Progress towards the validation of modeled environmental concentrations of engineered nanomaterials by analytical measurements
Bernd
Nowack
*a,
Mohamed
Baalousha
b,
Nikolaus
Bornhöft
a,
Qasim
Chaudhry
c,
Geert
Cornelis
d,
Jane
Cotterill
c,
Andreas
Gondikas
f,
Martin
Hassellöv
d,
Jamie
Lead
be,
Denise M.
Mitrano
a,
Frank
von der Kammer
f and
Tim
Wontner-Smith
c
aEmpa, Swiss Federal Laboratories for Materials Science and Technology, Lerchenfeldstrasse 5, CH-9014 St. Gallen, Switzerland. E-mail: nowack@empa.ch
bCenter for Environmental Nanoscience and Risk, University of South Carolina, SC 29028, USA
cFERA The Food and Environment Research Agency, Sand Hutton, York, YO41 1LZ, UK
dUniversity of Gothenburg, Department of Chemistry and Molecular Biology, Kemivägen 10, 41296 Gothenburg, Sweden
eSchool of Geography, Earth and Environmental Sciences, College of Life and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK
fDepartment of Environmental Geosciences, University of Vienna, Althanstrasse 14, 1090 Vienna, Austria
First published on 10th July 2015
Abstract
Environmental exposure modeling has been used extensively in the last years to obtain estimates of environmental concentrations of engineered nanomaterials (ENMs). In this perspective piece, we explore the issues when aiming to validate modeled environmental concentrations and propose options for both modelers and analytical chemists on how to proceed in the future to better compliment one another's efforts. In this context, validation means to determine the degree to which the simulation results from a model are accurate representations of the real world by comparison with analytical data. Therefore, for such a model validation procedure, analytical methods need to be available which provide information in the same subject area. Currently, a major issue with nanometrology is that a multitude of nanomaterials are present in natural systems but only some are ENMs; various other particles of natural origin are abundant in the same systems. The analytical tools available are not yet capable to distinguish the natural from engineered nanomaterials at the low ENM concentrations expected in complex environmental matrices. However, both modeling and analytical studies are able to provide an orthogonal view on nanomaterials: modeling is able to yield estimates of the presence of ENMs in various environmental compartments while analytics can provide physical characterization of ENMs in these systems with hints towards the total nanomaterial concentration. While we need to make strides to improve the two approaches separately, using the resulting data together in a mutually supportive way will advance the field of ENM risk assessment.
Nano impactModeling studies are used to obtain information on environmental exposure concentrations of engineered nanomaterials. All model systems, including those describing nanomaterial fate and transport, always call for a validation by analytical data. However, in this case, there are currently only very limited measurements available and, further complicating the issue, it is difficult to distinguish between natural and engineered nanomaterials in many circumstances. In this perspective article we raise the point that it is currently not possible to validate modeled data on engineered nanomaterial concentrations in the environment, but rather that modeling and analytics can be used in tandem to provide an orthogonal view on the presence of nanomaterials in the environment. |
Introduction
Engineered nanomaterials (ENMs) have become an integral component of the materials used in our society, with some materials used at volumes of tens of thousands of tons annually.1 Despite widespread use and exponentially increasing research on their environmental effects and behavior, very little analytical evidence exists about the presence (concentration, form, etc.) of these materials in the environment, for example in freshwaters and in technical systems such as wastewater treatment plants.2,3 In the last few years, several modeling studies have been published4–7 that provide the first predictions of their environmental concentrations on a large geographical scale and this exposure data has been used for environmental risk assessments.8 These modeled concentrations provide a preliminary idea of current ENM concentrations in the environment and are therefore a much-needed first step in providing an improved understanding of the actual risks that ENMs pose to the environment. The perception of some stakeholders that ENMs may pose a threat to the environment is fueled by many ecotoxicological studies that indeed find adverse effects to the ecosphere.9 ENM modeling studies, however, suggest that often ENM concentrations are many times lower than the effect levels found by the toxicity tests. A much more realistic picture of environmental ENM risk can be gained by using more precise data than figures which are based only on hazard values.To derive reliable findings from a model it has to be validated, which means to prove that it is an accurate representation of a real world system. Validity is determined with respect to the purposes for which the model was build, e.g. the ability for it to answer the questions on which the model was developed and within the precision capable considering the input data available at the time.10 For models predicting flows to the environment or environmental concentrations, analytical measurements (concentrations, characteristics) of ENM in these compartments are needed to ensure the models are based on realistic values. In the following sections, we will present the different ways that modeling and analytical technologies approach the determination of environmental exposure of ENM. It is from this perspective that we explore the challenges we are faced with when attempting to validate modeled environmental concentrations of ENMs and propose options for both modelers and analytical chemists on how to proceed, and collaborate, in the future.
The state of ENM exposure modeling
The first models to predict ENM concentrations in the environment were material flow analysis models (MFA) based on a life cycle perspective of products containing ENMs.11–13 Most of these models are top-down models, which start with the production of a certain mass of an ENM, distribute the mass to different product categories, and then identify the releases to the environment during production, use and disposal, and finally quantify the mass flows to technical and environmental compartments. Bottom-up models, starting with product usage and market penetration of nano-products, have also been developed.14,15 Behavior of ENMs during technical processes, e.g. in wastewater treatment plants or during waste incineration, can be described using transfer factors. It has been debated whether the concept of transfer factors is applicable to ENMs, particularly if they need to be extrapolated beyond the system based on which they were used.16–18 Uncertainty within the models originates from their limited coverage of realistic environmental fate processes, e.g. dissolution, agglomeration and sedimentation in various compartments. Current MFA-models do not distinguish between single ENMs, aggregated particles and ENMs attached to larger particles (including microbes) but rather track the total mass of a specific ENM through the system.Environmental fate models (EFMs) that include a mechanistic handling of agglomeration, hetero-agglomeration, sedimentation and other processes have been developed to allow a more process-based description.19–21 These models can be coupled to MFA models and may potentially enable a more accurate description of the actual form of the ENMs. However, they are strongly dependent on the input size distribution of the ENMs. To our knowledge, no data are currently available on the form and size distribution of ENMs entering the environment (either directly by release from products or indirectly through treatment systems).
The majority of published ENM exposure model scenarios only consider the engineered fraction of nanoparticles in the environment, but pigment TiO2 has also recently been modeled.6 Because pigment-TiO2 and nano-TiO2 occur simultaneously in samples from technical or environmental compartments, knowledge about the fraction of pigment-TiO2 is needed in order to relate the nano-TiO2 flows to those of the pigment form, which has a much higher production volume. However, so far no other model scenarios include other forms of nano-scale materials.
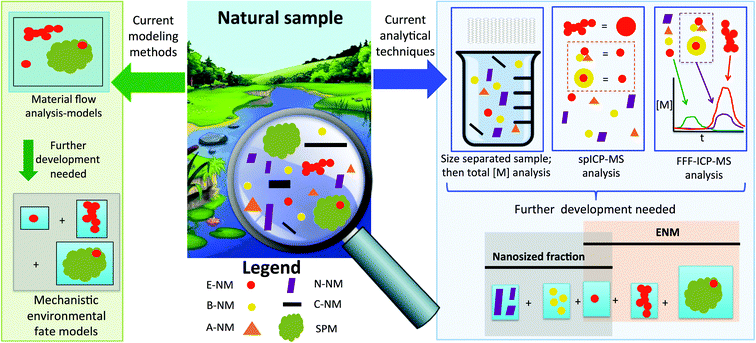
The state of ENM analytics in natural systems
The analysis and characterization of pristine ENMs in suspension is well established and a multitude of different methods can be used for a detailed description of materials.22 There has also been an improvement in our ability to measure ENM properties in complex media under laboratory conditions.23 However, for natural samples, the measurement difficulties are further increased from laboratory prepared complex samples because the ENMs only constitute a part of the nano-sized fraction in the matrix. Formed by natural geogenic, atmospheric and biological processes, environmental samples also contain other particles in the size range from 1–100 nm, such as e.g. clays and iron oxides.24–28 In addition, nanoparticles are also formed by combustion processes, both natural (e.g. forest fires) as well anthropogenic (e.g. fuel combustion).24,29 There are also other anthropogenic nanoparticles which are inadvertently produced or released: mechanical forces on a material matrix, such as sanding and polishing, lead to in high numbers of nano-sized particles even if the material does not contain any ENM.30,31 Another source of anthropogenic nano-sized particles are pigments, e.g. TiO2, that often have a particle size in the hundreds of nanometers yet also contain a certain fraction extending into the sub-100 nm range.32,33One of the main issues for environmental trace analysis is therefore that the ENMs are always present in a matrix together with many other particulate materials, including nano-scale materials (see Fig. 1), and are often composed of the same elements as the ENMs we are interested in detecting. Furthermore, the concentrations of ENMs are generally lower than the natural background34 which increases the difficulty of differentiating the particles of interest (ENMs) from their natural analogs.
Current sample pretreatment and measurement techniques attempt to focus on a certain size fraction (<1 μm, <0.45 μm, <0.2 μm, etc., depending on the separation method employed) but are not specific to ENMs. The situation is further complicated as sizing techniques are not able to specifically analyze ENMs adsorbed to larger particles because associated with larger particles will have been removed from the “nano” size fraction. Simply using a measurement of the nano-sized fraction as a proof of the presence or absence of ENMs will therefore give misleading results.
Even if the ENMs can be distinguished from natural NMs, sensitive techniques are required to analyze the low concentrations expected in environmental samples, often in the order of ng L−1 in aqueous samples or ng kg−1 in terrestrial samples.6 The only currently known techniques that are both selective and sensitive enough to analyze inorganic ENM in complex environmental samples are ICP-MS based, demonstrated thus far in natural freshwater35,36 and wastewater treatment effluent.37,38 Single particle ICP-MS (spICP-MS) is capable of providing number-based size distributions of ENM in these samples,37 but the capability to size the particles varies upon the element of interest.39 While the size of some nanomaterials (Au, Ag) can be measured down to a size of 6 nm or smaller,40 other ENMs, such as TiO2, can only be measured as small as ca. 50 nm.41 Moreover, as shown in Fig. 1 in the red dashed box, the standard spICP-MS method only measures the total mass of one element. So, for example, an Ag-NP cannot be distinguished from an Ag2S particle as the measured silver mass per particle is recalculated into a size. This pitfall also complicates the identification of separate components e.g. TiO2 ENMs in an environmental sample from other naturally occurring particles containing Ti.35
Fig. 1 shows how field flow fractionation (FFF) coupled to ICP-MS can distinguish particulate material which has a different hydrodynamic diameter (e.g. Ag and heteroagglomerated Ag-NP) and can assign the total mass of analyte associated with each diameter distribution, but we would not know the number of particles per (hetero)agglomerate. Coupling state-of-the art spICPMS techniques with FFF could provide the broadest suite of information where knowledge of the number of particles per aggregate allows distinguishing e.g. heteroagglomerated TiO2 ENM from naturally occurring Ti containing particles.2 However, the most recent developments in single particle time-of-flight ICP-MS (spTOF-ICP-MS)42 or data collection in micro-second dwell times43 promise simultaneous multi-element capability in real time and open up the possibility of possibly distinguishing ENMs from naturally occurring particles containing the same element at environmentally realistic concentrations. The latter technique could potentially distinguish homo- or heteroagglomerated particles from single particles, but large particles could possibly not be distinguished from large aggregates. Finally, equally sensitive methods are currently lacking for organic ENMs, such as carbon nanotubes, whereas for fullerenes chromatography methods with low ng L−1 detection limits have been suggested.44
To date, microscopy techniques have been widely used to measure NM size distribution, crystal structure and to obtain elemental composition of NM under investigation when coupled with X-ray energy dispersive spectroscopy (X-EDS) and electron energy loss spectroscopy (EELS). Microscopy techniques have not been widely used to acquire particle number concentration in the original samples material due to the inherent limitations of sample recovery with the sample preparation approaches. Recently, with the development of full recovery sample preparation techniques, it has been demonstrated that atomic force microscopy (AFM) and transmission electron microscope (TEM) can both be applied successfully to measure particle number concentration from a suspension at environmentally realistic NM concentrations (ca. 0.2–100 μg L−1 AuNPs).45 Although this new microscopy sample preparation approach enables fully quantitative recovery of NMs on the TEM grid and thus enables number concentration measurements, the ENM concentrations that current models predict are so low that obtaining a statistically significant count of nanoparticles in natural samples still proves challenging.
In combination with nano-specific techniques, traditional bulk analytical methods can provide useful information for identifying the presence of ENMs in complex samples. A prerequisite is that the presence of ENMs in the sample can be identified by changes in the composition of the sampled material compared to the natural background. An example, which is unfortunately not applicable to industrial materials, is the stable isotope labeling of ENMs and analysis with ICP-MS46 that provides extremely low detection limits for e.g. ZnO NPs below the natural background. Possible identifiers for unlabeled ENMs are the total elemental concentrations (if natural background of the ENM constituting element or a traceable impurity in the ENMs are very low compared to the amount of ENM introduced), elemental ratios and isotopic ratios. The elemental composition of natural colloids and nanomaterials are typically more diverse than their pristine manufactured counterparts, which often contain very few impurities, making signal specific identification of ENMs a possibility.2 Elemental ratios of Ti to Al and Nb have been used to determine the release of TiO2 ENMs from sunscreen products into surface waters during swimming.35 Isotopic ratios of certain elements exhibit geographic differences and may reveal the presence of industrial material.47 Similarly, when the mix of crystal structures of a mineral is different in ENMs than what is expected of the minerals in a certain geographic region, this difference could be used as a key for identifying the presence of ENMs.
In summary, bulk analysis contains information for both natural and engineered materials of all sizes and shapes and therefore, several criteria must be met in order for it to be used in detecting ENMs. First, the ENM must possess a property that enables a distinction from the natural background (e.g. an impurity not found in the natural particulate matter or an elemental or isotopic ratio that changes the respective ratio found in the background). The natural variation of this property in the background and the rate of change of introduced mass of ENM into the background determine the sensitivity of the method for the ENM. Secondly, a baseline for natural levels of a given element should be established, or, if the release has a temporal variation, time-resolved sampling and analysis might be required. Lastly, a great deal of information must be acquired on both local geochemical conditions and consumer products available in the local market in order to exclude influence from other, non-ENM sources. An example of the combination of bulk and nano-specific techniques coupled with the proper data interpretation has been shown to be a powerful tool for determining ENMs in natural samples, as seen in the case study for TiO2 from sunscreens.35
Transformation reactions
Another uncertainty further confounding the validation of modeled values by analytical measurements are transformation reactions that ENM can undergo.48,49 The current knowledge on mechanistic fate/transformation reactions of ENMs in the environment does not allow modelers to predict the likelihood of how (many) particles of a given ENM have been transformed. The newest model by Sun et al.,6 for example, considers sulfidation of nano-Ag in wastewater treatment plants as a sink for nano-Ag. However, recent toxicology data suggests it may be necessary to specifically model the further fate of the silver sulfides formed.50Many ENMs (e.g. Ag, iron-oxide and ZnO) are highly dynamic and may undergo redox reactions, dissolve and interact with other ions and re-precipitate as new nanomaterials. Solutions which initially contain no nanomaterials, but rather contain either ionic or bulk forms of a given metal, may eventually produce particulate matter similar to ENMs over time.51,52 This has taught us both that, i) ENMs cannot be assessed in their pristine composition in the environment, and ii) that many conventional compounds can also exist at the nanoscale.
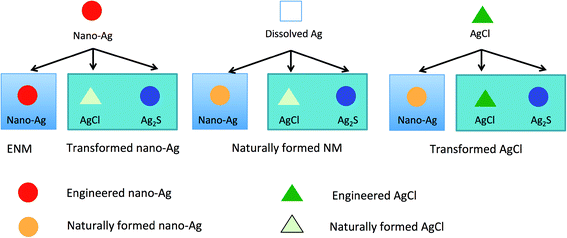
While analytical methods can in some cases provide very sensitive particle number concentration determinations in difficult media and even go so far as to provide clues as to in which form the ENM may occur (e.g. metallic Ag vs. Ag2S), it is unclear in which form they were initially released into the environment. Ag2S particles, for example, could also be formed from dissolved Ag+ and other Ag-forms and not only from nano-Ag.51,53,54 An example of this is shown in Fig. 2, where there are three silver forms presented-dissolved Ag+, nano-Ag(0) and AgCl. Nano-Ag can be transformed into different silver forms (AgCl, Ag2S). Likewise, dissolved silver can also be transformed into nano-Ag(0), AgCl and Ag2S. The same applies to AgCl. The presence of nano-sized Ag particles can therefore not be used to make any conclusions about the presence of engineered nano-Ag in the sample, since multiple starting materials produce the same material in the end.52 Also, a method specific for metallic nano-Ag(0) alone cannot be used to draw a conclusion regarding the presence of a ENMs in the sample. To be able to do this we would need to go beyond just identifying metallic nano-Ag but also to use some additional methods, e.g. by analyzing impurities to clearly identify a nano-Ag particle as engineered.
Validation of models by data
Mathematical models of natural systems always call for experimental validation by analytical data, either to prove that the model is accurately capturing the main aspects of the system or to show that significant deficiencies in the model still exist. Given the many assumptions that current models are required to make, performing a validation is clearly appropriate and recommended. However, we need to state that even in the absence of an operational model validation by analytical data, the models can still be validated conceptually, meaning that the underlying theories and assumptions of the conceptual model and the mathematics are correct.10 Such models have their validity by providing either prospective estimations (in case they refer to a future scenario) or being predictive in the sense that they allow to make statements about current exposure even in the absence of analytical data. Most of the models contain at least in some of the parameters worst-case assumptions and the predicted environmental concentrations therefore represent worst-case scenarios.The methodological approaches used in most of the models follow the procedures laid down in the chemicals risk assessment55 and thus the predicted concentrations derived by them are based on accepted methods. In a “process validation”, the underlying physical and chemical processes are validated, thus giving credibility to the model results. If a model assumption is that ENM are bound to sediments56 or other particles,19 a validation of sediment or particle behavior can serve as a validation for the prediction of the fate of ENM.
Aside from risk assessment, models may provide valuable information as to where nanomaterials are likely to first be spotted in the environment, thus suggesting possible/probable places for environmental chemists and nanometrologists to begin “nano-prospecting” to test their analytical methods in more complex matrices with the greatest change of finding nanomaterials. Spatially resolved models have been developed that can predict hotspots for ENM, either on a continental scale,57 in a river network56,58 or in a single river19 or in specific lakes.59 Such models will be very useful because the analytical measurements will be point measurements and thus for any validation predictions at specific locations will be needed.
When aiming to validate the concentrations predicted by ENM exposure and fate models, we need to take into account the factors discussed above: the presence of large concentrations of natural NMs in samples and methodological shortcomings in the current analytical methods to quantify all ENM forms, especially those bound to larger particles. In addition, mechanistic models predict number concentrations of a particular size distribution of ENM, whereas mass flow models predict mass concentrations. Some of the analytical methods discussed above can provide number concentrations, but in most cases, a validation of the ENM flow models would consist of, for example, measuring the ENM mass flows in wastewater, which would validate the model assumptions on production, use and release.
We have to raise the question about the need to model explicitly all different ENM species compared to just modeling an average ENM, so the justification for scientific models with a rigorous description of all processes compared to much simpler models relevant for risk assessment. The models used for risk assessment should only contain a level of detail needed in the risk assessment process and have to be compatible to the hazard data generated in ecotoxicological studies.
A comparison of modeled and measured values has been made by Gottschalk et al.11 There the authors stated that modeled and measured results are not always comparable due to the different forms and sizes of particles that analytics and modeling target. We can highlight the disparity with two examples:
Kiser et al.60 published data on nano-TiO2 concentrations in treated wastewater that are overlapping with the range predicted by modeling.7 So does this count as validation? On first sight yes, but not when delving into the matter: the size limit used in filtration was 700 nm, so the “nano-fraction” also includes larger sized particles. On the other hand, the filtration possibly removed nano-TiO2 adsorbed to larger flocs. The “real” nano-TiO2 concentration in the effluent can therefore either be lower, because pigment TiO2 has been erroneously included in the analysis or higher, because nano-TiO2 adsorbed to larger particles has been excluded from the analysis. Without more information on the actual TiO2 presence in wastewater a validation will not be possible. This means e.g. to have information on the distinction between pigment and nano-TiO2 or the analysis of nano-TiO2 associated with large flocs and/or improvement in the models to predict concentrations of nan-TiO2 and pigment TiO2 in different size classes.
The second example is the study by Mitrano et al. on nano-Ag in wastewater38 who reported about 100 ng l−1 of nano-Ag in treated wastewater effluent measured by spICP-MS. This concentration is again well within the range predicted by Gottschalk et al.7 and the technique used provides the size of the detected particles. So does this count as validation? Again yes on first sight but when we consider the published data on Ag behavior during wastewater treatment then the issue becomes complicated: it is likely that the measured Ag particles are in fact nano Ag2S, so transformed silver particles. The model by Gottschalk et al.7 at that time did not contain any transformation information while the newer update by Sun et al.6 does. In that model, Ag2S is no longer considered because the model is specific to metallic nano-Ag and sulfidation constitutes an elimination of metallic nano-Ag. In addition it has been shown that also dissolved Ag and AgCl are transformed during wastewater treatment into the same silver sulfide particles as nano-Ag.51,54 The presence of Ag-containing nanoparticles is therefore not a sufficient proof for the presence of nano-Ag but rather the consequence of the silver chemistry in wastewater.
A validation of more mechanistic environmental fate models may be possible to some extent in micro- or mesocosms because the initial system conditions can be controlled, the fate pathways are clearly defined, the system is a closed circuit and the experiment can be designed in order to tailor the analytical measurement needs to the ENMs added. Validation of the models will require that the methods are used on well-characterized starting samples of ENMs, e.g. with respect to isotopic, elemental and size signatures of materials in products and as they transform. In a natural sample, the system cannot be characterized with the same accuracy, especially with respect to the ENM entering the system. The assumption that the ENMs present in the sample have the same properties as pristine ENM will lead to misleading results as released and transformed ENM have completely different properties than pristine ENMs.61,62 With the expected improvement in the field of nanometrology in complex and environmental samples, analytics could also help provide additional data for the modeling community. Namely, as more precise characterization of nanomaterials is possible in the environment, we will be able to better define how materials age and transform over time in different environmental compartments. This chemical information will inform the further flow of materials between environmental sectors so that models can be adjusted regarding likely residence times and final fate.
We also need to question whether the challenges associated with the validation of modeled concentrations are specific to ENMs. Successful environmental fate model validations have been performed for organic pollutants and it has been shown, e.g. for DDT63 or PFOS,64 that model uncertainty is often related to uncertainties in emission rates, degradation rate constants and differences between wastewater treatment plants. For many metals, similar problems have historically been faced as those for ENM with which we are now presented: metals are present in many different species in the environment, each with different environmental behavior, e.g. dissolved metals, complexed metals, colloidal forms and metals associated with suspended solids. Fate models that include different reactivities of different metal species have been developed and successfully applied to simulate metals in the environment.65
The way forward
The view expressed in this perspective piece is that at a conceptual level the validation requirements of modeled concentrations are difficult to reconcile with the results that analytics will be able to provide – at least in the near future. There may well be many cases where we may never be able to trace a particular NM found in the environment back to whether it was emitted as an ENM or whether it is of natural origin. However, both modeling and analytics are able to provide an orthogonal view on nanomaterials: modeling can provide estimates of the presence of ENMs and analytics can provide estimates of the total NM concentration and their characterization. We need to look at the two methods separately and use their results in a mutually supportive way to move forward with the risk assessment of ENMs. We need to be cautious when trying to use one of the two approaches to validate the other as this is certainly going to be difficult if not impossible in the near future. On the other hand, under well controlled lab based or micro/mesocosm set-ups, as already reported in a few studies,66 experimental and fate-modeling approaches can and should be used in the near term to validate the basic assumptions of fate models.The way forward is therefore for the MFA models to be mainly improved on the side of input data to reduce the uncertainties that result from missing or conflicting knowledge. For the environmental fate models, the inclusion of particle-specific processes needs to be further developed. In the current state of the science, the results from the models and the available analytical data both provide the information necessary to obtain an up-to-date view on exposure of NMs and ENMs in the environment.
Acknowledgements
This work was supported by the European Commission within the Seventh Framework Programme (MARINA-grant agreement no. 263215). DMM is supported by NanoMILE (grant agreement no. NMP4-LA-2013-310451).References
- ANSES, Éléments issus des déclarations des substances à l'état nanoparticulaire. RAPPORT d'étude. ANSES (l'Agence nationale de sécurité sanitaire), 2013.
- F. von der Kammer, P. L. Ferguson, P. A. Holden, A. Masion, K. R. Rogers, S. J. Klaine, A. A. Koelmans, N. Horne and J. M. Unrine, Environ. Toxicol. Chem., 2012, 31, 32–49 CrossRef CAS PubMed.
- Y. Yang, Y. F. Wang, P. Westerhoff, K. Hristovski, V. L. Jin, M. V. V. Johnson and J. G. Arnold, Sci. Total Environ., 2014, 485, 441–449 CrossRef PubMed.
- N. C. Mueller and B. Nowack, Environ. Sci. Technol., 2008, 42, 4447–4453 CrossRef CAS.
- A. Keller, S. McFerran, A. Lazareva and S. Suh, J. Nanopart. Res., 2013, 15, 1–17 CrossRef.
- T. Y. Sun, F. Gottschalk, K. Hungerbühler and B. Nowack, Environ. Pollut., 2014, 185, 69–76 CrossRef CAS PubMed.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- F. Gottschalk, E. Kost and B. Nowack, Environ. Toxicol. Chem., 2013, 32, 1278–1287 CrossRef CAS PubMed.
- G. Hunt, I. Lynch, F. Cassee, R. D. Handy, T. F. Fernandes, M. Berges, T. A. J. Kuhlbusch, M. Dusinska and M. Riediker, Materials, 2013, 6, 1090–1117 CrossRef CAS PubMed.
- R. G. Sargent, Winter Simulation Conference, in Proceedings of the Winter Simulation Conference, Phoenix, Arizona, 2011, pp. 183–198 Search PubMed.
- F. Gottschalk, T. Y. Sun and B. Nowack, Environ. Pollut., 2013, 181, 287–300 CrossRef CAS PubMed.
- C. O. Hendren, M. Lowry, K. D. Grieger, E. S. Money, J. M. Johnston, M. R. Wiesner and S. M. Beaulieu, Environ. Sci. Technol., 2013, 47, 1190–1205 CAS.
- A. L. Dale, E. A. Casman, G. V. Lowry, J. R. Lead, E. Viparelli and M. Baalousha, Environ. Sci. Technol., 2015, 49, 2587–2593 CrossRef CAS PubMed.
- A. B. A. Boxall, Q. Chaudhry, C. Sinclair, A. D. Jones, R. Aitken, B. Jefferson and C. Watts, Current and future predicted environmental exposure to engineered nanoparticles, Central Science Laboratory, Sand Hutton, UK, 2007 Search PubMed.
- K. Tiede, P. Westerhoff, S. F. Hansen, G. J. Fern, S. M. Hankin, R. J. Aitken, Q. Chaudhry and A. Boxall, Review of the risks posed to drinking water by man-made nanoparticles. Food and Environment Research Agency, Sand Hutton, York, UK, 2010 Search PubMed.
- G. Cornelis, Environ. Sci.: Nano, 2015, 2, 19–26 RSC.
- A. L. Dale, G. V. Lowry and E. A. Casman, Environ. Sci.: Nano, 2015, 2, 27–32 RSC.
- A. Praetorius, N. Tufenkji, K.-U. Goss, M. Scheringer, F. von der Kammer and M. Elimelech, Environ. Sci.: Nano, 2014, 1, 317–323 RSC.
- A. Praetorius, M. Scheringer and K. Hungerbuhler, Environ. Sci. Technol., 2012, 46, 6705–6713 CrossRef CAS PubMed.
- J. A. J. Meesters, A. A. Koelmans, J. T. K. Quik, A. J. Hendriks and D. van de Meentt, Environ. Sci. Technol., 2014, 48, 5726–5736 CrossRef CAS PubMed.
- H. H. Liu and Y. Cohen, Environ. Sci. Technol., 2014, 48, 3281–3292 CrossRef CAS PubMed.
- M. Hassellov and R. Kaegi, in Environmental and Human Health Impacts of Nanotechnology, ed. Jamie R. Lead and Emma Smith, Blackwell Publishing Ltd. ISBN: 978-1-405-17634-7, 2009 Search PubMed.
- R. F. Domingos, M. A. Baalousha, Y. Ju-Nam, M. M. Reid, N. Tufenkji, J. R. Lead, G. G. Leppard and K. J. Wilkinson, Environ. Sci. Technol., 2009, 43, 7277–7284 CrossRef CAS.
- P. R. Buseck and K. Adachi, Elements, 2008, 4, 389–394 CrossRef CAS.
- B. K. G. Theng and G. D. Yuan, Elements, 2008, 4, 395–399 CrossRef CAS.
- M. F. Hochella and A. S. Madden, Elements, 2005, 1, 199–203 CrossRef CAS.
- J. Buffle, Environ. Chem., 2006, 3, 155–158 CrossRef CAS.
- J. R. Lead and K. J. Wilkinson, Environ. Chem., 2006, 3, 159–171 CrossRef CAS.
- C. Anastasio and S. T. Martin, in Nanoparticles and the Environment, ed. J. F. Banfield and A. Navrotsky, 2001, vol. 44, pp. 293–349 Search PubMed.
- D. Gohler, M. Stintz, L. Hillemann and M. Vorbau, Ann. Occup. Hyg., 2010, 54, 615–624 CrossRef PubMed.
- M. Vorbau, L. Hillemann and M. Stintz, J. Aerosol Sci., 2009, 40, 209–217 CrossRef CAS PubMed.
- Y. Yang, K. Doudrick, X. Y. Bi, K. Hristovski, P. Herckes, P. Westerhoff and R. Kaegi, Environ. Sci. Technol., 2014, 48, 6391–6400 CrossRef CAS PubMed.
- A. Weir, P. Westerhoff, L. Fabricius, K. Hristovski and N. von Götz, Environ. Sci. Technol., 2012, 46, 2242–2250 CrossRef CAS PubMed.
- M. F. Hochella, D. Aruguete, B. Kim and A. S. Madden, in Nature's Nanostructures, ed. A. S. Barnard and H. Guo, Pan Stanford Publishing, Singapore, 2012 Search PubMed.
- A. P. Gondikas, F. V. D. Kammer, R. B. Reed, S. Wagner, J. F. Ranville and T. Hofmann, Environ. Sci. Technol., 2014, 48, 5415–5422 CrossRef CAS PubMed.
- D. M. Mitrano, J. F. Ranville, A. Bednar, K. Kazor, A. S. Hering and C. P. Higgins, Environ. Sci.: Nano, 2014, 1, 248–259 RSC.
- J. Tuoriniemi, G. Cornelis and M. Hassellov, Anal. Chem., 2012, 84, 3965–3972 CrossRef CAS PubMed.
- D. M. Mitrano, E. K. Lesher, A. Bednar, J. Monserud, C. P. Higgins and J. F. Ranville, Environ. Toxicol. Chem., 2012, 31, 115–121 CrossRef CAS PubMed.
- S. Lee, X. Bi, R. B. Reed, J. F. Ranville, P. Herckes and P. Westerhoff, Environ. Sci. Technol., 2014, 48, 10291–10300 CrossRef CAS PubMed.
- G. Cornelis and M. Hassellov, J. Anal. At. Spectrom., 2014, 29, 134–144 RSC.
- R. J. B. Peters, G. van Bemmel, Z. Herrera-Rivera, H. P. F. G. Helsper, H. J. P. Marvin, S. Weigel, P. C. Tromp, A. G. Oomen, A. G. Rietveld and H. Bouwmeester, J. Agric. Food Chem., 2014, 62, 6285–6293 CrossRef CAS PubMed.
- O. Borovinskaya, B. Hattendorf, M. Tanner, S. Gschwind and D. Gunther, J. Anal. At. Spectrom., 2013, 28, 226–233 RSC.
- M. D. Montano, H. R. Badiei, S. Bazargan and J. F. Ranville, Environ. Sci.: Nano, 2014, 1, 338–346 RSC.
- M. Farré, S. Pérez, K. Gajda-Schrantz, V. Osorio, L. Kantiani, A. Ginebreda and D. Barceló, Open J. Mod. Hydrol., 2010, 383, 44–51 CrossRef PubMed.
- M. Baalousha, A. Prasad and J. R. Lead, Environ. Sci.: Processes Impacts, 2014, 16, 1338–1347 CAS.
- F. R. Khan, A. Laycock, A. Dybowska, F. Larner, B. D. Smith, P. S. Rainbow, S. N. Luoma, M. Rehkämper and E. Valsami-Jones, Environ. Sci. Technol., 2013, 47, 8532–8539 CAS.
- G. Bartov, A. Deonarine, T. M. Johnson, L. Ruhl, A. Vengosh and H. Hsu-Kim, Environ. Sci. Technol., 2012, 47, 2092–2099 CrossRef PubMed.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS PubMed.
- D. M. Mitrano, S. Motellier, S. Clavaguera and B. Nowack, Environ. Int., 2015, 77, 132–147 CrossRef CAS PubMed.
- L. Li, L. Hu, Q. Zhou, C. Huang, Y. Wang, C. Sun and G. Jiang, Environ. Sci. Technol., 2015, 49, 2486–2495 CrossRef CAS PubMed.
- R. Ma, C. Levard, J. D. Judy, J. M. Unrine, M. Durenkamp, B. Martin, B. Jefferson and G. V. Lowry, Environ. Sci. Technol., 2014, 48, 104–112 CrossRef CAS PubMed.
- D. M. Mitrano, E. Rimmele, A. Wichser, R. Erni, M. Height and B. Nowack, ACS Nano, 2014, 8, 7208–7219 CrossRef CAS PubMed.
- R. Kaegi, A. Voegelin, B. Sinnet, S. Zuleeg, H. Hagendorfer, M. Burkhardt and H. Siegrist, Environ. Sci. Technol., 2011, 45, 3902–3908 CrossRef CAS PubMed.
- E. Lombi, E. Donner, S. Taheri, E. Tavakkoli, A. K. Jamting, S. McClure, R. Naidu, B. W. Miller, K. G. Scheckel and K. Vasilev, Environ. Pollut., 2013, 176, 193–197 CrossRef CAS PubMed.
- ECHA, 2008.
- A. L. Dale, G. V. Lowry and E. A. Casman, Environ. Sci. Technol., 2015, 49, 7285–7293 CrossRef CAS PubMed.
- E. Dumont, A. C. Johnson, V. D. J. Keller and R. J. Williams, Environ. Pollut., 2015, 196, 341–349 CrossRef CAS PubMed.
- F. Gottschalk, C. Ort, R. W. Scholz and B. Nowack, Environ. Pollut., 2011, 159, 3439–3445 CrossRef CAS PubMed.
- A. A. Koelmans, J. T. K. Quik and I. Velzeboer, Environ. Pollut., 2015, 196, 171–175 CrossRef CAS PubMed.
- M. A. Kiser, P. Westerhoff, T. Benn, Y. Wang, J. Perez-Rivera and K. Hristovski, Environ. Sci. Technol., 2009, 43, 6757–6763 CrossRef CAS.
- S. Froggett, S. Clancy, D. Boverhof and R. Canady, Part. Fibre Toxicol., 2014, 11, 17 CrossRef PubMed.
- A. Al-Kattan, A. Wichser, S. Zuin, Y. Arroyo, L. Golanski, A. Ulrich and B. Nowack, Environ. Sci. Technol., 2014, 48, 6710–6718 CrossRef CAS PubMed.
- U. Schenker, M. Scheringer, M. D. Sohn, R. L. Maddalena, T. E. McKone and K. Hungerbuhler, Environ. Sci. Technol., 2009, 43, 128–134 CrossRef CAS.
- M. R. Earnshaw, A. G. Paul, R. Loos, S. Tavazzi, B. Paracchini, M. Scheringer, K. Hungerbuhler, K. C. Jones and A. J. Sweetman, Environ. Int., 2014, 70, 25–31 CrossRef CAS PubMed.
- S. P. Bhavsar, M. L. Diamond, L. J. Evans, N. Gandhi, J. Nilsen and P. Antunes, Environ. Toxicol. Chem., 2004, 23, 1376–1385 CrossRef CAS.
- A. L. Dale, G. V. Lowry and E. A. Casman, Environ. Sci. Technol., 2013, 47, 12920–12928 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |