143.6) | 73.37 (55.3, 91.44) |
| DHA | 112.8** (58.8, 166.7) | 297.0 (209.9, 384.1) |
| Total EPA + DPA | 232.3* (185.7, 279.0) | 169.2 (120.9, 217.4) |
| Total LC n-3 PUFA (EPA + DPA + DHA) | 345.1 (257.8, 432.3) | 466.2 (340.6, 591.8) |
| Brain | ||
| EPA | 3.17 (2.09, 4.24) | 2.06 (1.19, 2.94) |
| DPA | 7.93 (6.25, 9.61) | 10.77 (2.68, 18.87) |
| DHA | 210.2 (161.9, 258.6) | 213.1 (147.6, 278.6) |
| Total EPA + DPA | 11.1 (8.82, 13.37) | 12.84 (4.43, 21.25) |
| Total LC n-3 PUFA (EPA + DPA + DHA) | 221.3 (171.0, 271.5) | 225.9 (159.1, 292.7) |
| Gonadal adipose tissue | ||
| EPA | 74.08* (39.38, 108.8) | 38.87 (25.59, 52.15) |
| DPA | 29.66 (14.91, 44.4) | 21.02 (14.06, 27.98) |
| DHA | 21.78** (12.1, 31.46) | 47.38 (40.25, 79.53) |
| Total EPA + DPA | 103.7* (54.44, 153.0) | 59.89 (40.25, 79.53) |
| Total LC n-3 PUFA (EPA + DPA + DHA) | 125.5 (67.23, 183.8) | 107.3 (73.75, 140.8) |
| Retroperitoneal adipose tissue | ||
| EPA | 387.1** (231.0, 543.2) | 125.8 (26.49, 225.1) |
| DPA | 111.7** (79.84, 143.5) | 53.51 (12.73, 94.29) |
| DHA | 56.61 (39.65, 73.56) | 158.9 (17.63, 300.2) |
| Total EPA + DPA | 498.8** (312.2, 685.4) | 179.3 (39.67, 318.9) |
| Total LC n-3 PUFA (EPA + DPA + DHA) | 555.4* (368.9, 741.9) | 338.2 (57.33, 619.1) |
Discussion
A recent comparison of the appearance of EPA and DHA in plasma of healthy humans taking krill oil or polar-lipid rich oil from Nannochloropsis oculata over 10 hours following the oil consumption as part of a high fat meal found that when the subjects consumed the algal oil they had higher post-prandial EPA concentrations in their plasma than when they consumed the krill oil.23 In the current study, blood plasma of rats receiving the algal oil showed significantly higher amounts of EPA and lower amounts of DHA. This reflects the different distributions of EPA and DHA between algal oil (25% EPA and no DHA) and krill oil (15% EPA and 8% DHA).The focus of the current study was the longer-term appearance of EPA and DHA from krill oil and polar-lipid rich oil from Nannochloropsis oculata in tissues of rats. This is important as an extension of the previous human study because it is not generally feasible to biopsy tissues from humans. The fatty acids were measured in plasma, brain and liver, and two adipose depots since these represent transport, functional and storage pools of fatty acids, respectively.24 Krill oil contains both EPA and DHA and 40% phospholipids while the algal oil contains only EPA and 6% phospholipids and 9% glycolipids. When measuring total LC n-3 PUFA, there was no difference in plasma, brain, liver or gonadal adipose tissue between the two oils. Polar-lipid rich algal oil resulted in a significantly higher level of LC n-3 PUFA (as EPA) in retroperitoneal adipose tissue. There was an average 3-fold differential in EPA content of retroperitoneal adipose tissue between groups which is much greater than the difference in EPA content of the two oils.
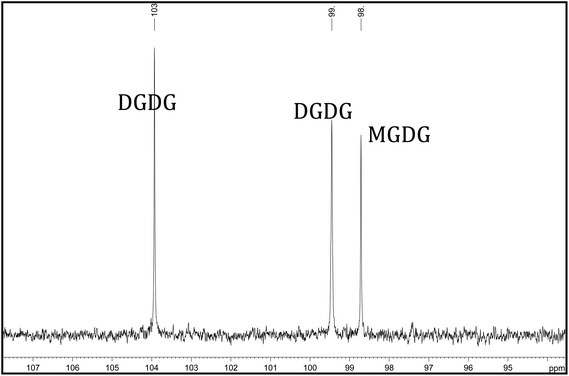
Glycolipids are a class of compounds containing one or more monosaccharides bound by a glycosidic linkage to a hydrophobic membrane-anchoring compound such as an acylglycerol or a sphingoid. Galactolipids are a type of glycolipid whose sugar group is galactose and in plants consist mainly of monogalactosyldiacylglycerols (MGDG) and digalactosyldiacylglycerols (DGDG) (Fig. 1) containing one or two saturated and/or unsaturated fatty acids linked to the glycerol moiety.35,36 Galactolipids are important food constituents in both animals and humans and are an important source of essential fatty acids.37 Both macro-algae38 and micro-algae22 contain glycolipids. MGDG and DGDG levels have been measured by 13C NMR (Fig. 2) in Nannochloropsis and found to be conjugated across the fatty acid spectrum.22 The role of galactolipids as intracellular messengers has been investigated by Wakelam39 and as anti-inflammatory agents by Lenti et al.40 and Bruno et al.41
In a study on the bioavailability and accumulation of lutein in mice, Gorusupudi and Vallikannan42 found that the percent of micellarization of lutein was higher with glycolipids than phospholipids and neutral lipids. Likewise, the mean plasma lutein response was higher for glycolipids than for phospholipids and neutral lipids. The authors postulated that these differences might be due to smaller micellar size with glycolipids that would favour absorption.
In this study, the presence of glycolipids in the polar-lipid rich algal oil and their different digestion and metabolism might explain the tissue uptake of the LC n-3 PUFA (EPA). While the total amount of polar lipids was lower in algal oil compared to krill oil (15% vs. 40%, respectively), tissue uptake was similar, and EPA uptake in retroperitoneal adipose tissue was higher with algal oil. Further research is needed to understand the specific function and mechanism of glycolipids in LC n-3 PUFA digestion, absorption and metabolism.
EPA, DPA and DHA concentrations did not differ between the brains of rats receiving the two oils. The feeding time used here was short (7 days) and the lack of effect on brain fatty acids reflects the relative insensitivity of the brain to dietary fatty acid modification.
Although the total LC n-3 PUFA content of the liver was not different between groups, animals in the algal oil group had a higher hepatic DPA concentration than those in the krill oil group. The sum of EPA plus DPA did show a significant difference between groups. This suggests some elongation of EPA to DPA occurs in the liver of rats in the algal oil group. This elongation would use EPA and may explain why hepatic EPA did not differ between the two groups of rats.
Total EPA concentration in retroperitoneal adipose tissue was higher in rats in the algal oil group compared with those in the krill oil group. Conversely DHA concentration was higher in gonadal adipose tissue of rats in the krill oil group. These differences reflect the differences in fatty acid content of the two oils.
One interesting observation made in the current study is that the EPA, DPA and DHA contents were higher in retroperitoneal than in gonadal adipose tissue in the rats in the algal oil group, although this was not seen in those in the krill oil group. The higher DPA in retroperitoneal adipose tissue of rats receiving algal oil may reflect local synthesis of DPA from EPA or may reflect that DPA (resulting from hepatic synthesis) is readily taken up by this adipose tissue store. Nevertheless some of the rats in the krill oil group did show high EPA, DPA and DHA contents in their retroperitoneal adipose tissue. These findings suggest that different adipose depots may take up and store LC n-3 PUFA differentially. There is support for this suggestion from the literature.43,45 First, de Heredia et al.43 reported much higher DHA in the mesenteric adipose tissue than in gonadal or subcutaneous adipose tissue of female rats fed a high fat diet containing some EPA and DHA. Secondly, Tou et al.44 reported higher (on average about 2-fold higher) EPA and DHA in retroperitoneal adipose tissue than in gonadal adipose tissue from female rats fed a high fat diet with various sources of preformed EPA and DHA. It is not clear what the mechanism underlying the differential enrichment of adipose tissue with LC n-3 PUFA is, but this may be important if dietary fatty acid interventions are to be used to influence adipose tissue biology. The current findings alongside those in the literature43,44 indicate that some adipose depots may be more sensitive than others to the influence of dietary LC n-3 PUFA.
One limitation of the current study is that there was no group that did not receive a LC n-3 PUFA rich oil. However, it is known that the EPA and DPA contents of most rat tissues are very low if the animals do not receive preformed EPA.43–45 Conversely the brain, and some other tissues like the heart, contain significant amounts of DHA even when the diet is very low in LC n-3 PUFA.44,45 This limitation does not detract from the main focus of this study, which was to observe whether the LC n-3 PUFA concentration of selected tissues would be higher in rats receiving polar-lipid rich oil from Nannochloropsis oculata than in those receiving krill oil.
Experimental
Ethics statement
The study was performed after approval by “The Israel Board for Animal Experiments” and in compliance with “The Israel Animal Welfare Act,” Ethics Approval Number IL-13-03-028. As such it adhered to the guidelines of the National Institute of Health and the Association for Assessment and Accreditation of Laboratory Animal Care.Animals and diets
Adult male and female Sprague-Dawley rats weighing approximately 250 g were used in the study, 10 from each sex, a number consistent with previously reported studies of this type. The number of animals was approved by the Ethics Committee to overcome individual differences and to ensure statistically significant results.Animals were housed under standard laboratory conditions, air conditioned and filtered with adequate fresh air supply (minimum 15 air changes per hour). Animals were kept in a climate controlled environment: the temperature range was between 20 and 24 °C and the relative humidity range was between 30 and 70% with a 12 hours light and 12 hours dark cycle. Animals were housed in polyethylene cages (3 rats per cage) measuring 35 × 30 × 15 cm, with a stainless steel top grill facilitating pelleted food and drinking water in a plastic bottle. Bedding was steam sterilized clean paddy husk (Harlan, Sani-chip) and was changed along with the cage at least twice a week.
Animals were fed ad libitum a commercial rodent diet (Certified Global 18% Protein Diet; Teklad, Madison, WI, USA). The diet contained (per kg diet) 180 g protein, 60 g fat (as soybean oil) and 440 g carbohydrate. Contributions to energy intake for protein, fat and carbohydrate were 24%, 18% and 58%, respectively. The fatty acid composition of the diet was as follows (g/100 g total fatty acid): palmitic acid (16:0): 11.7; stearic acid (18:0): 3.3; oleic acid (18:1n-9): 20; linoleic acid (18:2n-6): 51.7; α-linolenic acid (18:3n-3): 5.0.
Each day for 7 days the animals received 5 ml of supplement oil homogenized with 5 ml olive oil per kg body weight by oral gavage. Dilution and warming in a water bath to 35 °C before gavage was necessary because of the high viscosity of both the krill oil and the algal oil. Krill oil contained 23% EPA + DHA and 41% phospholipids (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EPA–DHA; Neptune Technologies) and algal oil 25% EPA and no DHA (Qualitas Health) with 6% phospholipids and 9% glycolipids. Therefore, over the course of the study, the animals were fed a total of 7.245 g kg−1 body weight EPA + DHA fatty acids from krill oil and 7.315 g kg−1 body weight EPA from algal oil. To put these amounts of oil and of LC n-3 PUFA into context, rats weighing 250 g eat about 25 g of food daily. In the current study, the diet contained about 60 g of fat per kg. Thus, these rats were eating about 2 g of fat from their diet each day. The amount of oil provided by gavage (10 ml kg−1 body weight each day) was 2.5 g each day for a 250 g rat. Thus the gavage slightly more doubled daily fat intake. As far as LC n-3 PUFA are concerned, a 250 g rat received about 0.26 g per day. Thus, LC n-3 PUFA contributed approximately 5.8% of total fat intake. This is higher than minimum recommendations made for humans which equate to about 0.5 to 1% of dietary fatty acids; for example intake of LC n-3 PUFA at the level of the minimum UK recommendation (0.45 g per day)12 by a woman or man consuming the average amount of fat for UK adults (60 and 80 g per day, respectively) would equate to an intake of about 0.8 and 0.6% of total dietary fatty acids, respectively. Contributions of LC n-3 PUFA from concentrated supplements, from prescription preparations and from fatty fish to fat intake would be greater than this. For example, the maximum prescribable dose of LC n-3 PUFA (4 g product providing 3.6 g EPA + DHA) equates to an LC n-3 PUFA contribution of 4.5% of total dietary fatty acids in a person consuming 80 g fat per day, and even more if that person is consuming a low fat diet. Finally, it is worth noting that in many experiments rodents are fed diets providing much more LC n-3 PUFA than used in the current study. For example, Yaqoob et al.45 fed rats diets providing 200 g fish oil per kg diet, 20% of which was EPA + DHA, resulting in EPA + DHA intakes of 1 g per day for a 250 g rat.
1 EPA–DHA; Neptune Technologies) and algal oil 25% EPA and no DHA (Qualitas Health) with 6% phospholipids and 9% glycolipids. Therefore, over the course of the study, the animals were fed a total of 7.245 g kg−1 body weight EPA + DHA fatty acids from krill oil and 7.315 g kg−1 body weight EPA from algal oil. To put these amounts of oil and of LC n-3 PUFA into context, rats weighing 250 g eat about 25 g of food daily. In the current study, the diet contained about 60 g of fat per kg. Thus, these rats were eating about 2 g of fat from their diet each day. The amount of oil provided by gavage (10 ml kg−1 body weight each day) was 2.5 g each day for a 250 g rat. Thus the gavage slightly more doubled daily fat intake. As far as LC n-3 PUFA are concerned, a 250 g rat received about 0.26 g per day. Thus, LC n-3 PUFA contributed approximately 5.8% of total fat intake. This is higher than minimum recommendations made for humans which equate to about 0.5 to 1% of dietary fatty acids; for example intake of LC n-3 PUFA at the level of the minimum UK recommendation (0.45 g per day)12 by a woman or man consuming the average amount of fat for UK adults (60 and 80 g per day, respectively) would equate to an intake of about 0.8 and 0.6% of total dietary fatty acids, respectively. Contributions of LC n-3 PUFA from concentrated supplements, from prescription preparations and from fatty fish to fat intake would be greater than this. For example, the maximum prescribable dose of LC n-3 PUFA (4 g product providing 3.6 g EPA + DHA) equates to an LC n-3 PUFA contribution of 4.5% of total dietary fatty acids in a person consuming 80 g fat per day, and even more if that person is consuming a low fat diet. Finally, it is worth noting that in many experiments rodents are fed diets providing much more LC n-3 PUFA than used in the current study. For example, Yaqoob et al.45 fed rats diets providing 200 g fish oil per kg diet, 20% of which was EPA + DHA, resulting in EPA + DHA intakes of 1 g per day for a 250 g rat.
Animals were sacrificed after 8 days. Blood was collected into EDTA as anticoagulant by cardiac puncture and plasma was prepared by centrifugation. Brain, whole liver, and retroperitoneal and gonadal adipose tissues were collected, weighed and snap frozen for further analysis.
Plasma fatty acid composition analysis
Total plasma fatty acids were analyzed as fatty acid methyl esters (FAMEs) obtained by direct transmethylation without previous extraction as described elsewhere.33 Plasma (100 μl) was added into a tube containing heptadecanoic acid (17:0; 5 μg) as internal standard and 1 ml 5% H2SO4 in methanol was added. The tubes were gassed with nitrogen, closed tightly and heated at 85 °C for 1.5 h with occasional shaking. After cooling, 1 ml of hexane was added, the tubes were mixed and the hexane layer was collected into a new tube, after a short centrifugation. The hexane extracts were dried down under nitrogen and then redissolved in a small volume of hexane. Gas chromatography was performed on a Varian 3800 gas chromatograph fitted with a BPX-70 column (30 m × 0.22 mm × 0.25 μm). Inlet temperature was 250 °C. Oven temperature was initially 170 °C and this was maintained for 5 min post-injection. Then the oven temperature was programmed to increase to 200 °C at the rate of 3 °C min−1, to hold at 200 °C for 10 min, and then to increase to 220 °C at the rate of 5 °C min−1. Total run time was 19 min. Helium was used as the carrier gas. FAMEs were detected by a flame ionization detector held at a temperature of 300 °C. The instrument was controlled by, and data collected using, Varian Star Workstation Advanced Application Software Version 6. FAMEs were identified by comparison of retention times with those of authentic standards run previously. Absolute concentrations of fatty acids were calculated using the 17:0 internal standard.Tissue fatty acid composition analysis
Fatty acids were analyzed in total lipid extracts from animal tissues; total lipid was extracted by homogenizing a known weight of tissue in 5 ml chloroform–methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol/vol) and collecting the top organic layer after centrifugation. A known amount of internal standard (free 21:0) was added to the lipid extracts which were then dried down under nitrogen gas. Toluene (0.5 ml) was added to redissolve the lipid. FAMEs were formed by incubation of the entire lipid extract with 1 ml methanol containing 2% (vol/vol) H2SO4 at 50 °C for 2 h. After allowing the tubes to cool, samples were neutralized by addition of 1 ml of a solution of 0.25 M KHCO3 and 0.5 M K2CO3. Then FAMEs were extracted into 1 ml hexane, dried down, redissolved in a small volume (150 μl) of hexane, and separated by gas chromatography. Gas chromatography was performed on a Hewlett Packard 6890 gas chromatograph fitted with a BPX-70 column (30 m × 0.22 mm × 0.25 μm). Inlet temperature was 300 °C. Oven temperature was initially 115 °C and this was maintained for 2 min post-injection. Then the oven temperature was programmed to increase to 200 °C at the rate of 10 °C min−1, to hold at 200 °C for 16 min, and then to increase to 240 °C at the rate of 60 °C min−1 and then to hold at 240 °C for 2 min. Total run time was 37 min. Helium was used as the carrier gas. FAMEs were detected by a flame ionization detector held at a temperature of 300 °C. The instrument was controlled by, and data collected using, HPChemStation (Hewlett Packard). FAMEs were identified by comparison of retention times with those of authentic standards run previously. Absolute concentrations of fatty acids were calculated using the 21:0 internal standard and information on the weight of tissue from which the lipid had been extracted. An intermediate step in the metabolism of DHA from EPA involves the production of docosapentaenoic acid (DPA, 22:5n-3).34 Total EPA levels are shown as the combination of EPA + DPA. Total LC n-3 PUFA content is the sum of EPA, DPA and DHA.
1 vol/vol) and collecting the top organic layer after centrifugation. A known amount of internal standard (free 21:0) was added to the lipid extracts which were then dried down under nitrogen gas. Toluene (0.5 ml) was added to redissolve the lipid. FAMEs were formed by incubation of the entire lipid extract with 1 ml methanol containing 2% (vol/vol) H2SO4 at 50 °C for 2 h. After allowing the tubes to cool, samples were neutralized by addition of 1 ml of a solution of 0.25 M KHCO3 and 0.5 M K2CO3. Then FAMEs were extracted into 1 ml hexane, dried down, redissolved in a small volume (150 μl) of hexane, and separated by gas chromatography. Gas chromatography was performed on a Hewlett Packard 6890 gas chromatograph fitted with a BPX-70 column (30 m × 0.22 mm × 0.25 μm). Inlet temperature was 300 °C. Oven temperature was initially 115 °C and this was maintained for 2 min post-injection. Then the oven temperature was programmed to increase to 200 °C at the rate of 10 °C min−1, to hold at 200 °C for 16 min, and then to increase to 240 °C at the rate of 60 °C min−1 and then to hold at 240 °C for 2 min. Total run time was 37 min. Helium was used as the carrier gas. FAMEs were detected by a flame ionization detector held at a temperature of 300 °C. The instrument was controlled by, and data collected using, HPChemStation (Hewlett Packard). FAMEs were identified by comparison of retention times with those of authentic standards run previously. Absolute concentrations of fatty acids were calculated using the 21:0 internal standard and information on the weight of tissue from which the lipid had been extracted. An intermediate step in the metabolism of DHA from EPA involves the production of docosapentaenoic acid (DPA, 22:5n-3).34 Total EPA levels are shown as the combination of EPA + DPA. Total LC n-3 PUFA content is the sum of EPA, DPA and DHA.
NMR analysis
13C NMR analysis of the galactolipids was performed by Spectral Services AG of Koln, Germany using a 500 MHz Avance.Statistical analysis
Data for male and female animals are combined. Since some data were not normally distributed all data are expressed as median and 90% confidence interval. The two-sample T-test and non-parametric Wilcoxon–Mann–Whitney Rank sum test for independent samples were applied for testing the statistical significance of the difference in all variables between krill oil and algae oil, overall and by sex. All tests applied were two-tailed, and a p value of 5% or less was considered statistically significant. Data were analyzed using the SAS® version 9.1 (SAS Institute, Cary, North Carolina).Conclusion
There were no differences in total LC n-3 PUFA levels in plasma, brain, liver and gonadal adipose tissue between animals given algal oil from Nannochloropsis oculata or krill oil. The algal oil resulted in a higher EPA content in retroperitoneal adipose tissue. It is concluded that tissue availability of LC n-3 PUFA from an algal oil containing 6% phospholipids and 9% glycolipids is similar to that from krill oil containing 40% phospholipids. This may indicate that, as reported in previous studies42 where the glycolipids MGDG and DGDG were shown to act synergistically to increase the absorption of lipids across the intestine, the glycolipids in the algal oil may promote effective delivery of EPA to plasma and tissues.Acknowledgements
Tissue fatty acid compositions were determined at the University of Southampton by Annette West under the supervision of Philip Calder. The NMR analysis was performed by Dr Bernd Diehl at Spectral Services AG, Koln, Germany. The authors wish to thank Philip Calder for help in drafting the manuscript.References
- British Nutrition Foundation, n-3 Fatty acids and human health, British Nutrition Foundation, London, 1999 Search PubMed.
- P. C. Calder and P. Yaqoob, Postgrad. Med., 2009, 121, 148–157 CrossRef PubMed.
- C. Campoy, M. V. Escolano-Margarit, T. Anjos, H. Szajewska and R. Uauy, Br. J. Nutr., 2012, 107(Suppl. 2), S85–S106 CrossRef CAS PubMed.
- D. W. Luchtman and C. Song, Neuropharmacology, 2013, 64, 550–565 CrossRef CAS PubMed.
- D. Mozaffarian and J. H. Wu, J. Am. Coll. Cardiol., 2011, 58, 2047–2067 CrossRef CAS PubMed.
- J. Delgado-Lista, P. Perez-Martinez, J. Lopez-Miranda and F. Perez-Jimenez, Br. J. Nutr., 2012, 107(Suppl. 2), S201–S213 CrossRef CAS PubMed.
- P. C. Calder, Eur. J. Pharmacol., 2011, 668, 550–558 Search PubMed.
- P. C. Calder, Br. J. Clin. Pharmacol., 2013, 75, 645–662 CAS.
- A. D. Dangour, V. A. Andreeva, E. Sydenham and R. Uauy, Br. J. Nutr., 2012, 107(Suppl. 2), S152–S158 CrossRef CAS PubMed.
- M. Gerber, Br. J. Nutr., 2012, 107(Suppl. 2), S228–S239 CrossRef CAS PubMed.
- A. Laviano, S. Rianda, A. Molfino and F. Rossi, Curr. Opin. Clin. Nutr. Metab. Care, 2013, 16, 156–161 CrossRef CAS PubMed.
- Scientific Advisory Committee on Nutrition/Committee on Toxicity Advice on fish consumption: Benefits and risks, TSO, London, 2004. Available: http://www.TSO.co.uk/bookshop (accessed 5 March 2014).
- P. Kris-Etherton, W. S. Harris and L. J. Appel, Circulation, 2002, 106, 2747–2757 CrossRef.
- J. C. Gigliottia, M. P. Davenport, S. K. Beamera, J. C. Toua and J. Jaczynski, Food Chem., 2011, 125, 1028–1036 CrossRef PubMed.
- W. Yongmanitchai and O. P. Ward, Phytochemistry, 1991, 30, 2963–2967 CrossRef CAS.
- A. Mendes, A. Reis, R. Vasconcelos, P. Guerra and T. L. da Silva, J. Appl. Phycol., 2009, 21, 199–214 CrossRef.
- A. D. Doughman, S. Krupanidhi and C. B. Sanjeevi, Curr. Diabetes Rev., 2007, 3, 198–203 CrossRef.
- S. A. MacKenzie, L. A. Belcher, G. P. Sykes, S. R. Frame, P. Mukerji and P. J. Gillies, Regul. Toxicol. Pharmacol., 2010, 58, 490–500 CrossRef CAS PubMed.
- B. Winther, N. Hoem, K. Berge and L. Reubsaet, Lipids, 2011, 46, 25–36 CrossRef CAS PubMed.
- J. P. Schuchardt, I. Schneider, H. Meyer, J. Neubronner, C. v. Schacky and A. Hahn, Lipids Health Dis., 2011, 10, 145–152 CrossRef CAS PubMed.
- A. Sukenik, in Chemicals from Microalgae, ed. Z. Cohen, CRC Press, Boca Raton, 1999, pp. 41–53 Search PubMed.
- J. C. Schneider, A. Livne, A. Sukenik and P. G. Roessler, Phytochemistry, 1995, 40, 807–814 CrossRef CAS.
- M. L. Kagan, A. L. West, C. Zante and P. C. Calder, Lipids Health Dis., 2013, 12, 102–112 CrossRef CAS PubMed.
- L. M. Browning, C. G. Walker, A. P. Mander, A. L. West, J. Madden, J. M. Gambell, S. Young, L. Wang, S. A. Jebb and P. C. Calder, Am. J. Clin. Nutr., 2012, 96, 748–758 CrossRef CAS PubMed.
- M. Lagarde, N. Bernoud, N. Brossard, D. Lemaitre-Delaunay, F. Thiès, M. Croset and J. Lecerf, J. Mol. Neurosci., 2001, 16, 201–204 CrossRef CAS.
- E. Murru, S. Banni and G. Carta, BioMed Res. Int., 2013, 965417, DOI:10.1155/2013/965417 , 13 pages.
- J. Edmond, J. Mol. Neurosci., 2001, 16, 181–193 CrossRef CAS.
- L. A. Popescu, B. Vîrgolici, D. Lixandru, D. Miricescu, E. Condruţ, O. Timnea, A. E. Ranetti, M. Militaru, M. Mohora and L. Zăgrean, Rom. J. Morphol. Embryol., 2013, 54, 785–790 Search PubMed.
- K. A. Balogun, C. J. Albert, D. A. Ford, R. J. Brown and S. K. Cheema, PLoS One, 2013, 8, e82399, DOI:10.1371/journal.pone.0082399.
- K. M. Bridges, J. C. Gigliotti, S. Altman, J. Jaczynski and J. C. Tou, J. Agric. Food Chem., 2010, 58, 2830–2837 CrossRef CAS PubMed.
- A. Sukenik, H. Takahashi and S. Mokady, Ann. Nutr. Metab., 1994, 38, 85–96 CrossRef CAS PubMed.
- F. S. Ghasemi, K. M. Linderborg, G. M. Turchini and A. J. Sinclair, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2014, 90, 23–26 CrossRef PubMed.
- C. Glaser, H. Demmelmair and B. Koletzko, PLoS One, 2010, 5, e12045, DOI:10.1371/journal.pone.0012045.
- H. Sprecher, Biochim. Biophys. Acta, 2000, 1486, 219–231 CrossRef CAS.
- P. Dörmann and B. Christoph, Trends Plant Sci., 2002, 7, 112–118 CrossRef.
- L. Andersson, C. Bratt, K. Arnoldsson, B. Herslöf, N. Olsson, B. Sternby and A. Nilsson, J. Lipid Res., 1995, 36, 1392–1400 CAS.
- L. P. Christensen, Foods Recent Pat. Food Nutr. Agric., 2009, 1, 50–58 CrossRef CAS.
- V. M. Dembitsky, O. A. Rozentsvet and E. E. Pechenkina, Phytochemistry, 1990, 29, 3417–3421 CrossRef CAS.
- J. O. Wakelam, Biochim. Biophys. Acta, 1998, 1436, 117–126 CrossRef.
- M. Lenti, C. Gentili, A. Pianezzi, G. Marcolongo, A. Lalli, R. Cancedda and F. D. Cancedda, Nat. Prod. Res., 2009, 23, 754–762 CrossRef CAS PubMed.
- A. Bruno, C. Rossi, G. Marcolongo, A. Di Lena, A. Venzo, C. P. Berrie and D. Corda, Eur. J. Pharmacol., 2005, 524, 159–168 CrossRef CAS PubMed.
- A. Gorusupudi and B. Vallikannan, Eur. J. Lipid Sci. Technol., 2012, 114, 710–717 CrossRef CAS.
- F. P. de Heredia, E. Larque, M. P. Portillo, M. Canteras, S. Zamora and M. Garaulet, Nutrition, 2008, 24, 1013–1022 CrossRef PubMed.
- J. C. Tou, S. N. Altman, J. C. Gigliotti, V. A. Benedito and E. L. Cordonier, Lipids Health Dis., 2011, 10, 179 CrossRef CAS PubMed.
- P. Yaqoob, E. J. Sherrington, N. M. Jeffery, P. Sanderson, D. J. Harvey, E. A. Newsholme and P. C. Calder, Int. J. Biochem. Cell Biol., 1995, 27, 297–310 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |