Extracting metal ions from water with redox active biopolymer electrodes†
Shimelis
Admassie
*ab,
Anders
Elfwing
b,
Andreas
Skallberg
c and
Olle
Inganäs
b
aDepartment of Chemistry, Addis Ababa University, PO Box 1176, Addis Ababa, Ethiopia. E-mail: shimadm09@gmail.com
bBiomolecular and Organic Electronics, Department of Physics, Chemistry and Biology (IFM), Linköping University, 581 83 Linköping, Sweden
cMolecular Surface Physics and Nanoscience, Department of Physics, Chemistry and Biology (IFM), Linköping University, 581 83 Linköping, Sweden
First published on 20th February 2015
Abstract
Renewable, environmentally friendly and cheap materials like lignin and cellulose have been considered as promising materials for use in energy storage technologies. Here, we report a new application for biopolymer electrodes where they can also be simultaneously used as ion pumps to purify industrial wastewater and drinking water contaminated with toxic metals. A ternary composite film consisting of a conducting polymer polypyrrole (PPy), biopolymer lignin (LG) and anthraquinonesulfonate (AQS) was synthesized by one-step galvanostatic polymerization from an aqueous electrolyte solution. X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDX) techniques revealed that lead ions can be extracted from a neutral aqueous solution containing lead ions by applying a potential, and can be released into another solution by reversing the polarity of the applied potential. Electrochemical quartz crystal microbalance was used to quantify the amount of metal ions that can be extracted and released.
Water impactThe high concentration of toxic heavy metals like lead, which are being released from industrial processes, is a serious challenge to our environment. The problem is even worse in developing countries since environmental standards are less stringent and storage batteries are being used to supply electricity. Available methods to treat such heavily contaminated wastewater employ expensive materials or regenerate additional waste during clean-up. We employed an electrochemical method using lignin and formed a cheap and renewable adsorbent system for treating water polluted with heavy metals like lead. Since the biopolymer electrode can also be used for charge storage, the battery or supercapacitor made of the biopolymer composites can store solar electricity and also remove heavy metals from polluted water. |
Introduction
With increasing population and industrialization, environmental pollution by toxic heavy metals like lead, mercury, cadmium and arsenic continues to be a serious challenge. This is also of greater concern for the industrial sector since more and more stringent regulations are placed on the utilization of these toxic materials. Among the top 20 hazardous substances, lead occupies the second place next to arsenic.1 A number of studies2–5 showed that depending on the level of exposure, lead affects the nervous system, kidneys, the immune system and the cardiovascular system. Particularly in young children, lead poisoning can result in damage to the central nervous system, causing behavioral problems and lowered IQs and it is also associated with high blood pressure in adults. As a result, many countries have taken steps to control the use of lead. The banning of lead in household paints6and the phase-out of lead in gasoline are the measures taken by some countries. Nonetheless, lead is still continually being introduced into the environment through processes like combustion of coal and oil, manufacturing of storage batteries, fertilizers, ammunition, lead smelting and mining.7–9 Because of these activities, the concentration of lead ions in industrial water reaches 200–500 mg L−1. These values are much higher than the maximum permissible level of 0.1–0.05 mg L−1.10 This calls for an intensified effort to develop cheap and environmentally friendly methods to reduce lead levels in drinking water as well as wastewater.Some of the approaches currently being used for removal of toxic heavy metals include coagulation,11 ion exchange,12 adsorption13and electrochemical14 methods. Among these methods, adsorption and ion exchange methods are extensively used mainly because of their low cost and simplicity. Hepel et al.15–17 in the late 1990s reported an electrochemically assisted removal of heavy metals using composite polypyrrole films with cation-exchange properties. They were able to monitor the adsorption and desorption of lead ions using an electrochemical quartz crystal microbalance for polypyrrole–melanine composite films.16 The main advantage of such an electrochemical method compared to other methods is that the adsorbent can simply be regenerated by applying a potential without leaving a secondary waste or requiring chemicals to regenerate the adsorbent. There have been continued studies on the extraction of heavy metals with polymer electrodes,18,19 but this is not a well-established technology. The potential application of the method for wastewater treatment did not progress well. In recent times, other researchers developed an electrically switched ion exchange (ESIX) technique based on nickel hexacyanoferrate (NiHCF) films deposited on electrodes for selective removal of alkali and alkaline earth cations such as Cs+ (ref. 20) and Ca2+ (ref. 21) and very recently of Ni2+.22
On the other hand, lignin alone and its composites with conducting polymers like polyaniline attracted great interest for developing low-cost water treatment technologies due to the large abundance of lignin in nature23–33 and the low cost of the material. However, almost all the reports in the literature use a physical adsorption method and there have been no reported work so far that employs the recently developed ESIX method. Recently, we developed an interpenetrating network of polypyrrole/lignin (PPy/LG) composites for energy storage applications.34–37 Our studies revealed that the electrochemical and charge storage properties of different lignins incorporated inside the PPy film show variations with the chemical compositions and source of the lignins where the specific capacitance and charge capacity values varied from 206 to 477 F g−1 and 30.4 to 69 mA h g−1.34,37 Moreover, for a ternary composite supercapacitor electrode consisting of phosphomolybdic acid, lignin and PPy, the specific capacitance and charge capacity values were significantly improved to 682 F g−1 and 128 mA h g−1, respectively.35 Similarly, we showed that when anthraquinonesulfonate was used instead phosphomolybdic acid in the same ternary system, values as high as 186 mA h g−1 were obtained.36 During our studies on this application, we noted that the PPy/LG composites also do have ion-exchange properties. Many of these electrode materials rely on proton exchange and have been studied in aqueous acidic media. For treatment of water, it is essential to operate close to neutral pH. For this purpose, we have modified our synthesis of the biopolymer electrode. Using the method developed in our laboratory, we studied the cation dynamics of the polypyrrole/lignin/anthraquinonesulfonate (PPy/LG/AQS)36 composites and here we report the synthesis, characterization and potential application of the composite for removal of lead ions.
Experimental
Sodium chloride, sodium salts of anthraquinone-2-sulfonic acid and lignosulfonic acid and analytical-grade lead nitrate were purchased from Sigma Aldrich and the required solution was prepared using 18 MΩ deionized water.Cyclic voltammetry and galvanostatic experiments were carried out using an AutolabPGStat 10 (EchoChemie, the Netherlands). Quartz crystal microbalance studies were carried out with a Q-Sense E4 using gold deposited quartz crystals. The frequency change was monitored during the deposition of the material, and analysed using the Sauerbrey equation to deduce the mass. The PPy/LG and PPy/AQS/LG composites were synthesized by galvanostatic polymerization at a current density of 250 μA cm−2 (until a charge of 187.5 mC cm−2) in a three-electrode system consisting of a gold working electrode, a Ag/AgCl/KCl (3.0 M NaCl) reference electrode and a platinum wire counter electrode from a solution consisting of 0.025 M Py and 5 mg mL−1 LG, and 0.025 M Py, 2.5 mM AQS and 5 mg mL−1 LG in 0.1 M NaCl aqueous solution. After this, the electrode was removed from the polymerization solution, rinsed gently and transferred to monomer and dopant free 0.1 M NaCl aqueous solution. Repetitive cyclic voltammograms were then recorded by scanning the electrode potential between 0.6 V and −1.0 V vs. Ag|AgCl until a steady response was obtained. After this, the biopolymer electrode was ready for use. Metal extraction from an aqueous sample of lead and its release into a lead free solution was performed by applying a potential of −0.4 V and +0.4 V vs. Ag|AgCl for 6 and 12 minutes, respectively.
XPS measurements were performed using a Microlab 310F instrument equipped with a hemispheric analyzer using unmonochromatized Al Kα photons (1486.6 eV). Fixed analyzer pass energies for Pb4f and survey spectra were 50 and 100 eV, respectively. The binding energy scales of the spectra were aligned to the C 1s peak (285 eV). The surface morphology and composition were characterized by a scanning electron microscope (Leo 1550 Gemini, Zeiss) equipped for energy dispersive X-ray spectroscopy (X-Max, Oxford Instrument). The samples (organic film on gold covered silicon wafer) were analyzed using an acceleration voltage of 20 kV.
Results and discussion
One of the important properties of conducting polymers (CPs), such as polypyrrole (PPy), is their ability to act as electrically switchable cations or anion exchange materials depending on the sizes of the dopant ions. PPy normally exists in its oxidized state soon after its formation by oxidative electropolymerization. When it is reduced, the polymer becomes electrically neutral. If the dopant ions inserted during the oxidative polymerization are small and mobile ions, like chloride or perchlorate, they will be expelled from the film leaving the polymer film in its neutral state. Hence, upon continuous cycling between the oxidized and reduced states of the polymer film, the small counter ions are continually ejected and inserted.34,35 On the other hand, if PPy is doped with large and immobile negatively charged counter ions like hexasulfonated calix[6]arenes (C6S),38–40 heparin,15 and sodium dodecyl sulfonate,35 the film retains the immobile counter anions upon reduction, and cations from the electrolyte solutions will be inserted. These cations are subsequently released when the film is oxidized. As a result, the film acts as a cation exchanger. However, the cation exchange properties of such a film are also dependent on the pH of the medium. For instance, Hepel et al.15 showed that the PPy/heparin film acts as a cation exchanger in neutral media and as an anion exchanger in acidic media. Similarly, our earlier results34,35 also showed that the PPy/LG film largely acts as an anion exchanger along with protons in acidic media.To utilize our composite material as a cation exchanger, PPy incorporating the sodium salt of lignosulfonic acid (LG) was galvanostatically polymerized from an aqueous solution of 0.1 M NaCl as a supporting electrolyte. The composite film was cycled for 50 cycles at 50 mV s−1 in a monomer free 0.1 M NaCl solution and the resulting voltammogram is shown in Fig. 1(a). However, as opposed to our previous results34–36 for the PPy/LG composites studied in an acidic environment, in neutral media a decrease in both the anodic and cathodic peak currents was observed, indicating poor stability and loss of electroactivity of the film during subsequent scans.
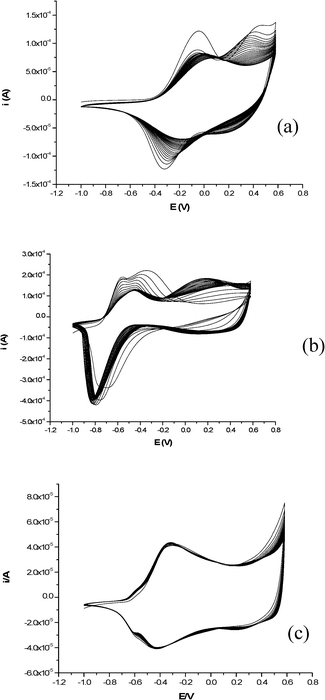 | ||
| Fig. 1 Cyclic voltammograms of (a) PPy/LG, (b) PPy/AQS, and (c ) PPy/AQS/LG films cycled for 50 cycles at 50 mV s−1 in a monomer free 0.1 M NaCl solution. | ||
When PPy incorporating anthraquinonesulfonate (AQS) was polymerized galvanostatically from an aqueous solution of 2.5 mM AQS in 0.1 M NaCl as a supporting electrolyte and the composite film was cycled for 50 cycles at 50 mV s−1 in a monomer free 0.1 M NaCl solution (Fig. 1(b)), the film showed better stability than the PPy/LG system. Moreover, when AQS is simultaneously incorporated in the PPy film along with LG and cycled under similar conditions, the stability of the ternary system is significantly improved. This is clearly seen in the cyclic voltammograms of the composite film (Fig. 1(c)) cycled for 50 cycles in monomer free 0.1 M NaCl solution as a supporting electrolyte. Besides the stabilizing effect, AQS has an added advantage in keeping the composite film negatively charged when the polymer backbone is in its reduced state by remaining intact in the film due to its large size. Hence, it facilitates cation insertion and expulsion upon subsequent electrochemical switching of the polymer film between its oxidized and reduced states. The metal binding properties of LG promote the extraction and release of metallic ions by an applied potential. Several processes including ion exchange, surface adsorption, and complexation have been suggested to explain the mechanisms involved. Detailed studies are required to arrive at a quantitative and mechanistic understanding of the sorption of metal ions by lignin. Here, we propose a simplified redox reaction for the electrochemically assisted metal extraction of lead ions:
| 2PPy+(AQS−/LGS−) + Pb2+ + 2e = PPy(AQS−/LGS−)2Pb2+ |
Fig. S1† depicts the effect of scan rates on the peak currents for the major oxidation and reduction peaks of the composite films. For PPy doped with AQS, the major redox peaks observed were due to the redox activity of AQS (Fig. S1(a)†). But, when LG is incorporated simultaneously with AQS, the AQS peaks are suppressed and the dominant peaks observed are mainly due to the redox activity of the PPy and lignin composites(Fig. S1(c)†). In both cases, the peak currents were found to vary linearly with the scan rate (Fig. S1(b) and (d)†) suggesting that a surface confined redox process took place in the composites.
The cyclic voltammograms of the composite films in the absence and presence of lead ions were recorded to determine the potential window for extraction and release of lead ions using the composites films without reducing lead ions to metallic lead (Fig. 2). The composite films showed different behaviors for the reduction of lead. For both PPy/AQS and PPy/AGS/LG composites in the absence of lead ions, the cyclic voltammograms do not show redox activity at potentials lower than −0.8 V. But, when lead ions were introduced into the PPy/AQS film in addition to the broad peaks at anodic potentials, a strong reduction current was observed (Fig. 2(a)ii). This strong reduction current is attributed to the electrochemical reduction of lead ions to metallic lead which is not a favorable condition for an electrochemically assisted metal extraction and release process.
 | ||
| Fig. 2 Cyclic voltammograms of PPy/AQS films in the absence (a(i)) and presence (a(ii)) of lead ions and PPy/AQS/LG in the absence (b(i)) and presence (b(ii)) of lead ions. | ||
However, in the PPy/AQS/LG composites (Fig. 2(b)ii) such a strong reduction current was not observed indicating that lignin binds the lead ions and inhibits the electrodeposition of metallic lead. A similar inhibition effect of lignosulfonates in the electrodeposition of lead has been reported in the literature.42 On the other hand, in the anodic potential region, new redox peaks appear when lead ions were present in the electrolyte solutions (Fig. 2(b)ii). These peaks are presumably due to the redox activity of the lead ions complexed with the lignosulfonates and quinone groups in AQS and LG.
XPS measurements were done to qualitatively monitor the extraction and release of lead ions by applying cathodic and anodic potentials to the composite films. The XPS survey and high resolution Pb4f spectra including those of the as-prepared composite film are shown in Fig. 3(a) and (b). The presence of Pb4f, Pb4d and Pb4p peaks (Fig. 3(a)ii) when a cathodic potential was applied to the film confirms the insertion of lead ions. When the potential was reversed to anodic potential, these peaks disappeared (Fig. 3(a)iii), which was just similar to the as-prepared film (Fig. 3(a)i), confirming that the inserted lead ions were released into the solution.
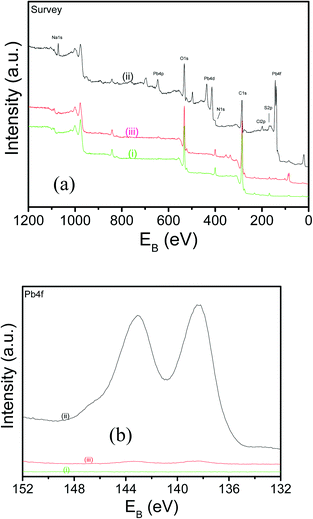 | ||
| Fig. 3 XPS survey and high resolution Pb4f spectra of (a(i) and b(i)) the as-prepared composite film, after adsorption (a(ii) and b(ii)) and release (a(iii) and b(iii)) of lead ions. | ||
The SEM-EDX images and spectra of the adsorbent films before adsorption, after adsorption and release of lead ions are depicted in Fig. 4. Similar to the film before adsorption (Fig. 4(a)i and (b)i), no lead ions were observed in the composite film after releasing the adsorbed lead ions (Fig. 4(a)iii and (b)iii) by applying an anodic potential on the film. Also the SEM-EDX results clearly confirm the presence of lead ions when a cathodic potential was applied to the film (Fig. 4(a)ii and (b)ii). Hence, these results further complement the XPS data and the fact that lead ions can be extracted and released by applying cathodic and anodic potentials, respectively, is very well ascertained.
 | ||
| Fig. 4 SEM-EDX images and spectra of (a(i) and b(i)) the as-prepared composite film, after adsorption (a(ii) and b(ii)) and after release (a(iii) and b(iii)) of lead ions. | ||
EQCM is a powerful technique to quantitatively determine the ion-dynamics in the composite films. The masses recorded at various frequencies for the PPy/AQS/LG electrode are depicted in Fig. S2.† Uniform and linear depositions were observed at all frequencies with deposition masses in the range ≈ 110 μg and a film thickness of about 0.7 μm. Fig. 5 (a) and (b) depict the changes in the amounts of sodium and lead ions that were incorporated and released from 0.1 M NaCl and 2.0 mM Pb(II) in 0.1 M NaCl solution at the PPy/AQS/LG film when the potential was switched between +0.4 and −0.4 V.
It was observed that the sodium salt of lignosulfonic acid had a high sodium ion exchange capacity, where up to 3.1 μg of sodium ions were exchanged with an adsorption capacity of 28.2 mg g−1 (Fig. 5(a)). Similarly, when 2.0 mM Pb(II) ions were added, the mass of the metal ion exchanged increased to 9.0 μg due to the additional lead ions (Fig. 5(b)). No significant ion exchange dynamics was observed for the PPy/AQS system.
In order to further determine the adsorption capacity of lead on the composite film, the ion dynamics was investigated by varying the concentration of lead ions from 0.05 M to 0.4 M Pb(II) ions in water. The observed ion-dynamics and mass changes for each concentration investigated are shown in Fig. S3,† where the mass changes increased from 3.1 to 6.9 μg as the concentration of lead ions increased from 0.05 M to 0.4 M. The adsorption capacities at each concentration were also plotted against the concentration of lead ions in the solution (Fig. 6).
It was found that the adsorption capacity initially increased with increasing concentration of lead ions and reached a steady value with a maximum adsorption capacity of 69 mg g−1.
Conclusions
We demonstrated the use of biopolymer electrodes for metal extraction from water. This could be used in a system where ions are extracted from one liquid stream and released into another liquid stream. Pb is cleaned from the first stream, and then released into a second liquid stream with a higher concentration of the metal ion in the second stream. Hence, the biopolymer electrode may serve as an ion pump system. When the charge storage properties of the biopolymer electrode are combined with the ion extraction properties, renewable and cheap electricity from windmills at high speed, or photovoltaic modules at maximum illumination, can be stored while metal ions are extracted from one liquid phase to be released into another. The discharge can be done when needed, as in a distributed system of charge storage.43Acknowledgements
This work was supported by the Knut and Alice Wallenberg Foundation through the project Power Papers, a Wallenberg Scholar grant to O.I, and the Swedish Science Council VR through the Swedish Research Links program.Notes and references
- J. Moros, I. Llorca, M. L. Cervera, A. Pastor, S. Garrigues and M. de la Guardia, Anal. Chim. Acta, 2008, 613, 196 CrossRef CAS PubMed.
- P. A. Meyer, M. J. Brown and H. Falk, Mutat. Res., 2008, 659, 166 CAS.
- H. Needleman, Annu. Rev. Med., 2004, 55, 209 CrossRef CAS PubMed.
- G. Flora, D. Gupta and A. Tiwari, Interdiscip. Toxicol., 2012, 5, 47 CAS.
- D. A. Gidlow, Occup. Med., 2004, 54, 76 CrossRef CAS PubMed.
- H. L. Needleman, Am. J. Public Health, 1998, 88, 1871 CrossRef CAS PubMed.
- S. Tunali, T. Akar, A. S. Ozcan, I. Kiran and A. Ozcan, Sep. Purif. Technol., 2006, 47, 105 CrossRef CAS PubMed.
- K. Conrad and H. C. B. Hansen, Bioresour. Technol., 2007, 98, 89 CrossRef CAS PubMed.
- E. Eren, B. Afsin and Y. Onal, J. Hazard. Mater., 2009, 161, 677 CrossRef CAS PubMed.
- A. S. Özcan, Ö. Gök and A. Özcan, J. Hazard. Mater., 2009, 161, 499 CrossRef PubMed.
- F. A. de Mello, M. Marchesiello and P. X. Thivel, Sep. Purif. Technol., 2013, 107, 109 CrossRef PubMed.
- B. Alyüz and S. Veli, J. Hazard. Mater., 2009, 167, 482 CrossRef PubMed.
- C. Aydiner, M. Bayramoglu, B. Keskinler and O. Ince, Ind. Eng. Chem. Res., 2008, 48, 903 CrossRef.
- K. Dermentzis, J. Hazard. Mater., 2010, 173, 647 CrossRef CAS PubMed.
- J. Hepel, S. Bruckenstein and M. Hepel, Microchem. J., 1997, 55, 179 CrossRef CAS.
- M. Hepel, Z. Xingmin, R. Stephenson and S. Perkins, Microchem. J., 1997, 56, 79 CrossRef CAS.
- M. Hepel and L. Dentrone, Electroanalysis, 1996, 8, 996 CrossRef CAS.
- M. Antilén, M. Á. González, M. Pérez-Ponce, M. Gacitúa, M. Angélica del Valle, F. Armijo, R. del Río and G. Ramírez, Int. J. Electrochem. Sci., 2011, 6, 901 Search PubMed.
- M. Antilén and F. Armijo, J. Appl. Polym. Sci., 2009, 113, 3619 CrossRef.
- B. Sun, X. G. Hao, Z. D. Wang, G. Q. Guan, Z. L. Zhang, Y. B. Li and S. B. Liu, J. Hazard. Mater., 2012, 233–234, 177 CrossRef CAS PubMed.
- C. Weidlich and K.-M. Mangold, Electrochim. Acta, 2011, 56, 3481 CrossRef CAS PubMed.
- Z. Wang, Y. Feng, X. Hao, W. Huang, G. Guan and A. Abudul, J. Hazard. Mater., 2014, 274, 436 CrossRef CAS PubMed.
- A. Demirbas, J. Hazard. Mater., 2004, 109, 221 CrossRef CAS PubMed.
- A. Demirbas, J. Hazard. Mater., 2008, 157, 220 CrossRef CAS PubMed.
- S. Babel and T. A. Kurniawan, J. Hazard. Mater., 2003, 97, 219 CrossRef CAS.
- S. E. Bailey, T. J. Olin, R. M. Bricka and D. D. Adrian, Water Res., 1999, 33, 2469 CrossRef CAS.
- X. Guo, S. Zhang and X.-Q. Shan, J. Hazard. Mater., 2008, 151, 134 CrossRef CAS PubMed.
- W. S. Peternele, A. A. Winkler-Hechenleitner and E. A. G. Pineda, Bioresour. Technol., 1999, 68, 95 CrossRef CAS.
- D. Mohan, C. U. Pittman Jr. and P. H. Steele, J. Colloid Interface Sci., 2006, 297, 489 CrossRef CAS PubMed.
- M. Ahmaruzzaman, Adv. Colloid Interface Sci., 2011, 166, 36 CAS.
- Q.-F. Lu, J.-J. Luo, T.-T. Lin and Y.-Z. Zhang, ACS Sustainable Chem. Eng., 2014, 2, 465 CrossRef CAS.
- J. Yang, J.-X. Wu, Q.-F. Lu and T.-T. Lin, ACS Sustainable Chem. Eng., 2014, 2, 1203 CrossRef CAS.
- Y. Ge, D. Xiao, Z. Li and X. Cui, J. Mater. Chem. A, 2014, 2, 2136 CAS.
- G. Milczarek and O. Inganäs, Science, 2012, 335, 1468 CrossRef CAS PubMed.
- S. Admassie, A. Elfwing, E. W. H. Jager, Q. Bao and O. Inganäs, J. Mater. Chem. A, 2014, 2, 1974 CAS.
- D. H. Nagaraju, T. Rebis, R. Gabrielsson, A. Elfwing, G. Milczarek and O. Inganäs, Adv. Energy Mater., 2014, 4, 1300443 Search PubMed.
- S. Admassie, T. Y. Nilsson and O. Inganäs, Phys. Chem. Chem. Phys., 2014, 16, 24681 RSC.
- J. Wu, W. M. Mullett and J. Pawliszyn, Anal. Chem., 2002, 74, 4855 CrossRef CAS.
- L. T. T. Kim, C. Gabrielli, A. Pailleret and H. Perrot, Electrochim. Acta, 2011, 56, 3516 CrossRef CAS PubMed.
- M. N. Akieh, S. F. Ralph, J. Bobacka and A. Ivaska, J. Membr. Sci., 2010, 354, 162 CrossRef CAS PubMed.
- M. Quan, D. Sanchez, M. F. Wasylkiw and D. K. Smith, J. Am. Chem. Soc., 2007, 129, 12847 CrossRef CAS PubMed.
- L. Muresan, L. Oniciu and R. Wiart, J. Appl. Electrochem., 1993, 23, 66 CrossRef CAS.
- O. Inganäs and S. Admassie, Adv. Mater., 2014, 26, 830 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The effect of the scan rate on peak currents, mass changes during the growth of the composite films and mass changes for different concentrations of lead ions are presented in the ESI. See DOI: 10.1039/c4ew00097h |
| This journal is © The Royal Society of Chemistry 2015 |


