Arsenic removal using a sulfonated poly(ether ether ketone) coated hollow fiber nanofiltration membrane
Jianfeng
Song
ab,
Mengxi
Zhang
ab,
Alberto
Figoli
c,
Yong
Yin
a,
Baolong
Zhao
a,
Xue-Mei
Li
*a and
Tao
He
*ad
aLaboratory for Membrane Materials and Separation Technology, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China. E-mail: lixm@sari.ac.cn; het@sari.ac.cn; Fax: +86 21 20325034; Tel: +86 21 20325162
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cInstitute on Membrane Technology, ITM-CNR, University of Calabria, Via P. Bucci, Cubo 17/C, 87030 Rende (CS), Italy
dSchool of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210, China
First published on 22nd July 2015
Abstract
A new composite hollow fiber nanofiltration (NF) membrane with a thin sulfonated poly(ether ether ketone) (SPEEK) active layer was prepared and used for arsenic removal. The effects of the feed concentration, pH and transmembrane pressure on arsenic removal were systematically examined. The obtained SPEEK hollow fiber NF membrane could achieve an As(V) rejection of over 95% and water permeability of 11 L m−2 h−1 bar−1. These excellent As(V) rejection and high water permeability were attributed to the highly negatively charged surface of the SPEEK membrane, which could enhance the As(V) rejection through the Donnan exclusion mechanism or electrostatic repulsion between the negatively charged arsenate species and the negatively charged SPEEK membrane surface. A preliminary techno-economic assessment of a 1000 m3 h−1 plant suggests that using the proposed SPEEK hollow fiber membrane, the treatment cost was 0.15 US$ per m3, which thus could be economically viable for arsenic removal applications in China.
Water impactArsenic contaminated drinking water is a major health risk for millions of people throughout the world. Most of them live in underdeveloped cities or rural areas. An efficient nanofiltration process with low energy consumption and financial cost can be used for arsenic removal from groundwater. In this work, a composite hollow fiber nanofiltration membrane with sulfonated poly(ether ether ketone) coating was developed and evaluated for arsenic removal. The estimated overall water treatment cost could be affordable for many Chinese communities. |
1. Introduction
Arsenic occurs naturally in groundwater in many parts of the world, particularly in South and South East Asia (e.g., China, India, Bangladesh, and Thailand), North America (e.g., USA and Canada), and parts of Europe (e.g. Italy and Spain).1–4 Most arsenic compounds are toxic. Chronic exposure to arsenic at a low level can cause lung and bladder cancer, muscular weakness, and black-foot disease.5 In 1993, the World Health Organisation (WHO) adjusted the maximum contaminant level (MCL) of arsenic in drinking water from 50 to 10 μg L−1.Many treatment methods, including coagulation, adsorption, ion exchange, bacterial treatment, and membrane processes, can be applied for arsenic removal.4,6,7 Cost, efficiency, technical complexity and physical footprint are amongst the most important criteria for selecting these treatment methods.8 Among these methods, pressure-driven membrane processes such as reverse osmosis (RO) and nanofiltration (NF) can offer probably the highest treatment efficiency. Moreover, these membrane processes are also compact, can be fully automated, and do not require extensive maintenance.9–11 Compared to RO, the NF process operates at a much lower pressure and thus is more economical. However, NF membranes are commercially available mostly in spiral wound modules. Spiral wound modules are prone to channel clogging. Thus, they require extensive pre-treatment by either sand filtration or a low pressure membrane process such as microfiltration and ultrafiltration, which adds significant treatment cost.8,9 Therefore, cost-effective NF membranes with high stability under a wide pH range are highly desirable.
Hollow fiber membranes have well-defined flow channels and variable inner diameters. They show advantages over spiral wound membrane modules because of easy cleaning, high tolerance to turbidity and particulates, and consequently less demand for pre-treatment. The preparation of hollow fiber NF membranes has been reported either by using direct spinning or direct spinning followed by surface coating. Direct spinning has been widely studied using a single polymer solution12 or simultaneous co-extrusion of two polymeric solutions.13,14 Coating an ultrathin layer onto an existing membrane could also be achieved by interfacial polymerization,15 UV-photo grafting,16 and direct dip-coating.17,18 In particular, dip-coating has been employed for the preparation of NF hollow fiber membranes with a thin active layer of sulfonated polyether ether ketone (SPEEK) on a commercial polyethersulfone (PES) support.17 The resulting composite membranes showed high sodium sulfate rejection and low magnesium chloride rejection. These SPEEK coated PES membranes could be used to separate sodium chloride from glyphosate.18 The same membrane showed a stable performance (for more than 2000 h) owing to the cross-linking of the active coating layer.19
In an aqueous solution, inorganic arsenic can be present as either As(III) or As(V). The transformation between As(III) and As(V) depends on the redox condition of the solution.20,21 In a reducing condition such as an underground aquifer, arsenic exists mostly in the form of arsenite or As(III). However, once groundwater has been extracted to the surface, As(III) can be readily oxidized by oxygen in air into arsenate or As(V). Because of the prevalent oxidation of As(III) to As(V) when exposed to air, this work will focus exclusively on the removal of As(V).
Based on the Donnan exclusion mechanism, SPEEK composite NF membranes can potentially offer high removal of multivalent ions such as HAsO42−.21 However, the actual separation performance of the SPEEK NF membranes towards As(V) is largely unknown, particularly in the presence of interfering ions. In this report, the SPEEK membrane performance is investigated for As(V) removal in the presence of interfering ions, including Na+, Ca2+, Cl−, NO3−, SO42−, and others. The effects of the arsenic concentration, pH and operational pressure on the rejection of As(V) were investigated. Rejections of other cations and anions were determined in order to assess the role of the Donnan exclusion mechanism.22–25 Finally, the treatment cost related to As(V) removal was estimated to evaluate the potential of the present SPEEK hollow fiber NF membrane for drinking water production from arsenic contaminated groundwater.
2. Experimental
2.1 Materials and membranes
SPEEK powder (VESTAKEEP 4000P) was kindly provided by Evonik Degussa. The PES hollow fiber ultrafiltration membrane was kindly provided by Altrateck Co. Ltd. The key characteristics of this PES hollow fiber membrane are summarized in Table 1. All other chemicals (e.g., Na3AsO4·12H2O, methanol, Na2SO4, and H2SO4) were from Sinopharm Corporation Ltd and were of analytical grade. De-ionized water was used to prepare all experimental solutions.| Properties | Unit | Value |
|---|---|---|
| ID/OD | mm | 0.78/1.28 |
| MWCO | Da | 70000 |
| Pure water permeability | L m−2 h−1 bar−1 | 400 ± 50 |
| Burst pressure | bar | >10 |
| Maximal Temp. | °C | 40 |
2.2 Preparation of composite hollow fiber nanofiltration membrane
The detailed procedure for the preparation of SPEEK (SPEEK-S48) and the composite hollow fiber NF membrane was reported elsewhere.18 Briefly, SPEEK coating solution (concentration 1.5 wt%) in methanol was filtered and dip-coated onto the inner surface of the hollow fiber PES UF membrane. The initial composite membrane was dried and cured for an hour at 65 °C. The membrane was then stored under ambient environment for subsequent analysis and filtration experiments.2.3 Characterization of the composite membrane
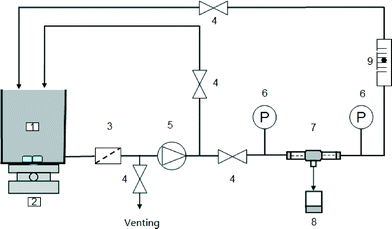
A hollow fiber membrane module was prepared by epoxy potting six fibers (effective length of 16–20 cm) into a nylon tube (inner diameter/outer diameter = 9/12 mm). A schematic diagram of the hollow fiber NF set-up is shown in Fig. 1.17,18A Na2SO4 feed solution of 500 mg L−1 was used. The pressure and flow rate at the lumen side were controlled manually. The cross-flow velocity in the lumen was kept at 0.25 m s−1. The solution temperature was maintained at 25 ± 2 °C. At each operating condition, the system was stabilised for at least 1 h before permeate and feed sample collection for rejection determination and permeability measurement. All experiments were conducted in duplicate and the deviation between two replicates was always less than 10%.
A stock solution containing 9 mg L−1 As(V) in de-ionized water was prepared and used for NF filtration experiments. This stock solution was then diluted to obtain a working solution with a desirable As(V) concentration to study the effects of interfering ions and pH on arsenic rejection. To study the effects of pH, the solution pH was adjusted by adding a small volume of either 1 M HCl or 1 M NaOH solution. The trans-membrane pressure was set at 4 bar.
The permeate flux (J) was determined by the permeate weight through the membrane at unit time and unit membrane area. The water permeability was calculated by dividing the permeate flux by the trans-membrane pressure. The observed rejection (Robs) was determined as reported in eqn (1):
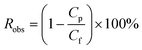 | (1) |
2.4 Water quality analysis
The conductivity and pH were measured using a Mettler Toledo (LE703) conductivity meter and a Sartorius pH meter (PB-10), respectively. A Shimadzu inductively coupled plasma atomic emission spectrometer (ICP AES, ICPE-9000) was used to determine the cation and As(V) concentration. The total hardness was determined using the disodium ethylenediamine tetraacetate (EDTA-2Na) titration method. COD was determined by digestive degradation and measured using a spectrophotometer (Hach DR2800) based on standard methods. All samples were analyzed in triplicate and the standard deviation of 3 replicate measurements was always less than 10%.2.5 Scanning electron microscopy26 and zeta potential
Prior to scanning electron microscopy26 analysis, the samples were coated with a thin layer of gold. A field emission scanning electron microscope (FESEM, HITACHI S-4800) (for high magnifications) was used for characterizing the membrane morphology.The surface zeta potential of the membrane was measured using a Surpass (Anton Paar) electro-kinetic analyser.
3. Results and discussion
3.1 Characteristics of SPEEK coated composite NF membrane
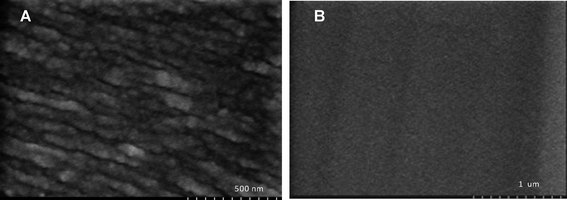
Fig. 2 shows the inner surface of the PES hollow fiber membrane before and after SPEEK coating. The PES surface shows aligned spindle-like polymer aggregates with width of about 20–50 nm (Fig. 2A). The pores are distinctly shown as the gap between the polymer aggregates. After coating with a SPEEK solution of 1.5 wt%, the membrane inner surface shows a very smooth surface (Fig. 2B). This observation indicates that SPEEK is homogeneously coated on the PES membrane support.The PES membrane was negatively charged with the surface zeta potential in the range of −12 to −35 mV (Fig. 3). After coating with SPEEK, the membrane surface was much more negatively charged and the surface zeta potential was in the range of −105 to −120 mV. This significant change in surface charge is attributed to the deprotonation of the sulfonate group of SPEEK. The results in Fig. 3 also confirm that SPEEK surface coating was successful.
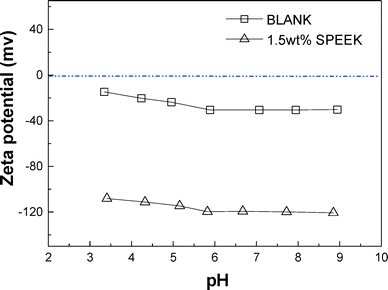 | ||
| Fig. 3 Zeta potential of the support PES ultrafiltration membrane (BLANK) and SPEEK composite NF membrane at different pH. | ||
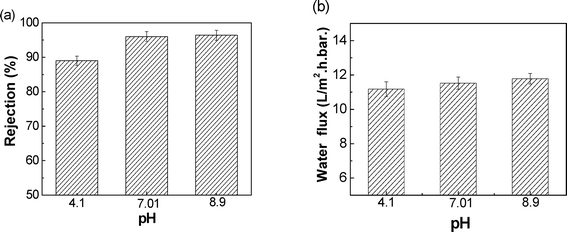
SPEEK coating also resulted in a marked reduction in water permeability. Indeed, as can be seen in Table 2, the SPEEK coated membrane water permeability was 11.5 L m−2 h−1 bar−1 while the water permeability of the PES membrane (which was used as the base) was 400 L m−2 h−1 bar−1. In addition, inorganic salt rejections by the SPEEK coated membrane were comparable to those of a traditional NF membrane (Table 2). The high rejection of Na2SO4 evidenced the integrity of the coating layer. Based on the surface charge of the NF membrane, a high rejection of Na2SO4 and low rejection of NaCl were expected, according to Donnan exclusion. A rejection of 34.0% towards CaCl2 was not in line with the effect of the Donnan exclusion; in fact, the large amount of sulfonic acid groups on the main chain of the SPEEK polymer presumably should allow the transport of the bivalent Ca2+ through the coating; therefore, a value lower than the measured rejection value was expected. Consequently, this deviation may indicate that Donnan exclusion was not the only separation mechanism for the SPEEK membrane; other separation mechanisms may also play a role.
3.2 Removal of arsenic
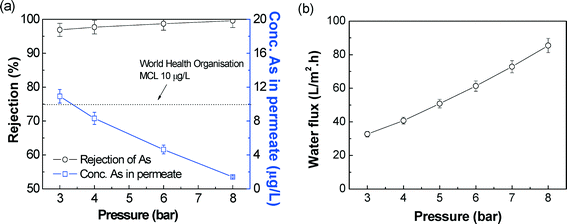 | ||
| Fig. 6 Effect of operating pressure on As(V) rejection (a) and water flux (b) of SPEEK NF membrane. Feed concentration: 360 μg L−1 at 25 °C. | ||
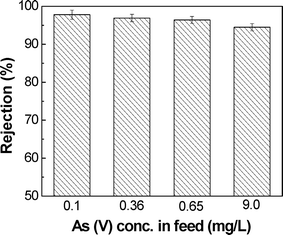
These results demonstrated that the SPEEK NF membrane has shown high rejection of arsenic without the presence of interfering ions, and the Donnan exclusion can rationally be applied to understand the rejection of As.
3.3 Effects of interfering ions on As(V) removal
Arsenic was spiked into tap water and used as a model solution to illustrate the effect of interfering anions and cations on As rejection using the SPEEK hollow fiber NF membrane. Table 3 lists the major water quality data analyzed in the NF experiments. The pH of the feed tap water was adjusted to 8.78. The permeate arsenic concentration was 9.0 μg L−1, below the MCL required by WHO. The rejection is similar to the one obtained without the presence of other ions. A significant reduction in turbidity (75%) and conductivity (32.7%) was observed with a slight decrease in pH. The reduction of turbidity and conductivity is a direct result of membrane rejection. As reported in Table 3, for monovalent ions, e.g. Cl− and NO3− , the rejection was below 20%. The rejection of Si was close to that of Cl−, which was probably due to the predominant presence of HSiO4− (ref. 30) at pH 8.78. Surprisingly, SO42− rejection was 86.4% and was below the value when no interfering ions were present in the bulk feed solution. The rejection of Na+ was about 30%, which is comparably lower than the rejection of Ca2+ (51.5%) and Mg2+ (43.1%). For trivalent ferric ions, a rejection over 94% was found, which is expectable because ferric may associate with OH− to form colloidal particles to be rejected by the membrane.31| Analytes | Feed | Permeate | Rejection (%) | |
|---|---|---|---|---|
| pH | — | 8.78 | 8.67 | — |
| Turbidity | NTU | 0.4 | 0.1 | 75.0 |
| Conductivity | μs cm−1 | 574 | 383 | 32.7 |
| COD | mg L−1 | 12.9 | 5.90 | 54.3 |
| As | μg L−1 | 380 | 9.0 | 98 |
| Ca | mg L−1 | 69.1 | 33.5 | 51.5 |
| Fe | mg L−1 | 1.9 | <0.1 | >94 |
| Mg | mg L−1 | 8.91 | 5.05 | 43.3 |
| Na | mg L−1 | 31.4 | 22.3 | 28.9 |
| Si | mg L−1 | 5.22 | 4.30 | 17.6 |
| Cl− | mg L−1 | 66.5 | 54.3 | 18.3 |
| HCO3− | mg L−1 | 0.12 | 0.09 | 25.0 |
| NO3− | mg L−1 | 32.0 | 27.6 | 13.8 |
| SO42− | mg L−1 | 45.5 | 6.5 | 85.7 |
The hydration radii (Table 4) of the main cations and anions are significantly smaller (0.2–0.43 nm) than the pore radius of the SPEEK NF membrane (1.2–3.3 nm).17,18 Thus, steric exclusion of the cations and anions is not significant. Suppose that the Donnan exclusion effect is still valid for the model system, the rejection of SO42− would be much higher than 86.4% (compared to the results listed in Table 2), and the rejection of positively charged divalent ions, Ca2+ and Mg2+, would be much lower than those listed in Table 3. In addition, the rejection of Na+ would be much higher than the value listed in Table 3 (referring to the rejection values for NaCl reported in Table 2). The rejection value (51.5%) for Ca2+ in the model solution is significantly higher than that without interfering ions (34.0%). The above results seem to be contradictory to that expected by Donnan exclusion alone. The other striking phenomenon was that for the negatively charged SPEEK membrane, the rejection of monovalent anions (e.g., Cl− and NO3−) is even much lower than that of monovalent cations. The discrepancy between the Donnan exclusion and the observed results indicates that, for a mixture solution, other separation mechanisms may play an important role as well.
| Species | Hydration radii (nm) | D (10−9 m−2 s−1) |
|---|---|---|
| Ca2+ | 0.41 | 0.92 |
| Mg2+ | 0.428 | 0.71 |
| Na+ | 0.36 | 1.33 |
| Cl− | 0.33 | 2.03 |
| NO3− | 0.335 | 1.90 |
| HCO3− | 0.20–0.22 | 1.19 |
| HAsO42− | 0.200–0.202 | 0.32 |
| SO42− | 0.379 | 1.07 |
By analyzing the data in Tables 3 and 4, the relationship between the hydration radii of various ions and their rejection rates is not apparent. For example, Mg2+ has the largest hydration radius among the cations but its rejection is lower than Ca2+; for anions, the smallest HAsO42− has the highest rejection. Furthermore, there is no monotonic correlation between the diffusivity and rejection either. In order to explain such results, the separation mechanisms have to be reviewed.
Saliha et al.32 has incorporated the steric/electric model with dielectric exclusion, and their results show that the surface charge density in the nanopores within a NF membrane is lower than the experimentally determined streaming potential. This difference could be caused by the adsorption of counter ions,33 or even more significantly of multivalent ions.34 For a single salt, the adsorption of counter ions is probably less important. However, when interfering ions are present, the adsorption of counter ions partially weakens the surface charges of the membrane, leading to reduced rejection of SO42− and increased rejection of bivalent cations.
Moreover, according to dielectric exclusion principles, the exclusion energy is proportional to the square of the charge. Therefore, it is expectable that a higher rejection value for bivalent than monovalent ions (both cations and anions) was observed. For the bivalent anions HAsO42− and SO42−, although a similar effect of dielectric exclusion was anticipated, the much lower diffusivity of HAsO42− than SO42− is more effective in retaining the former anions, leading to a higher rejection. Similarly, the higher rejection of Na+ than Cl− or NO3− could be explained by the greater diffusivity of the former. The slightly higher rejection of HCO3− may have originated from the association with cations such as Ca2+ and Mg2+. Overall, based on the principles of dielectric exclusion theory, the data in Table 3 can be rationally explained, indicating that in the presence of interfering ions, dielectric exclusion is the main mechanism for the separation performance of SPEEK NF membranes.
3.4 Cost evaluation
A preliminary cost estimation for the removal of arsenic from drinking water sources was conducted assuming a treatment capacity of 1000 m3 h−1 and a working time of 300 d per year. The assumptions and key cost parameters for such a treatment system are listed as follows:(1) membrane permeability: 10 L m−2 h−1 bar−1; operation pressure: 5 bar, which is lower than the burst pressure of the hollow fiber membrane (13 bar); membrane lifetime: 5 years, because the underground drinking water quality is rather high;
(2) module geometry: 8 inch × 1.5 m with an area of 35 m2; cost of the hollow fiber membrane: 70 $ per m2; this cost is an optimal evaluation of the membrane in large scale production; the cost includes the ultrafiltration membrane and coating layer and is predicted to be more realistic than the previous optimistic value;19
(3) water recovery rate: 80%; this is realistic because the TDS and scaling potential of surface water is generally low;
(4) lifetime of the membrane system: 20 years; cost of a membrane system: 3 times that of the membranes;36
(5) labor cost: 80k$ per year for 4 full time operators for a large plant because the membrane plant is fully automatic;
(6) the cost of electricity is assumed to be 0.1$ per kW h for rural areas.30 The cost breakdown of the nanofiltration system (Fig. 7) shows that the membrane, system depreciation and power takes up to 71% of the total treatment cost for producing drinking water (0.15$ per m3) from groundwater contaminated with arsenic, while manpower, maintenance and pre-treatment only add up to 29%. Reduction in the membrane and pre-treatment costs is the main issue for further improvement. It should be noted that the costs for interest rate, land, and other peripheral facilities were not taken into consideration, and this may affect slightly the total cost. The membrane cost is rather optimistic at this stage since the cost of the NF membrane is just marginally higher than the market price of the ultrafiltration membrane ($35–50).35,37 However, the price of the membrane and system depreciation could be reduced by constant improvement and increase in market capacity.
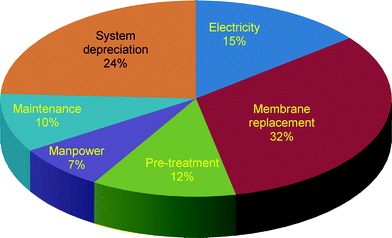 | ||
| Fig. 7 Cost breakdown of the NF membrane in removal of arsenic from drinking water. The total cost for producing drinking water is 0.15 $ per m3. | ||
4. Conclusions
The performance of the SPEEK coated composite NF membrane for arsenic removal from drinking water was evaluated. The membrane showed a high rejection (above 95%) to As(V) with a water permeability of about 11 L m−2 h−1 bar−1. At higher pH of 9, high trans-membrane pressure (8 bar) and low feed concentration (360 μg L−1), the membrane arsenic rejections were high (99.61%). The rejection properties of the membrane toward As in the presence of interfering ions were studied. Results showed that dielectric exclusion could rationally explain the rejection deviation from the Donnan exclusion effect, where the presence of interfering ions and the valence of the ions played the major role. Finally, a cost estimation show that the treatment cost using the proposed SPEEK coated hollow fiber membrane for a plant of 1000 m3 h−1 is about 0.15 US$ per m3. This result indicates that SPEEK composite NF membranes can be viable candidates for arsenic removal from groundwater.Acknowledgements
The authors thank the National Natural Science Foundation of China (Project No. 21176119), the National Key Basic Research Program of China (973 Program) (Project No 2012CB932800), and TMSR from Chinese Academy of Sciences (Project No. XDA02020100) for partial financial support.References
- R. Nickson, J. Mcarthur, W. Burgess, K. M. Ahmed, P. Ravenscroft and M. Rahmann, Nature, 1998, 395, 338–338 CrossRef CAS PubMed.
- A. H. Smith, P. A. Lopipero, M. N. Bates and C. M. Steinmaus, Science, 2002, 296, 2145–2146 CrossRef CAS PubMed.
- Z. Ning, D. T. Lobdell, R. K. Kwok, Z. Liu, S. Zhang, C. Ma, M. Riediker and J. L. Mumford, Toxicol. Appl. Pharmacol., 2007, 222, 351–356 CrossRef CAS PubMed.
- P. Ravenscroft, H. Brammer and K. Richards, Arsenic Pollution: A Global Synthesis, RGS-IBG Book Series, 2009.
- C. J. Chen, C. W. Chen, M. M. Wu and T. L. Kuo, Br. J. Cancer, 1992, 66, 888–892 CrossRef CAS PubMed.
- E. O. Kartinen and C. J. Martin, Desalination, 1995, 103, 79–88 CrossRef CAS.
- M. Mukherjee and S. De, Environ. Sci.: Water Res. Technol., 2015, 1, 204–217 CAS.
- R. B. Johnston, S. Hanchett and M. H. Khan, Nat. Geosci., 2010, 3, 2–3 CrossRef CAS PubMed.
- M. T. Uddin, M. S. I. Mozumder, A. Figoli, M. A. Islam and E. Drioli, Indian J. Chem. Technol., 2007, 14, 441–450 CAS.
- P. Mondal, S. Bhowmick, D. Chatterjee, A. Figoli and B. Van Der Bruggen, Chemosphere, 2013, 92, 157–170 CrossRef CAS PubMed.
- M. C. Shih, Desalination, 2005, 172, 85–97 CrossRef CAS PubMed.
- Y. Yang, X. Jian, D. Yang, S. Zhang and L. Zou, J. Membr. Sci., 2006, 270, 1–12 CrossRef CAS PubMed.
- T. He, M. H. V. Mulder, H. Strathmann and M. Wessling, J. Membr. Sci., 2002, 207, 143–156 CrossRef CAS.
- S. P. Sun, K. Y. Wang, N. Peng, T. A. Hatton and T.-S. Chung, J. Membr. Sci., 2010, 363, 232–242 CrossRef CAS PubMed.
- M. Frank, G. Bargeman, A. Zwijnenburg and M. Wessling, Sep. Purif. Technol., 2001, 22–23, 499–506 CrossRef.
- S. Béquet, J.-C. Remigy, J.-C. Rouch, J.-M. Espenan, M. Clifton and P. Aptel, Desalination, 2002, 144, 9–14 CrossRef.
- T. He, M. Frank, M. H. V. Mulder and M. Wessling, J. Membr. Sci., 2008, 307, 62–72 CrossRef CAS PubMed.
- J. Song, X.-M. Li, A. Figoli, H. Huang, C. Pan, T. He and B. Jiang, Water Res., 2013, 47, 2065–2074 CrossRef CAS PubMed.
- J. Song, X.-M. Li, Z. S. Li, Y. Yin, B. L. Zhao, D. F. Kong, G. Chen and T. He, Desalination, 2015, 355, 83–90 CrossRef CAS PubMed.
- A. Figoli, A. Cassano, A. Criscuoli, M. S. I. Mozumder, M. T. Uddin, M. A. Islam and E. Drioli, Water Res., 2010, 44, 97–104 CrossRef CAS PubMed.
- A. Seidel, J. J. Waypa and M. Elimelech, Environ. Eng. Sci., 2001, 18, 105–113 CrossRef CAS.
- T. Urase, J. I. Oh and K. Yamamoto, Desalination, 1998, 117, 11–18 CrossRef CAS.
- E. M. Vrijenhoek and J. J. Waypa, Desalination, 2000, 130, 265–277 CrossRef CAS.
- W. R. Bowen, B. Cassey, P. Jones and D. L. Oatley, J. Membr. Sci., 2004, 242, 211–220 CrossRef CAS PubMed.
- W. R. Bowen and A. W. Mohammad, Chem. Eng. Res. Des., 1998, 76, 885–893 CrossRef CAS.
- G. Zelmanov and R. Semiat, Desalination, 2014, 333, 107–117 CrossRef CAS PubMed.
- W. R. Bowen, T. A. Doneva and H. Yin, Desalination, 2002, 145, 39–45 CrossRef CAS.
- W. R. Bowen, S. Y. Cheng, T. A. Doneva and D. L. Oatley, J. Membr. Sci., 2005, 250, 1–10 CrossRef CAS PubMed.
- T. Y. Inan, H. Doğan, E. E. Unveren and E. Eker, Int. J. Hydrogen Energy, 2010, 35, 12038–12053 CrossRef CAS PubMed.
- P. Mondal, T. Anh Thi Kim and B. Van Der Bruggen, Desalination, 2014, 348, 33–38 CrossRef CAS PubMed.
- Y. Wang, J. Duan, S. Liu, W. Li, J. Van Leeuwen and D. Mulcahy, Sep. Purif. Technol., 2014, 135, 64–71 CrossRef CAS PubMed.
- B. Saliha, F. Patrick and S. Anthony, Chem. Eng. Sci., 2009, 64, 3789–3798 CrossRef CAS PubMed.
- V. Silva, A. Martin, F. Martinez, J. Malfeito, P. Pradanos, L. Palacio and A. Hernandez, Chem. Eng. Sci., 2011, 66, 2898–2911 CrossRef CAS PubMed.
- Z. Ma, M. Wang, X. Gao and C. Gao, Front. Environ. Sci. Eng., 2014, 8, 650–658 CrossRef CAS.
- C. M. Nguyen, S. Bang, J. Cho and K. Kim, Desalination, 2009, 245, 82–94 CrossRef CAS PubMed.
- U. Atikol and H. S. Aybar, Desalination, 2005, 184, 253–258 CrossRef CAS PubMed.
- R. W. Baker, Membrane Technology and Applications, 3rd edn, John Wiley and Sons Ltd., West Sussex, UK, 2012 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |