Biofilm formation of anoxygenic photosynthetic bacteria induced by phototaxis for enhancing hydrogen production†
Xingzu
Wang
a,
Kaiji
Xie
a,
Xiang
Cheng
b,
Yiwei
Ren
*a and
Chunli
Wan
*c
aKey Laboratory of Reservoir Aquatic Environment, Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences, no. 266 Fangzheng Avenue, Shuitu Hi-tech Industrial Park, Shuitu Town, Beibei District, Chongqing, 400714, China. E-mail: 48123897@qq.com; Fax: +86 23 63063972; Tel: +86 23 63063972
bCollege of Environmental Science & Engineering, Beijing Forestry University, 35 Tsinghua East Road, Beijing, 100083, China
cDepartment of Environmental Science and Engineering, Fudan University, Shanghai, 200433, China
First published on 1st April 2015
Abstract
In this study, the potential application of phototaxis to promote the formation of anoxygenic photosynthetic bacteria (APB) biofilms and hydrogen production was evaluated in batch and continuous experiments. The batch experiments showed that the amount of biofilm biomass on the illuminated sidewall reached 0.86 mg cm−2, which was 26-fold higher than that of the biofilm on the unlighted sidewall. The analyses of living cell fluorescence labeling and intracellular c-di-GMP suggested that sidewall illumination led to aggregation of APB cells to form flocs on the illuminated sidewall by upregulating intracellular c-di-GMP levels, but not on the illuminated liquid surface. These findings suggested that phototaxis and surface sensing modulated the biofilm formation of APB. Further, a continuous photorotating biological contactor reactor with mirror coating was developed to improve APB biofilm formation. APB biomass and hydrogen production was significantly higher than those in the control without mirror coating, indicating the feasibility of applying reflected light-induced biofilm formation of APB for improvement of hydrogen production.
Water impactAnoxygenic photosynthetic bacteria (APB) are considered as the most promising candidates for large-scale hydrogen production because of their capability to use various organic pollutants. However, APB could not efficiently form biofilms and be washed out with an effluent from a reactor, which led to low biomass and hydrogen production performance. This study evaluates the feasibility and mechanisms of APB biofilm formation induced by phototaxis. Phototaxis and surface sensing could enable APB with poor biofilm-forming ability to form a stable biofilm by upregulating intracellular c-di-GMP levels, which significantly promotes APB biomass retention and consequential hydrogen production in a continuous photobioreactor. This study is potentially suitable for practical applications in photohydrogen production from wastewater. |
1. Introduction
Photohydrogen production processes with anoxygenic photosynthetic bacteria (APB) have been regarded as efficient systems for energy recovery from organic wastewater.1 The economic production of hydrogen from wastewater shows attractive prospects because producing bioenergy from wastewater could reduce wastewater treatment costs.2–4 To achieve rapid and stable hydrogen production, APB are expected to be cultivated in a photobioreactor to produce hydrogen continuously. However, photohydrogen production performance remains relatively poor because of low cell concentration and light energy utilization attributed to shielding effects. One solution to the problems for the improvement in photohydrogen production performance is optimization of the immobilized bioreactor design.5 In general, available bioreactors can be classified as biofilm or cell entrapment. The former is apparently a more reasonable cell immobilization approach than the latter because the efficiency of light energy utilization is important in photohydrogen fermentation bioreactors.3,6APB cannot efficiently form biofilms or flocs, and it is difficult to achieve a high quantity of biofilm biomass in photobioreactors.7,8 According to the Derjaguin–Landau–Verwey–Overbeek theory, APB fail to overcome the total energy barrier to flocculate effectively. This condition arises because the contribution of the van der Waals interaction energy to the total interaction energy could be ignored due to the small effective Hamaker constant.9 Steunou et al. reported a two-component system called EmbRS, which controlled the biofilm formation of the purple bacterium Rubrivivax gelatinosus.10 They showed that R. gelatinosus represses extracellular matrix production and biofilm formation through the EmbRS system to avoid formation of fast-sinking aggregates. Material flow analysis during continuous photohydrogen production showed that APB were not efficiently separated from the supernatant and were continuously washed out with the effluent due to poor flocculation. In this case, most of the substrates were utilized for APB cell growth rather than hydrogen production, resulting in a low hydrogen yield.11 The poor flocculation capability of APB limits the utilization of these bacteria in continuous-flow bioreactors. Alternatively, pure cultivation of APB can produce sufficient extracellular polymeric substances (EPS) and form mats after addition of certain chemicals. For instance, the addition of toxic substances such as Cu(II), Cr(VI), Cd(II), or 2,4-dichlorophenol stimulates EPS production by Rhodopseudomonas acidophila.12Rhodopseudomonas faecalis overcame the total energy barrier and flocculated effectively under high concentrations of L-cysteine.13 High salinity significantly enhances the floc-forming capability of Rhodovulum sp. PS88.14 However, these methods are not always suitable for real wastewater treatment processes because the added substances usually trigger the reduction of APB biomass or the replacement of APB with other non-photosynthetic microorganisms.15
To develop new strategies for biofilm control, the adaptive pathways that facilitate the development and maintenance of bacterial biofilms need to be understood. Taxis (such as chemotaxis, energy taxis and phototaxis) is among the best-characterized signaling systems in biology.16 It is a mechanism by which bacteria move towards optimal environments and is implicated in biofilm formation and symbiosis. There is evidence for the impact of taxis on surface interactions and biofilm formation in bacterial species. For instance, Marinobacter adhaerens HP15 has been demonstrated to attach to a diatom via chemotaxis. The bacteria then induce the formation of transparent exopolymeric particles and aggregates.17 The chemotaxis of Escherichia coli leads to cellular swarming and collective swimming in active suspensions, as well as formation of biofilms on solid surfaces.18 The Azospirillum brasilense chemotaxis-like Che1 signal transduction pathway has been shown to modulate changes in adhesive cell surface properties.19 These changes consequently affect cell-to-cell aggregation and flocculation behavior. In addition, Shewanella oneidensis MR-1 is capable of swimming towards electrodes via energy taxis and is known to cause rapid cell aggregation, which ultimately results in attachment and biofilm formation.20 The accumulation of bacteria near surfaces is a key step during the initial stages of biofilm formation, and it has been suggested that bacterial taxis plays an important role in bacteria–surface interactions. However, most previous reports have focused on floc or biofilm formation involved in chemotaxis or energy taxis. At present, studies on the effects of phototaxis on biofilm formation in photofermentative hydrogen production is still lacking.
This research aims to investigate the feasibility and mechanism of APB biofilm formation induced by phototaxis and surface sensing. First, the mechanism with respect to biofilm formation was investigated in batch experiments using both liquid surface and sidewall illumination. Then, a photorotating biological contactor (PRBC) process assisted by mirror-coated disks was carried out to improve the continuous hydrogen production and the removal of organic pollutants from synthetic wastewater. The strong experimental evidence of EPS and c-di-GMP levels is discussed with respect to APB biofilm formation.
2. Methods
2.1. Microorganism and medium
Rhodopseudomonas palustris W1, isolated from an anaerobic moving bed biofilm reactor that treats textile wastewater,21 was used in this study. The culture medium for biofilm formation and hydrogen production contained the following (g L−1): sodium acetate, 3.0; K2HPO4, 0.2; NaCl, 1.0; MgSO4·7H2O, 0.2; FeSO4·7H2O, 0.1; glutamate, 2.0; trace solution, 10 mL L−1. The trace solution contained the following (mg L−1): MnSO4·7H2O, 10; ZnSO4·7H2O, 50; H3BO3, 10; CaCl2, 10; Na2MoO4, 10; CoCl2·6H2O, 200; AlK(SO4)2, 10. The pH level of the synthetic wastewater was adjusted to 7.0.2.2. Batch experiment
Two identical reactors (5 cm × 5 cm × 5 cm) with working and headspace volumes of 100 and 25 mL, respectively, were made of polymethyl methacrylate. Reactor 1 and Reactor 2 were illuminated via sidewall and liquid surface illumination, respectively (Fig. 1). The reactors with culture media were sterilized for 15 min at 121 °C prior to start-up. R. palustris W1 inoculated into both bioreactors was cultivated statically under an anaerobic atmosphere of argon and an illumination intensity of approximately 250 W m−2 (illuminated with an incandescent lamp). The effects of light irradiation on biofilm formation and hydrogen evolution were monitored under varying light intensities. Five different light intensities (50, 150, 250, 300, and 400 W m−2) were studied. Each experiment was carried out in triplicate. | ||
| Fig. 1 Schematic of Reactor 1 with sidewall illumination and Reactor 2 with liquid surface illumination. | ||
2.3. Continuous experiment
Two identical laboratory-scale PRBC reactors were used for the continuous experiment (Fig. 2). Each reactor had a total reaction volume of 3.0 L and consisted of three stages, with eight polyvinyl chloride disks. For PRBC1, the surface of each disk was roughened with a 5 grit grade sandpaper and then coated with acrylic aerosol light reflecting spray paint (Guangzhou Comma Car Care Accessories Co., Ltd., Guangdong, China). The roughness average, water contact angle, and light reflectance on the disk surface with mirror coating were approximately 1.1 μm, 92.5° and 46.4%, respectively. For PRBC2, the disks roughened with a 5 grit grade sandpaper were not sprayed with any reflecting paint and were used as controls. The roughness average, water contact angle, and light reflectance on the disks without mirror coating were approximately 1.5 μm, 87.2° and 5.5%, respectively. Illumination was provided by two incandescent lamps placed 0.5 m apart. The illumination intensity on the outside surface of the reactors was maintained at 50 W m−2 (1000 lx). A low light intensity was used because of the low light attenuation in the PRBC reactors.22 In order to attain uniformity of feedstock and repeatability of all experiments, the same medium was used as synthetic wastewater for continuous hydrogen production. The pH level of the influent to the reactors was adjusted to 7.0, and the hydraulic retention time of the PRBC was set to 15 h. The PRBC, synthetic wastewater, and pipelines were not sterilized. Continuous experiments were conducted at a constant temperature of 25 ± 2 °C.2.4. Measurement and staining of EPS
EPS were extracted by using ethylenediaminetetraacetic acid disodium.23 Polysaccharide (PS) content was determined by using the anthrone method with glucose as a standard. Protein (PN) content was measured using the Lowry method with bovine serum albumin as a standard.EPS staining was performed according to the modified procedure by Chen et al.24 Biofilms were centrifuged to remove the supernatant, washed twice with 1× phosphate-buffered saline (PBS) buffer (pH 7.2), and kept fully hydrated in 2 mL centrifuge tubes covered with an aluminum foil. For PN staining, 0.1 M sodium bicarbonate buffer (100 μL) was added to keep the amine group in its non-protonated form. Fluorescein isothiocyanate solution (1.0 g L−1, 10 μL) was then added, and the mixture was stirred at room temperature for 1 h. For PS staining, 100 μL of calcofluor white (100 mg L−1, Sigma-Aldrich) was added, after which the mixture was incubated for another 30 min to bind with β-linked PS. Next, 100 μL of Nile red (5 mg L−1, Sigma-Aldrich) was added, and the mixture was incubated for 30 min to stain the lipids. After each staining stage, the samples were washed twice with PBS (0.1 M, pH 7.2) to remove excess dyes. Finally, the stained samples were placed onto glass slides to observe the distribution of EPS by using a fluorescence microscope (CX41, Olympus, Japan).
2.5. Extraction and quantification of intracellular c-di-GMP
Extraction of c-di-GMP was amended according to the procedures by Simm et al.25 Approximately 100 mg (wet weight) of the cells (equivalent to 9 mL of culture with an OD660 of 1.8) was harvested by centrifugation at 9000g for 10 min. The pellet was resuspended in 15 mL of water. Lysozymes and glass beads (0.1 mm) were added and the mixture was incubated at 37 °C for 1 h. The lysates were extracted twice by using double volumes of ethanol. The extracts were lyophilized using a vacuum dryer and then resuspended in 3 mL of water. Finally, 1 mL of the supernatant was loaded into a chromatography vial for high-performance liquid chromatography (HPLC) analysis to measure c-di-GMP concentration. HPLC (Agilent 1260, Agilent Co. Ltd., USA) was performed with a C18 column at 40 °C, with detection at 254 nm using a diode array detector. Runs were performed in mixed solvent (95% solvent A as 0.9% NaCl and 5% solvent B as 100% acetonitrile) at 1 mL min−1.262.6. Fluorescence in situ hybridization (FISH)
Oligonucleotide probe Rpal686 was used to target R. palustris,27 and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) was used to label all microorganisms. The probe Rpal686 had the sequence 5′-CTCACCTCTGCCATACTC-3′ and was linked with tetramethylrhodamine (TAMRA). In situ hybridization was performed as described by Honda et al.28 Briefly, a sample was first homogenized by using a vortex mixer. The sample was then placed in a hybridization well on a gelatin-coated microscopic slide plate. The sample was fixed with 4% paraformaldehyde for 1 h and stored in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of PBS (0.1 M, pH 7.2) and 99% ethanol at −20 °C. The concentration of deionized formamide in the hybridization buffer was 20%, whereas that of NaCl was 0.9 mol L−1. After hybridization, the cells were washed with PBS and stained by using DAPI staining solutions (20 μM, Sigma) for 10 min at 35 °C in the dark. The stained cells were investigated by using a fluorescence microscope (CX41, Olympus, Japan). Thirty microscopic fields per sample were randomly chosen when images were taken. Quantification of R. palustris was carried out using image analysis software Image-Pro Plus 6.0.
1 mixture of PBS (0.1 M, pH 7.2) and 99% ethanol at −20 °C. The concentration of deionized formamide in the hybridization buffer was 20%, whereas that of NaCl was 0.9 mol L−1. After hybridization, the cells were washed with PBS and stained by using DAPI staining solutions (20 μM, Sigma) for 10 min at 35 °C in the dark. The stained cells were investigated by using a fluorescence microscope (CX41, Olympus, Japan). Thirty microscopic fields per sample were randomly chosen when images were taken. Quantification of R. palustris was carried out using image analysis software Image-Pro Plus 6.0.
2.7. Biomass of biofilm
The biomass of the biofilm (mg cm−2) was obtained according to the method described by Jeswani and Mukherji.29 Samples of the biofilm (1.0 cm × 1.0 cm) were taken from the central area in the disc or sidewall. For batch experiments, the biofilm sample was vortex mixed with 5 mL of phosphate buffer in the presence of beads to obtain a uniform suspension. The absorbance at 660 nm of the cell suspension was determined using a spectrophotometer (SP-1105, China). The dry cell weight was estimated using a calibration curve derived from the relationship between the absorbance at 660 nm and the dry cell weight.30 For continuous experiments, the amount of suspended solids per cm2 of the biofilm was detected, which was indicative of the total biomass of the biofilm.2.8. Dil labelling
Samples were first collected from different regions of Reactor 1 and Reactor 2 by using a Pasteur pipette. The collected samples were stained with 5 μg mL−1 Dil solution (Molecular Probes, Invitrogen, USA) for 15 min in the dark at 33 °C with agitation. After labelling, the cells were washed thrice and then resuspended in PBS. The labelled cells were observed by using a fluorescence microscope.2.9. Other analyses
Hydrogen in the biogas was determined by using a gas chromatograph (SC-3000, Chuanyi Analyzer Co., Ltd., Chongqing) equipped with a thermal conductivity detector (TCD) and a 2 m packed column with porous polymer beads. Argon gas with a flow rate of 25 mL min−1 was used as the carrier gas. The temperatures at the injection port, column oven and TCD were maintained at 100 °C, 55 °C and 100 °C, respectively. The electric current for the TCD was 80 mA.Acetate concentration was determined using a gas chromatograph (7890A, Agilent, USA) fitted with a HP-FFAP capillary column (30 m × 0.25 mm × 0.25 μm) and a flame ionization detector. The temperature was programmed as follows: 80 °C for 5 min, then increased to 200 °C at 10 °C min−1. The ion concentration was analyzed by inductively coupled plasma mass spectrometry (HITACHI, P-4010).
Bacteriochlorophyll a (BChl a) concentration in the effluent was analyzed according to the method described by Casamayor et al.31 Pigments were extracted with an acetone–methanol mixture (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). The concentration of BChl a was determined with the absorbance at 770 nm (extinction coefficients of 76 mM−1 cm−1) using a UV–Vis spectrophotometer (UV-2550, Shimadzu, Japan).
2, v/v). The concentration of BChl a was determined with the absorbance at 770 nm (extinction coefficients of 76 mM−1 cm−1) using a UV–Vis spectrophotometer (UV-2550, Shimadzu, Japan).
2.10. Statistical analysis
Experimental results are expressed as mean ± standard deviation. Data were analyzed by a one way ANOVA test (using SPSS 17.0 statistical software; StatSoft Inc., Tulsa, OK).3. Results and discussion
3.1. Effect of illumination methods on biofilm formation and hydrogen production
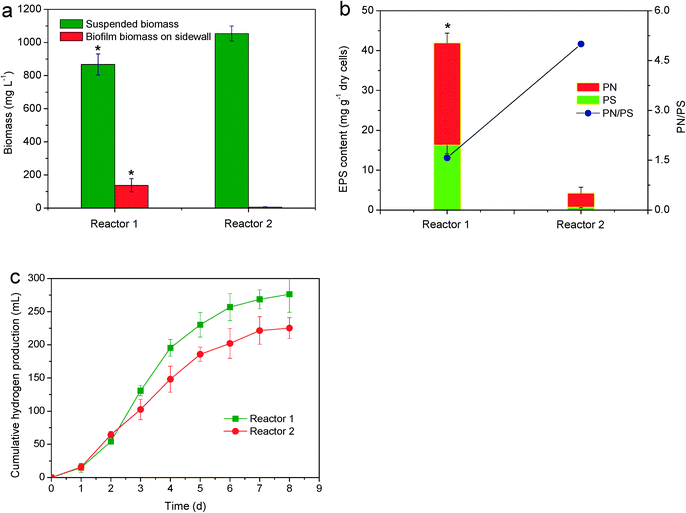
To test the feasibility of phototaxis-induced biofilm formation, Reactor 1 with sidewall illumination was used to improve biofilm formation and hydrogen production and Reactor 2 with liquid surface illumination was used for comparison. Fig. 3a shows that the amount of suspended biomass in Reactor 2 with liquid surface illumination was slightly higher than that in Reactor 1 with sidewall illumination. However, almost no biofilm was detected on the sidewall of Reactor 2. In contrast, the amount of biofilm biomass on the illuminated sidewall of Reactor 1 reached 137 mg L−1 (0.86 mg cm−2), which was 26-fold higher than that of the biofilm on the sidewall of Reactor 2 with liquid surface illumination (P < 0.05). This biofilm formation was also confirmed by macroscopic observations (Fig. S1†). In Reactor 1 with sidewall illumination, R. palustris was able to form purple biofilms on the illuminated sidewall which was maintained over the entire operation period. In Reactor 2 with liquid surface illumination, almost no floc or biofilm was observed on the sidewall. Likewise, no floc or biofilm was observed on the nearby liquid surface or bottom part of Reactor 2. It appeared that sidewall illumination was able to induce biofilm formation by R. palustris on the illuminated sidewall.EPS refer to sticky biopolymers secreted by microorganisms with extracellular PS and PN as two major components. These biopolymers attach to the cell surface to form a matrix structure that can facilitate cell-to-cell interaction and further strengthen the microbial architecture.32Fig. 3b shows the respective profiles of PS and PN contents in the biofilm from the illuminated sidewall of Reactor 1 and cells from the sidewall of Reactor 2 during 15 h of incubation. The PN and PS contents of the cells in Reactor 2 were approximately 3.5 and 0.7 mg gdry cells−1, respectively. Meanwhile, the EPS content in the biofilm from Reactor 1 remained nearly constant at 25.6 mg gdry cells−1 for PN and 16.3 mg gdry cells−1 for PS. The total EPS content in the biofilm from the illuminated sidewall of Reactor 1 was nine-fold higher than that in the cells from the sidewall of Reactor 2 (P < 0.05). These observations indicate that EPS secretion induced by phototaxis and surface sensing was a direct reason for the biofilm formation of APB.
Fig. 3c shows the difference between the hydrogen production by R. palustris in Reactor 1 with sidewall illumination and that in Reactor 2 with liquid surface illumination. The lag phases of hydrogen production in Reactor 1 and Reactor 2 do not show significant differences. The levels of cumulative hydrogen production at the end of incubation in Reactor 1 and Reactor 2 were 276.3 and 225.2 mL, respectively. Cumulative hydrogen production in Reactor 1 was 22.7% higher than that in Reactor 2. This result may be related to the lifestyle of R. palustris. Although the amount of total biomass in Reactor 1 and Reactor 2 did not show significant differences (P > 0.05), more bacteria were attached to the illuminated sidewall of Reactor 1, and the amount of biofilm biomass in Reactor 1 was significantly higher than that in Reactor 2 (P < 0.05). This condition is beneficial for enhancing bacterial hydrogen production activity. Biofilm formation may reduce the energy for bacterial physiological activity. As a result, more energy is available for photofermentative metabolism. Increases in hydrogen production and nitrogenase activity have been reported through APB immobilization.33 Therefore, the superiority of sidewall illumination over liquid surface illumination can be clearly attributed to the enhancement of biofilm formation and hydrogen production.
3.2. Effect of light intensity on biofilm formation and hydrogen production
To confirm the relationship between light and biofilm formation, the effects of light intensities (50, 150, 250, 300, and 400 W m−2) on biofilm formation as well as hydrogen production were studied. The effect of light intensities on biomass and cumulative hydrogen production is shown in Fig. 4. At low light intensities (50–300 W m−2), the amount of suspended and biofilm biomass of APB cells increased with the light intensity increasing and then reached a peak value under a light intensity of 300 W m−2 (Fig. 4a). The positive correlation between biofilm biomass and light intensity further demonstrates that light illumination from the sidewall influenced the enhancement of biofilm formation by R. palustris. When the light intensity was further increased to 400 W m−2, the growth of suspended APB cells was impaired, but the biofilm biomass exhibited a constant increase. This implies that the increase in biofilm biomass did not rely on the concentration of suspended biomass. | ||
| Fig. 4 Effect of light intensity on (a) biomass and (b) hydrogen production (*P < 0.05). The reactor was illuminated via sidewall illumination. | ||
An increase in the light intensity from 50 W m−2 to 300 W m−2 resulted in an increase in the cumulative hydrogen production. However, the cumulative hydrogen production decreased when the light intensity was higher than 300 W m−2 (Fig. 4b). The trend for hydrogen production was similar to that for total biomass formation. In this study, an optimum light intensity of 300 W m−2 (6000 lx) was obtained for the maximum cumulative hydrogen production (301.6 mL). The intensity was slightly higher than the reported optimal range of 4000 lx to 5000 lx in other studies.34–36 The difference may be attributed to shading of the biofilm on the illuminated sidewall. Therefore, optimization of the immobilized bioreactor design is needed for improving photohydrogen production performance.
3.3. Cell aggregation process and intracellular c-di-GMP levels
To study the mechanism of biofilm formation induced by phototaxis and surface sensing, the biofilm from the illuminated sidewall of Reactor 1 and the suspended cells from the liquid surface of Reactor 2 were compared by cell aggregation and c-di-GMP levels. The process of phototaxis-induced biofilm formation on the illuminated sidewall of Reactor 1 was observed on the basis of living cell fluorescence labelling, and that for the suspended cells from the illuminated liquid surface of Reactor 2 was also presented for comparison. In Reactor 1 (Fig. 5a), APB cells swarmed towards the region near the illuminated sidewall 5 h after inoculation, thus increasing the cell intensity. As the cell density increased, the APB cells formed small aggregates on the illuminated sidewall. The amount of aggregates gradually increased, and the number of individual cells decreased (10 h). After 15 h of incubation, the biofilm aggregates assembled and progressively expanded to occupy most of the available space. Finally, macroscopic APB biofilms formed and maintained stability on the illuminated sidewall. The development of these biofilms exhibited a collective nature, which is plausibly associated with the phenomenon of quorum sensing, a process through which certain genes or behaviors are only expressed in response to a sufficiently high cell density.37 Unexpectedly, APB in Reactor 2 were also motile towards light and caused high cell density near the illuminated liquid surface. However, the APB cells still were not able to form aggregates, which can be observed microscopically as individual motile cells, even under high-cell-density conditions over a period of 15 h (Fig. 5b). This observation indicates that R. palustris was capable of forming biofilms on the illuminated sidewall in response not only to light illumination but also to solid surface hindered phototactic movement. Therefore, we speculate that phototactic movement and contact with light radiators promoted the biofilm formation of R. palustris. This result is in good agreement with that of Pratt and Kolter,38 who proposed a model for the initiation of E. coli biofilm formation. In their model, chemotaxis was dispensable, but the movement towards the surface was required to overcome surface repulsion and trigger surface sensing.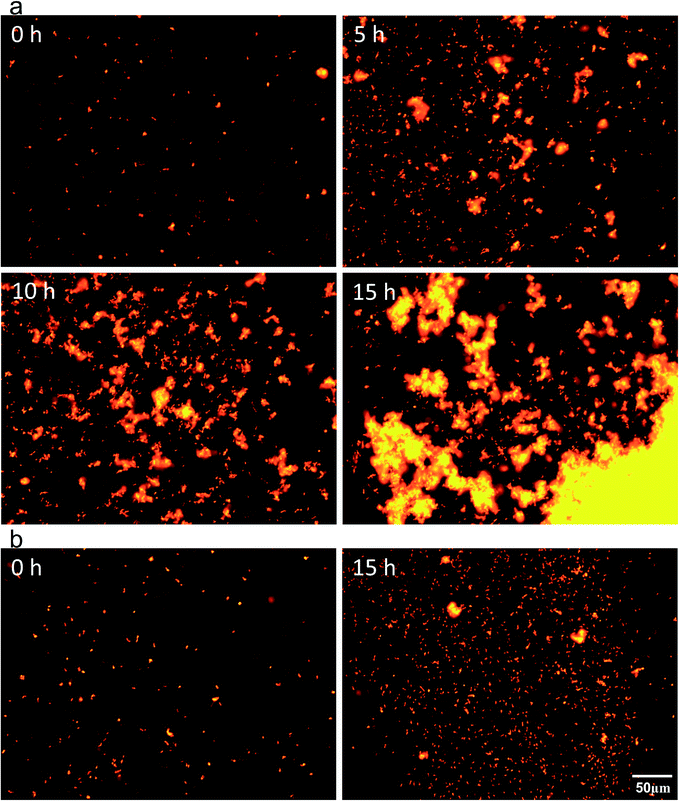 | ||
| Fig. 5 R. palustris cell aggregation process near (a) the illuminated sidewall of Reactor 1 and (b) illuminated liquid surface of Reactor 2 at different stages. Live cells were stained with Dil. | ||
Several enzymes encoding surface-regulated genes have the capability to modulate the levels of the second messenger c-di-GMP.39 c-di-GMP is an important signaling molecule in the transition between planktonic and sessile forms of bacterial life.40 To further study the mechanism of biofilm formation on the illuminated sidewall, c-di-GMP levels in R. palustris were quantified by HPLC analysis (Fig. S2†). The c-di-GMP contents in the biofilm from Reactor 1 remained at high levels (8.5 μg gdry cells−1), whereas a lower c-di-GMP pool was observed in the biomass from Reactor 2 (0.98 μg gdry cells−1). For example, the c-di-GMP contents in the biofilm from the illuminated sidewall of Reactor 1 were 7.7 times higher than those in the cells from the illuminated liquid surface of Reactor 2 at 15 h. One possible reason is that on the illuminated sidewall of Reactor 1, the frequent cell–surface contact mediated by the phototactic movement of APB may trigger surface sensing, which promotes the synthesis of c-di-GMP and inhibits the hydrolysis of c-di-GMP. Previous studies have shown that surface sensing systems in bacteria are activated when an individual bacterium comes into contact with the solid phase. Such contact can induce c-di-GMP production and stimulate biofilm formation.40 In the present study, surface sensing could not be triggered near the illuminated liquid surface in Reactor 2 because the phototactic cells did not come into contact with the solid light radiators. In addition, a high proportion of pGpG was observed in cells growing near the illuminated liquid surface (data not shown), which indicates that intracellular c-di-GMP was subjected to hydrolysis in the presence of light illumination and in the absence of a solid surface. The hydrolysis of c-di-GMP also explains why the high-intensity R. palustris near the liquid surface in Reactor 2 did not form flocs under lighted conditions. This result is consistent with the fact that blue light induces c-di-GMP hydrolysis. Kanazawa et al. reported that light-excited PapB in R. palustris enhanced the phosphodiesterase activity of PapA, which degraded c-di-GMP into pGpG.41 Hence, we speculate that phototactic movement and surface sensing do not solely upregulate the concentration of cellular c-di-GMP; rather, their influence on the c-di-GMP level depends on their synergy and the signals they process. Thus, phototaxis and surface sensing may synergistically modulate R. palustris c-di-GMP content, which in turn regulates PS and PN gene expression following biofilm formation.
3.4. Continuous hydrogen production in PRBC
To overcome the disadvantages of poor biofilm formation by R. palustris and the light shielding by biofilms, we designed a PRBC1 with mirror-coated disks and a PRBC2 control without mirror coating. The FISH technique was applied to characterize the main populations present in the biofilm samples collected from PRBC1 and PRBC2 at different stages. The visualization of R. palustris hybridizing with the Rpal686 probe and the total bacteria stained with DAPI is shown in Fig. S3.† The abundance of R. palustris in the total biomass was also calculated using the average values of images taken for the pixel areas of the TAMRA-labelled probe and DAPI-stained cells. Fig. 6a shows that the amount of biofilm biomass in PRBC1 increased simultaneously from 1.5 mg cm−2 to 4.2 mg cm−2, whereas the abundance of R. palustris slightly decreased from 99.4% to 78.5% during the start-up period and then maintained an almost constant value. In contrast, the amount of biofilm biomass in PRBC2 increased from 1.2 mg cm−2 to 6.2 mg cm−2, whereas the abundance of R. palustris dramatically decreased from 90.1% to 13.6% (Fig. 6b). Based on the calculation of biofilm biomass and the abundance of R. palustris, the biomass amount of R. palustris in PRBC1 (3.3 mg cm−2) was 2.5-fold higher than that in PRBC2 (0.84 mg cm−2) (P < 0.05). The results show that APB could successfully attach to the disks with mirror coating and form a stable biofilm in PRBC. The abundance of R. palustris was comparatively close to that of Rhodopseudomonas in a developed purple MFC effluent (72.2%), which applied illuminated microbial fuel cells to achieve high power densities.42 The amount of attached biomass of R. palustris in the PRBC was approximately 2.6-fold higher than that in a flat panel photobioreactor (0.92 mg cm−2),43 indicating that reflected light-induced biofilm formation has potential for applications in APB immobilization.As shown in Fig. 6c, the EPS content and the PN/PS ratio in the biofilm from PRBC1 simultaneously increased from 235 mg m−2 to 851 mg m−2 and from 1.13 to 1.78, respectively. In contrast, the EPS content in the biofilm from PRBC2 increased sharply from 534 mg m−2 to 1782 mg m−2, but the PN/PS ratio decreased from 0.85 to 0.56 with an increase in time from 10 days to 30 days (Fig. 6d). In fact, the PN/PS ratio in the biofilm from PRBC1 was of a similar level to that of R. palustris present in Fig. 3b because the biofilm from PRBC1 contained large amounts of R. palustris (78.5%). In contrast, the PN/PS ratio in the biofilm from PRBC2 was only 0.56, which was significantly less than the ratio of 1.57 for R. palustris W1. Fig. 7a and b further illustrate EPS distribution in the biofilms from PRBC1 and PRBC2 at 30 days. In PRBC2 without mirror coating, β-polysaccharides were distributed throughout the biofilm, whereas proteins and lipids exhibited obvious non-uniform distributions. Meanwhile, proteins accumulated more uniformly in the biofilm from PRBC1, whereas β-polysaccharides and lipids accumulated in the biofilm in the shape of a block. The protein matrix provided an architectural structure that enhanced the mechanical stability of the biofilm in PRBC1. These differences in EPS composition and distribution may be related to the significant difference in APB abundance on disks with and without mirror coating.
 | ||
| Fig. 7 Images of the biofilm grown in (a) PRBC1 and (b) PRBC2, stained with Nile red (lipids, red), fluorescein isothiocyanate (proteins, green), and calcofluor white (β-polysaccharides, blue). | ||
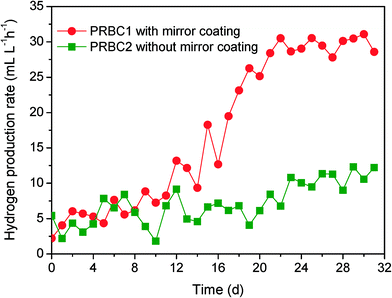
The hydrogen production rate in the biofilm under the same operating conditions was also explored and detailed (Fig. 8). The hydrogen production rate of PRBC1 slightly increased within the 12 h inoculation period and then increased rapidly from 13.2 mL L−1 h−1 to 30.5 mL L−1 h−1. The hydrogen production rate reached a constant value after approximately 22 days. In contrast, a low hydrogen production rate (12.33 mL L−1 h−1) was observed in PRBC2, even when the run time was prolonged to 30 days. Compared to PRBC2 without mirror-coated disks, PRBC1 with mirror-coated disks produced more hydrogen (P < 0.05) because more APB attached to the disk surface. In addition, PRBC1 showed a higher pollutant removal efficiency and a higher hydrogen yield compared to PRBC2. The COD removal efficiency and hydrogen yield in PRBC1 reached 85.4% and 0.60 mol of H2 per mol of acetate, whereas the corresponding figures in PRBC2 were 67.2% and 0.26 mol of H2 per mol of acetate (Table 1). The concentrations of BChl a in the effluents from PRBC1 and PRBC2 were 1.2 μmol L−1 and 9.8 μmol L−1, indicating that more APB biomass was washed out in PRBC2. Thus, the observed low COD removal and hydrogen production in PRBC2 may result from APB biomass withdrawal from the reactor. This result was in good agreement with that of Xie et al.,6,44 who demonstrated that the APB biofilm process could be operated at organic removal and hydrogen production efficiencies higher than those of the suspended growth processes because cell immobilization could retain APB biomass without biomass overflow. Although the hydrogen production rate of PRBC1 was close to that of other studies, illumination (1000 lx) in the present work was significantly lower than the reported range of 3000 lx to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lx in other continuous photobioreactors using a halogen or tungsten lamp as a light source.45–47 This is because PRBC possessed a thin liquid film over the biofilm on the discs, which can avoid light decay through the synthetic wastewater. Photohydrogen production is considered as the most promising alternative for large-scale hydrogen production due to its capability to utilize wastewater as feedstock whereby wastewater is stabilized at the same time. However, one major problem encountered in photohydrogen production from industrial wastewater is the dark color of the wastewater, which could sharply reduce light penetration.3,48 Notably, the thickness of liquid film over the APB biofilm on the discs of PRBC, which equaled to the light path, almost did not increase when the photobioreactor was scaled up. From this point of view, PRBC processes assisted by mirror-coated disks may be considered as promising options for continuous photohydrogen production from colored wastewater.
000 lx in other continuous photobioreactors using a halogen or tungsten lamp as a light source.45–47 This is because PRBC possessed a thin liquid film over the biofilm on the discs, which can avoid light decay through the synthetic wastewater. Photohydrogen production is considered as the most promising alternative for large-scale hydrogen production due to its capability to utilize wastewater as feedstock whereby wastewater is stabilized at the same time. However, one major problem encountered in photohydrogen production from industrial wastewater is the dark color of the wastewater, which could sharply reduce light penetration.3,48 Notably, the thickness of liquid film over the APB biofilm on the discs of PRBC, which equaled to the light path, almost did not increase when the photobioreactor was scaled up. From this point of view, PRBC processes assisted by mirror-coated disks may be considered as promising options for continuous photohydrogen production from colored wastewater.
| Acetate removal (%) | COD removal (%) | Hydrogen yield (mol of H2 per mol of acetate) | Concentration of BChl a in effluent (μmol L−1) | |
|---|---|---|---|---|
| PRBC1 | 92.7 ± 6.8 | 85.4 ± 4.6 | 0.60 ± 0.08 | 9.8 ± 0.4 |
| PRBC2 | 87.5 ± 7.5 | 67.2 ± 6.5 | 0.26 ± 0.12 | 1.2 ± 0.5 |
4. Conclusion
Both phototactic movement and surface sensing may be responsible for the biofilm formation of R. palustris, which did not occur in single-stimulation experiments. In particular, phototactic movement and surface sensing together modulate R. palustris c-di-GMP content, which in turn regulates PS and PN production following biofilm formation. Furthermore, APB can successfully attach to the disks with mirror coating and form a stable biofilm in PRBC, thus resulting in high APB cell concentrations in the bioreactors for hydrogen production and removal of organic pollutants. Therefore, the reflected light-induced biofilm formation of APB has potential for applications in the area of simultaneous photohydrogen production and organic wastewater purification.Acknowledgements
This study was supported by the National Natural Science Foundation of China (no. 51008025, 51478040) and the Open Project of the Key Laboratory of Reservoir Aquatic Environment,Chongqing Institute of Green and Intelligent Technology, Chinese Academy of Sciences (no. RAE2014CB05B).References
- A. Adessi and D. R. Philippis, Int. J. Hydrogen Energy, 2014, 39, 3127–3141 CrossRef PubMed.
- V. Gadhamshetty, A. Sukumaran and N. N. Khandan, Crit. Rev. Environ. Sci. Technol., 2011, 41, 1–51 CrossRef.
- T. Y. Wu, J. X. W. Hay, L. B. Kong, J. C. Juan and J. M. Jahim, Renewable Sustainable Energy Rev., 2012, 16, 3117–3122 CrossRef PubMed.
- S. S. Mirza, J. I. Qazi, Q. Zhao and S. Chen, Biotechnol. Biofuels, 2013, 6, 144 CrossRef PubMed.
- C. Y. Chen, C. H. Liu, Y. C. Lo and J. S. Chang, Bioresour. Technol., 2011, 102, 8484–8492 CrossRef PubMed.
- G. J. Xie, B. F. Liu, J. Ding, W. Q. Guo and N. Q. Ren, Biomass Bioenergy, 2012, 44, 122–129 CrossRef PubMed.
- G. J. Xie, B. F. Liu, D. F. Xing, J. Nan, J. Ding, H. Y. Ren, W. Q. Guo and N. Q. Ren, RSC Adv., 2012, 2, 2225–2228 RSC.
- T. Y. Jeong, G. C. Cha, I. K. Yoo and D. J. Kim, Int. J. Hydrogen Energy, 2007, 32, 525–530 CrossRef PubMed.
- X. M. Liu, G. P. Sheng and H. Q. Yu, Environ. Sci. Technol., 2007, 41, 4620–4625 CrossRef.
- A. S. Steunou, S. Liotenberg, M. N. Soler, R. Briandet, V. Barbe, C. Astier and S. Ouchane, MicrobiologyOpen, 2013, 2, 431–446 CrossRef PubMed.
- G. J. Xie, B. F. Liu, H. Y. Ren, D. F. Xing, J. Nan and N. Q. Ren, GCB Bioenergy, 2014, 6, 621–628 CrossRef PubMed.
- G. P. Sheng, H. Yu and Z. Yue, Appl. Microbiol. Biotechnol., 2005, 69, 216–222 CrossRef PubMed.
- G. J. Xie, B. F. Liu, D. F. Xing, J. Nan, J. Ding and N. Q. Ren, Biotechnol. Biofuels, 2013, 6, 64 CrossRef PubMed.
- M. Watanabe, K. Sasaki, Y. Nakashimada, T. Kakizono, N. Noparatnaraporn and N. Nishio, Appl. Microbiol. Biotechnol., 1998, 50, 682–691 CrossRef.
- S. Chitapornpan, C. Chiemchaisri, W. Chiemchaisri, R. Honda and K. Yamamoto, Water Sci. Technol., 2012, 65, 504–512 CrossRef CAS PubMed.
- V. Sourjik and N. S. Wingreen, Curr. Opin. Cell Biol., 2012, 24, 262–268 CrossRef CAS PubMed.
- E. C. Sonnenschein, D. A. Syit, H. P. Grossart and M. S. Ullrich, Appl. Environ. Microbiol., 2012, 78, 6900–6907 CrossRef CAS PubMed.
- K. Drescher, J. Dunkel, L. H. Cisneros, S. Ganguly and R. E. Goldstein, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 10940–10945 CrossRef PubMed.
- P. Siuti, C. Green, A. N. Edwards, M. J. Doktycz and G. Alexandre, FEMS Microbiol. Lett., 2011, 323, 105–112 CrossRef PubMed.
- H. W. Harris, M. Y. El-Naggar and K. H. Nealson, Biochem. Soc. Trans., 2012, 40, 1167–1177 CrossRef PubMed.
- X. Z. Wang, X. Cheng and D. Z. Sun, Appl. Microbiol. Biotechnol., 2008, 80, 907–915 CrossRef PubMed.
- X. Z. Wang, X. Cheng, D. Z. Sun, Y. W. Ren and G. H. Xu, J. Chem. Technol. Biotechnol., 2014, 89, 1545–1552 CrossRef PubMed.
- G. P. Sheng, H. Q. Yu and Z. Yu, Appl. Microbiol. Biotechnol., 2005, 67, 125–130 CrossRef PubMed.
- M. Y. Chen, D. J. Lee, J. H. Tay and K. Y. Show, Appl. Microbiol. Biotechnol., 2007, 75, 467–474 CrossRef PubMed.
- R. Simm, M. Morr, A. Kader and U. Römling, Mol. Microbiol., 2004, 53, 1123–1134 CrossRef PubMed.
- M. Hyodo, Y. Sato, Y. Hayakawa and D. K. R. Karaolis, Nucleic Acids Symp. Ser., 2005, 49, 117–118 CrossRef PubMed.
- K. Izu, F. Nakajima, K. Yamamoto and F. Kurisu, Syst. Appl. Microbiol., 2001, 24, 294–302 CrossRef PubMed.
- R. Honda, K. Fukushi and K. Yamamoto, J. Biotechnol., 2006, 125, 565–573 CrossRef PubMed.
- H. Jeswani and S. Mukherji, Bioresour. Technol., 2012, 111, 12–20 CrossRef PubMed.
- R. W. Pott, C. J. Howe and J. S. Dennis, Bioresour. Technol., 2013, 130, 725–730 CrossRef PubMed.
- E. O. Casamayor, J. Mas and C. Pedrós-Alió, FEMS Microbiol. Ecol., 2001, 42, 427–437 CrossRef PubMed.
- A. Bridier, R. Briandet, V. Thomas and F. Dubois-Brissonnet, Biofouling, 2011, 27, 1017–1032 CrossRef PubMed.
- D. Shi, M. Brouers, D. Hall and R. Robins, Planta, 1987, 172, 298–308 CrossRef PubMed.
- K. Sasikala, C. V. Ramana and P. R. Rao, Int. J. Hydrogen Energy, 1991, 16, 597–601 CrossRef.
- Z. Jamil, M. S. Mohamad Annuar, S. Ibrahim and S. Vikineswary, Int. J. Hydrogen Energy, 2009, 34, 7502–7512 CrossRef PubMed.
- P. Castillo, J. P. Magnin, M. Velasquez and J. Willison, Energy Procedia, 2012, 29, 357–366 CrossRef CAS PubMed.
- C. Solano, M. Echeverz and I. Lasa, Curr. Opin. Microbiol., 2014, 18, 96–104 CrossRef PubMed.
- L. A. Pratt and R. Kolter, Mol. Microbiol., 1998, 30, 285–293 CrossRef.
- C. J. Gode-Potratz, R. J. Kustusch, P. J. Breheny, D. S. Weiss and L. L. McCarter, Mol. Microbiol., 2011, 79, 240–263 CrossRef PubMed.
- J. R. O'Connor, N. J. Kuwada, V. Huangyutitham, P. A. Wiggins and C. S. Harwood, Mol. Microbiol., 2012, 86, 720–729 CrossRef PubMed.
- T. Kanazawa, S. Ren, M. Maekawa, K. Hasegawa, F. Arisaka, M. Hyodo, Y. Hayakawa, H. Ohta and S. Masuda, Biochemistry, 2010, 49, 10647–10655 CrossRef CAS PubMed.
- T. J. Park, W. Ding, S. Cheng, M. S. Brar, A. P. Ma, H. M. Tun and F. C. Leung, AMB Express, 2014, 4, 22 CrossRef PubMed.
- Y. J. Wang, Q. Liao, Y. Z. Wang, X. Zhu and J. Li, Bioresour. Technol., 2011, 102, 6902–6908 CrossRef CAS PubMed.
- G. J. Xie, B. F. Liu, H. Q. Wen, Q. Li, C. Y. Yang, W. L. Han, J. Nan and N. Q. Ren, Int. J. Hydrogen Energy, 2013, 38, 7780–7788 CrossRef CAS PubMed.
- C. M. Lee, G. J. Hung and C. F. Yang, Bioresour. Technol., 2011, 102, 8350–8356 CrossRef CAS PubMed.
- H. Y. Ren, B. F. Liu, G. J. Xie, L. Zhao and N. Q. Ren, GCB Bioenergy, 2014, 6, 599–605 CrossRef CAS PubMed.
- I. Eroglu, A. Tabanoglu, U. Gunduz, E. Eroglu and M. Yucel, Int. J. Hydrogen Energy, 2008, 33, 531–541 CrossRef CAS PubMed.
- K. Seifert, M. Waligorska and M. Laniecki, Int. J. Hydrogen Energy, 2010, 35, 4085–4091 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ew00019j |
| This journal is © The Royal Society of Chemistry 2015 |