Occurrence, seasonal variation and removal efficiency of antibiotics and their metabolites in wastewater treatment plants, Jiulongjiang River Basin, South China
Han
Zhang
a,
Miaomiao
Du
a,
Hongyou
Jiang
b,
Dandan
Zhang
a,
Lifeng
Lin
a,
Hong
Ye
a and
Xian
Zhang
*a
aKey Lab of Urban Environment and Health, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, P. R. China. E-mail: xzhang@iue.ac.cn; Fax: +86 592 6190518; Tel: +86 592 6190519
bInstitute of Agro-Products Processing and Nuclear-Agricultural Technology, Hubei Academy of Agricultural Sciences, Wuhan, Hubei 430064, China
First published on 26th November 2014
Abstract
Wastewater treatment plants (WWTPs) are regarded as one of the most important sources of antibiotics in the environment. The occurrence, seasonal variation and removal efficiency of 21 antibiotics and 10 metabolites, including five sulfonamides and three of their metabolites, six quinolones, two macrolides, two β-Lactams and five tetracyclines and seven of their metabolites, were investigated in five WWTPs in different seasons in the Jiulongjiang River Region, South China. 16 antibiotics and 6 metabolites in summer and 14 antibiotics and 6 metabolites in winter were found, respectively. The most frequently detected antibiotics were sulfamethazine, sulfamethoxazole, n-acetyl sulfamethazine, n-acetyl sulfamethoxazole, ofloxacin, cephalexin monohydrate and cephradine; of these, the concentration of cephradine was the highest in most of the influent and effluent samples. The highest level of total antibiotics was found in Longyan City WWTPs, where there are more population and swine farms. Seasonal variation of the antibiotics in wastewater samples was also studied. The concentrations of antibiotics in winter were higher than those in summer. The antibiotics could not be removed completely by the WWTPs, and the mean removal efficiency ranged from −71.6 to 56.3%. Of all the antibiotics, the tetracyclines were removed comparatively more efficiently, probably due to their adsorption to sludge. The low removal efficiency of antibiotics in WWTPs could be one of the important reasons for the presence of antibiotics in the environment in Jiulongjiang Region.
Environmental impactAntibiotic residues in the environment have become an important environmental issue because they pose a potential threat to human health. Wastewater treatment plants are regarded as one of the most important sources of antibiotics in the environment. It is very important to study fate of antibiotics and their metabolites in wastewater treatment plants, which is useful to assess potential environmental risk. This paper investigates occurrence, seasonal variation and removal efficiency of 21 antibiotics and 10 metabolites in five wastewater treatment plants in different seasons in the Jiulongjiang River Region, South China. |
1. Introduction
Antibiotics have important uses in both human and veterinary medicine for their antibacterial properties and as growth promoters. As their consumption has increased, they are being detected in all sectors of the environment. Antibiotics in environmental residuals have attracted much public attention in the last decade.1 Previous studies have clearly shown that the municipal wastewater treatment plants (WWTPs) are an important pollution source of antibiotics released into the environment.2 Antibiotics are suspected to present an environmental risk, e.g., fostering bacterial resistance.3,4 For this reason, antibiotics in WWTPs have been subject to increased investigation in the last decade.5–7 The conventional wastewater treatment plants appear to be unable to completely eradicate these antibiotics. For example, the removal efficiency of tetracycline varied from 12% (ref. 8) to 80% (ref. 9) in wastewater treatment plants. The incomplete removal of some of these antibiotics during conventional wastewater treatment can be directly linked to their presence not only in surface waters and sediments but also in soils.Most antibiotics are excreted unaltered or as metabolites in feces and urine.10,11 These antibiotics having been poorly absorbed or metabolized, as much as 30–90% can be excreted. However, the current studies indicate that metabolites of antibiotics can be persistent, and accumulate in food12 and drinking supplies,13 including ground waters14 which were originally expected to be impervious to considerable contamination. For example, approximately 40% of a sulfamethoxazole (SMZ) is transformed into N4-acetyl-sulfamethoxazole (ASMZ) in urine.15 But there is evidence that ASMZ is transformed back to the parent compound during wastewater treatment.16 Oxytetracycline, tetracycline, and chlortetracycline may be metabolized into their 4-epimers of 4-epioxytetracycline (EOTC), 4-epitetracycline (ETC) and 4-epichlortetracycline (ECTC). Although the antimicrobial effects of the 4-epimers are lower than that of tetracyclines or even disappeared, their toxicities are heavier than that of tetracyclines (TCs).17 With increasing and poorly regulated use of antibiotics in the world, the accumulation of antibiotics and their metabolites in animals and humans can thus represent an environmental and toxicological threat, with wastewaters as central transfer media.
The Jiulongjiang River is the second largest water system in Fujian Province, southern China. It is the most important source of drinking water and industrial and agricultural activities. With the rapid growth of the urban population in the Jiulongjiang River basin, there are numerous inputs of medical antibiotics from municipal wastewater treatment systems into the river. In our previous studies,18 we found that levels of antibiotics in water of the Jiulongjiang River were very high. Besides, it was found that antibiotics in the upstream of Jiulongjiang mainly came from swine wastewater, but those in the downstream of Jiulongjiang from WWTPs.19 However, little information on antibiotics in wastewater treatment plant of the Jiulongjiang River is available. In this study, we examined the occurrence and fate of selected antibiotics and their metabolites in influents and effluents from five WWTPs in the Jiulongjiang River Basin. Specifically, we collected and analyzed the samples from both summer and winter seasons. The results were used to evaluate distribution characteristics and seasonal variation of the selected antibiotics and their metabolites from WWTPs.
2. Materials and methods
2.1. Chemicals and materials
The antibiotics and their metabolites in this study including five sulfonamides and three of their metabolites, five tetracyclines and seven of their metabolites, six quinolones, two macrolides, and two β-Lactams were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). More details are shown in Table 1. Stock solutions (100.00 mg L−1) were prepared by dissolving 0.500 mg standard antibiotics in 5 mL 10% methanol solution, individually. They were kept in brown glass vials in a refrigerator (4 °C). Oasis HLB (500 mg, 6 mL) extraction cartridges were purchased from Waters Corporation (Milford, MA, USA). HPLC grade methanol and acetone were purchased from TEDIA Company (Fairfield, OH, USA). Milli-Q water (Millipore, USA) was used throughout the study. Unless otherwise indicated, chemicals used in the analysis were of analytical grade.| Class | Compound | Abbreviation |
|---|---|---|
| Sulfonamides (SAs) | Sulfadiazine | SD |
| Sulfadimethoxine | SDM | |
| Sulfamerazine | SM1 | |
| Sulfameter | SMT | |
| Sulfamethazine | SM2 | |
| Sulfamethoxazole | SMZ | |
| Metabolites of sulfonamide (MSAs) | n-Acetyl sulfamethazine | ASM |
| n-Acetyl sulfadiazine | ASD | |
| n-Acetyl sulfamethoxazole | ASMZ | |
| Quninolones (QNs) | Ciprofloxacin hydrochloride | CIP |
| Norfloxacin | NOF | |
| Sarafloxacin hydrochloride | SARA | |
| Ofloxacin | OF | |
| Danofloxacin mesylate | DALA | |
| Enrofloxacin | ENX | |
| Macrolide (MLs) | Roxithromycin | ROX |
| Erythromycin | ERY | |
| β-Lactams (β-Ls) | Cephalexin monohydrate | CEFM |
| Cephradine | CEFD | |
| Tetracyclines (TCs) | Tetracycline | TC |
| Oxytetracycline | OTC | |
| Chlorotetracycline | CTC | |
| Methacycline | MTC | |
| Doxycycline | DXC | |
| Metabolites of tetracyclines (MTCs) | Anhydrotetracycline | ATC |
| 4-Epitetracycline | ETC | |
| 4-Epi-anhydrotetracycline | EATC | |
| α-Apo-oxytetracycline | α-OTC | |
| 4-Epioxytetracycline | EOTC | |
| Isochlortetracycline | ICTC | |
| Demeclocycline | DMCTC |
2.2. Sampling sites and sample collection
As is shown in Fig. 1, five wastewater treatment plants (S1–S5) in the Jiulongjiang River Basin were chosen. S1 is situated in Longyan City. S2 is located in Zhangping City. S3 is sited at Hua'an County. S4 is situated in Changtai County and S5 is located in Zhangzhou City. The WWTPs employ similar treatment processes: the primary (mechanical) process using screens, settling tanks and skimmers to remove particles, coupled with secondary biological treatment. Detailed information of five WWTPs is shown in Table 2.| Sequence | City | WWTPs | Treatment techniques | Sewage source | Treatment capacitya (104 m3 d−1) | Population (104) |
|---|---|---|---|---|---|---|
| a A2/O represents anaerobic-anoxic-oxic. A/B represents adsorption bio-degradation. OD represents oxidation ditch. dom represents domestic. | ||||||
| 1 | Longyan | S1 | A2/O | dom | 13.1 | 30 |
| 2 | Zhangping | S2 | A2/O | dom | 1.5 | 5 |
| 3 | Hua'an | S3 | A2/O | dom | 0.8 | 3 |
| 4 | Changtai | S4 | OD | dom | 1.3 | 5 |
| 5 | Zhangzhou | S5 | A/B | dom | 6.7 | 18 |
Wastewater samples were collected as “grab samples” from five WWTPs. For the grab sampling program, the raw wastewater influents and the final effluents from the WWTPs were sampled in sequence according to the hydraulic retention of the sewage water treatment. Three replicate samples were collected for laboratory analysis. During the sample collection, the sampling bottles were rinsed with the sample three times before a final sample was collected. 4 L of each sample was collected. All samples were kept in the dark at −18 °C until analysis. All samples were collected in July 2012 (summer) and January 2013 (winter).
2.3. Extraction and analysis
The water samples were filtered through 0.45 μm glass fiber filters within 24 h after the sample collection. Antibiotic compounds were extracted according to the method of Zhang et al.18 Briefly, 500 mL of water sample was acidified to pH = 3.0 by adding HCl, followed by addition of 0.2 g Na2EDTA. The samples were extracted using Oasis HLB (500 mg, 6 mL) extraction cartridges. The HLB columns were conditioned with 6.0 mL of acetone, 6.0 mL of methanol and 6.0 mL of 0.1% formic acid and 5 mmol L−1 ammonium acetate (CH3COONH4), followed by loading of the sample at a flow rate of 5 mL min−1. The cartridges were dried under nitrogen and eluted with 6 mL methanol. Finally, the target fraction was collected in a 10 mL test tube, volume reduced to almost dryness under a gentle nitrogen stream, and then re-dissolved in 10% methanol solution to a final volume of 1.0 mL. Final extracts were transferred to 2 mL amber vials for LC-MS/MS analysis.Sulfonamides (SAs), quinolones (QNs), macrolides (MLs) and β-Lactams (β-Ls) were analyzed by the LC-MS/MS system (ABI 3200Q TRAP) as described previously with slight revision. Briefly, an Inertsil ODS-SP column (4.6 mm × 150 mm, 5 μm, GL Science Inc. Japan) was maintained at 40 °C, with injection volumes of 20 μL. Purified water with 0.1% formic acid (v/v) (phase A) and methanol (phase B) were used as chromatographic mobile phases at a total flow rate of 1 mL min−1. Tetracyclines and their metabolites were analyzed by the UPLC-MS/MS system with the same mobile phases at a total flow rate of 0.2 mL min−1. Detailed information on the method of antibiotics detection has been described previously.19
2.4. Quality assurance and quality control
Recoveries of the 31 target compounds were determined for wastewater by the standard addition method at 100 ng L−1 in triplicate. Limits of detection (LODs) of the antibiotics were determined as the lowest concentrations resulting in a signal-to-noise (S/N) ratio of 3. Limits of quantification (LOQs) were calculated with a S/N ratio of 10. Main analytical parameters are shown in Table 3.| Compounds | Recoverya (%) (n = 3) | LODb (ng L−1) | LOQc (ng L−1) |
|---|---|---|---|
| a Recovery – at 100 ng L−1. b LOD – limit of detection. c LOQ – limit of quantitation. | |||
| SD | 88.8 ± 2.8 | 0.50 | 0.8 |
| SDM | 40.6 ± 3.6 | 0.27 | 0.91 |
| SM1 | 59 ± 3.0 | 0.25 | 0.83 |
| SMT | 53.5 ± 3.5 | 0.27 | 0.5 |
| SM2 | 70.1 ± 4.5 | 0.27 | 0.91 |
| SMZ | 50.7 ± 2.3 | 0.04 | 0.14 |
| ASM | 92.3 ± 1.3 | 0.67 | 1.18 |
| ASD | 70.0 ± 2.4 | 0.50 | 0.87 |
| ASMZ | 73.7 ± 5.1 | 0.20 | 0.7 |
| CIP | 120.9 ± 1.2 | 5.0 | 8.7 |
| NOF | 78.4 ± 7.6 | 5.0 | 10 |
| SARA | 65.8 ± 3.0 | 1.0 | 3.22 |
| OF | 52.9 ± 8.6 | 0.67 | 2.56 |
| DALA | 125 ± 2.1 | 4.3 | 13.3 |
| ENX | 72.2 ± 3.4 | 1.5 | 3.91 |
| ROX | 59.0 ± 4.1 | 0.33 | 1.33 |
| ERY | 102 ± 2.5 | 5.0 | 15.4 |
| CEFM | 89.4 ± 4.7 | 5.0 | 11.1 |
| CEFD | 94.1 ± 4.2 | 5.0 | 8.7 |
| TC | 118 ± 5.6 | 0.67 | 2 |
| OTC | 126 ± 2.5 | 0.32 | 1 |
| CTC | 64.2 ± 7.0 | 2.0 | 8 |
| ATC | 66.6 ± 1.8 | 12 | 40 |
| MTC | 112 ± 3.7 | 5.0 | 10 |
| ETC | 109 ± 5.1 | 1.82 | 6.4 |
| EATC | 64.2 ± 4.3 | 5.0 | 13.9 |
| DXC | 120 ± 3.6 | 3.0 | 10 |
| α-OTC | 65.7 ± 5.1 | 4.2 | 13.7 |
| EOTC | 125 ± 4.2 | 0.67 | 2 |
| ICTC | 124 ± 7.1 | 1.0 | 3.35 |
| DMCTC | 97.9 ± 8.2 | 5.0 | 32.3 |
All data generated from the analysis were subject to strict quality control procedures. With each set of samples to be analyzed, a solvent blank, a standard and a procedure blank were run in sequence to check for background contamination, peak identification and quantification. In addition, surrogate standards were added to all the samples to monitor matrix effects.
2.5. Statistical analysis
Two-way analysis of variance (ANOVA) was used to examine the seasonal differences. The relationship analyses were performed using SPSS 15.0 for windows (SPSS Inc.).3. Results and discussion
3.1. Occurrence of antibiotics in summer
Table 4 shows the concentrations of selected antibiotics in influent and effluent samples from five WWTPs in summer. Among 31 analytes, 16 antibiotics and 6 metabolites including three SAs and three of their metabolites, five QNs, two macrolides, two β-Lactams and four TCs and three of their metabolites were detected.| Influent | Effluent | |||||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean | Median | Frequency (%) | Range | Mean | Median | Frequency (%) | |
| a ND represents not detectable (below the limit of detection). | ||||||||
| SD | 2.30–36.0 | 18.9 | 20.4 | 100 | 1.46–28.4 | 14.1 | 15.8 | 100 |
| SM2 | ND-251 | 60.7 | 13.1 | 80 | ND-184 | 46.2 | 11.2 | 80 |
| SMZ | 5.41–14.5 | 44.5 | 32.9 | 100 | 2.96–137 | 42.7 | 28.5 | 100 |
| ASM | 8.50–51.5 | 22.6 | 9.36 | 100 | 2.50–52.9 | 24.7 | 12.2 | 100 |
| ASD | 2.70–19.3 | 10.9 | 10.9 | 100 | 4.30–17.6 | 8.10 | 5.70 | 100 |
| ASMZ | 72.7–2312 | 125 | 96.0 | 100 | 38.7–162 | 136 | 129 | 100 |
| CIP | ND-55.8 | 22.3 | 12.5 | 60 | ND-49.8 | 16.5 | 0 | 40 |
| NOF | ND-130 | 59.7 | 34.8 | 80 | 13.1–172 | 74.5 | 56.0 | 100 |
| OF | 129–91.5 | 20.1 | 21.7 | 100 | 12.0–24.6 | 37.6 | 18.9 | 100 |
| ENX | ND-11.0 | 2.20 | 0 | 20 | ND-10.9 | 2.20 | 0 | 20 |
| ROX | 6.50–42.0 | 18.8 | 16.1 | 100 | 12.7–54.5 | 23.5 | 15.6 | 100 |
| ERY | 1.12–1.67 | 1.40 | 1.50 | 100 | 1.20–4.40 | 2.00 | 1.50 | 100 |
| CEFM | 54.1–155 | 114 | 139 | 100 | 25.0–116 | 76.7 | 98.5 | 100 |
| CEFD | 92.5–214 | 161 | 165 | 100 | 21.4–136 | 105 | 132 | 100 |
| TC | ND-175 | 47.9 | 0 | 40 | ND-34.8 | 10.9 | 0 | 40 |
| OTC | ND-75.8 | 19.8 | 0 | 40 | ND-37.8 | 14.2 | 11.9 | 60 |
| CTC | ND-103 | 12.3 | 0 | 40 | ND-39.6 | 30.8 | 0 | 40 |
| DXC | ND-51.0 | 13.1 | 0 | 40 | ND-87.3 | 17.5 | 0 | 20 |
| ETC | ND-48.8 | 9.8 | 0 | 20 | 0 | 0 | 0 | 0 |
| EOTC | ND-40.3 | 12.4 | 0 | 40 | ND-37.4 | 14.1 | 11.9 | 60 |
| ICTC | 3.9–131 | 56.4 | 48.3 | 100 | ND-310 | 107 | 86.9 | 80 |
In the five WWTPs, three out of five SAs were detected in influent and effluent samples, and SDM and SM1 were not found in all WWTPs. SD and SMZ were detected in all influents and effluents of WWTPs. The detection frequencies of SM2 were both 80% in the effluent and influent samples, with the maximum concentration of 251 ng L−1 in the influent sample. The concentration levels of SAs in the present study coincide with those in WWTP effluents in Spain20 and Beijing.21 Three metabolites of SAs were ubiquitous in all samples of influent and effluent of WWTPs. ASM, ASD and ASMZ concentrations in influent samples were in the range of 8.50–51.5 ng L−1, 2.70–19.3 ng L−1 and 72.7–232 ng L−1, respectively.
There were four QNs observed in the wastewater of the investigated WWTPs. In influent samples, OF was the most frequently detected (100%) with the maximum concentration of 91.5 ng L−1. The second in influent samples is NOF (80%), with a mean concentration of 34.8 ng L−1 and a maximum concentration of 130 ng L−1. The detection frequencies of CIP in influent and effluent samples were 60% and 40%, respectively. Among QNs, OF and NOF are the dominant compounds, which have been proven to be widely used in medical treatments.22 Compared to our results, NOF levels in WWTP effluents in Sweden, France, Greece, and Italy were lower (30–80 ng L−1) but OF levels were higher (120–580 ng L−1).23 A similar concentration range for NOF has been recorded as 0.10–0.46 μg L−1 in influent samples from five WWTPs in Hong Kong and Shenzhen24 and 0.054–0.26 μg L−1 (ref. 25) in influent samples from four WWTPs in the Pearl River Delta. In addition, the reported concentration of CIP (0.028–0.32 μg L−1) is similar to our result (0.015–0.14 μg L−1). In contrast, in Spain, much higher concentrations of CIP have been reported, 0.160–13.6 μg L−1.26
ERY and ROX were the macrolides detected in all influent and effluent samples in this study. The concentrations of ERY ranged from 1.10 to 4.40 ng L−1 in influents and from 1.20 to 1.80 ng L−1 in effluents. ROX was found ranging from 12.7 to 54.5 ng L−1 in influents and from 6.54 to 42.0 ng L−1 in effluents. However, higher concentration ranges such as 54–360 ng L−1 for ROX and 51–300 ng L−1 for ERY in WWTPs effluent samples in Beijing have been reported.21 In this study, β-Lactams including CEFM and CEFD were detected in all samples and the highest concentrations found were 147 and 175 ng L−1, respectively.
Among five targets of TCs, four TCs were found including TC, OTC, CTC and DXC. In influent samples, the detection frequencies of all four TCs were 40%. In effluent samples, the highest detection frequency was of OTC (60%), with the maximum concentration of 37.8 ng L−1 and the lowest detection frequency was of DXC (20%), with the maximum concentration of 87.3 ng L−1. In South China, levels of TCs in WWTPs were higher than those in this study.27 In this study, levels of TCs were much lower than those in the raw WWTP influents in the USA at concentrations between 0.1 and 0.6 mg L−1.28 In Canada, the remaining concentration of tetracycline in WWTP effluents was reported to be nearly 1.0 μg L−1.29 Once TCs were extensively used for both human and veterinary medicine in China. But now TCs are mostly used in treating animal disease in this region. From metabolites of TCs, three out of seven were detected in influent samples. Although the detection frequency of TC was 40%, ICTC, as a metabolite of TC, was found in all WWTP influents. This is probably because CTC is converted to isochlortetracycline (ICTC) under alkaline conditions, while the epimerisation has been found to be catalyzed in acidic solutions in a pH range from 2 to 6.30
3.2. Occurrence of antibiotics in winter
The concentrations of selected antibiotics are shown in Table 5 in influent and effluent samples from five WWTPs in winter. 20 out of 31 analytes were detected in the analyzed samples of WWTPs including three sulfonamides and three of their metabolites, four quinolones, one macrolides, two β-Lactams and four tetracyclines and three of their metabolites.| Influent | Effluent | |||||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean | Median | Frequency (%) | Range | Mean | Median | Frequency (%) | |
| a ND represents not detectable (below limit of detection). | ||||||||
| SD | 8.60–32.1 | 20.0 | 25.5 | 100 | 3.60–26.3 | 16.8 | 22.7 | 100 |
| SM2 | 5.30–259 | 67.0 | 31.0 | 100 | ND-233 | 62.3 | 31.6 | 80 |
| SMZ | 13.5–152 | 59.3 | 46.8 | 100 | 11.4–145 | 52.1 | 34.5 | 100 |
| ASM | 4.00–43.5 | 19.3 | 12.2 | 100 | 5.0–39.8 | 17.2 | 6.70 | 100 |
| ASD | ND-51.4 | 21.6 | 22.7 | 80 | ND-51.4 | 21.6 | 22.7 | 80 |
| ASMZ | 82.7–299 | 149 | 136 | 100 | 88.7–208 | 131 | 123 | 100 |
| CIP | ND-15.5 | 7.50 | 9.50 | 60 | ND-13.0 | 6.50 | 9.40 | 60 |
| NOF | ND-46.2 | 16.5 | 12.2 | 60 | ND-13.0 | 11.6 | 0 | 40 |
| OF | 6.0–39.5 | 23.0 | 17.7 | 100 | 5.70–53.4 | 35.1 | 38.2 | 100 |
| ENX | ND-16.8 | 9.80 | 10.5 | 80 | ND-18.5 | 10.5 | 10.9 | 80 |
| ROX | 29.7–63.7 | 41.5 | 35.9 | 100 | 59.3–104 | 78.2 | 71.0 | 100 |
| CEFM | 33.4–822 | 240 | 133 | 100 | 65.2–187 | 142 | 147 | 100 |
| CEFD | 131.0–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
460 | 357 | 100 | 155.7–369 | 304 | 353 | 100 |
| TC | ND-124 | 45.2 | 16.5 | 60 | ND-101 | 41.0 | 19.9 | 60 |
| OTC | ND-167 | 64.1 | 6.70 | 60 | ND-178 | 65.1 | 7.10 | 80 |
| CTC | ND-261 | 83.7 | 35.3 | 60 | ND-154 | 57.7 | 24.9 | 60 |
| DXC | 17.3–36.4 | 26.4 | 25.5 | 100 | ND-36.2 | 14.0 | 16.1 | 60 |
| ETC | ND-87.7 | 21.8 | ND | 40 | ND-82.1 | 23.0 | 9.70 | 60 |
| EOTC | ND-287 | 81.2 | 6.30 | 60 | ND-296 | 81.5 | 5.60 | 60 |
| ICTC | ND-233 | 98.6 | 31.0 | 80 | ND-651 | 250 | 105 | 80 |
Among the five SAs, three out of five were observed in all influent samples including SM2, SMZ and SD. The highest concentration was of SM2 up to 259 ng L−1 in influent samples. In effluent samples, SD and SMZ were detected in all samples. The detected frequency of SM2 was 80% in effluents. At the same time, metabolites of three SAs were also found in WWTPs. Among three metabolites of SAs, the concentration of ASMZ was the highest up to 297 ng L−1. Among QNs, OF was the most frequently detected (100%), with the maximum of 53.4 ng L−1. The second was ENX (80%), with a maximum concentration of 18.5 ng L−1 in S5 effluent. However, SARA and DALA were not found in all samples. The detected frequency of CIP was 60%, while NOF was only 40%. Macrolides and β-Lactams were detected in all WWTP samples except ERY. The level of CEFD was the highest up to 1185 ng L−1. A similar level (670–2900 ng L−1) was observed in WWTP influents from Hong Kong and Shenzhen, southern China.24
There were four TCs found in WWTPs in winter. Among four TCs, DXC was the most frequently detected (100%) in effluent samples. TC, OTC and CTC were found with the detection frequency of 60%. In effluent samples, OTC was the most frequently detected (80%), with the maximum concentration of 178 ng L−1, and then the detected frequencies of TC, DXC and CTC were 60%. MTC was not detected in all samples. Similar to metabolites of TCs in summer, there were three MTCs found in winter. ICTC, as a metabolite of TCs, was found in all WWTPs except S5 with the maximum of 651 ng L−1. Similar to ICTC, the highest concentration in S1was of EOTC up to 296 ng L−1. The main degradation products of TCs detected in WWTPs were their epimers ETC, ECTC and EOTC, which is in agreement with the reported literature that TCs can be excreted in the form of their 4-epimers.31 Especially for ETC, the concentration was nearly as much as that of TC. Dehydrated products and 4-epianhydrotetracyclines of TC and CTC tended to be lower than their parent TCs.
3.3. Seasonal variation of antibiotics in influents and effluents
As shown in Fig. 2, the total levels of antibiotics showed significant seasonal fluctuations both in winter and in summer in all wastewater samples (p < 0.05, F = 9.97). The total levels of antibiotics in winter were higher than those in summer in all WWTPs. For example, the total concentration of antibiotics in the winter season in S4 influent was 6.5 times higher than that in summer.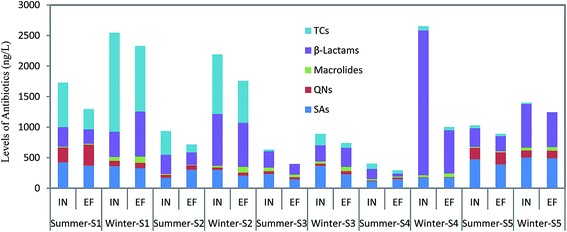 | ||
| Fig. 2 Seasonal variation and spatial distribution of antibiotics in five WWTPs of the Jiulongjiang River Region. | ||
Statistical analysis showed that there were not significant seasonal (p > 0.001) variations for five kinds of SAs, except SDM and SM1. For three metabolites of SAs, no significant variations were found between the two sampling events, although the total levels of SA metabolites in winter were slightly higher than those in summer. Among six quinolones, two quinolones (SARA and DALA) were not detected in winter, but only SARA was not found in summer. The total concentrations of quinolones in summer were higher than those in winter, although the frequencies of detection were higher in winter than those in summer. This is probably because quinolones are frequently used for treatment of intestines infection in summer. Two macrolides showed significant (p < 0.05, F = 4.18) seasonal variations between two sampling events. The levels of ROX in winter were significantly (p < 0.05, F = 6.87) higher than those in summer. ERY was not detected in winter, but the detected frequency of ERY was 100% in summer. The case of two β-Lactams, was similar to the macrolides. Four TCs showed significant seasonal variations (p < 0.05, F = 6.24). The total concentrations of tetracyclines in influent samples in winter (ranging from 17.3 to 482 ng L−1) were 2 times those in the summer season (from ND to 231 ng L−1). Detected frequencies of four tetracyclines were 40%, and they were only observed in S1 and S2 influent samples in summer. However, all four TCs were detected in all influent samples, except S4 and S5. The case of metabolites of TCs was similar to TCs. The total concentration of metabolites in summer was slightly lower than that in winter, although there was not significant variation in winter and in summer.
The different occurrences of antibiotics between the two sampling events may be explained by the consumption level of antibiotics and climatic conditions. Antibiotics showed highest concentrations in winter and lowest concentrations in summer in the WWTP influents. Previous reports have also revealed that the level of antibiotics in the WWTP influents is greater in winter than in other seasons,32–34 indicating a higher consumption of antibiotics in winter. Because of the cold weather during the winter, human beings and livestock used much more antibiotics to cure the infection of the respiratory tract. In addition, water consumption of urban residents usually is much more in summer than that in winter. Besides, summer is the typical flood and high water season of the Jiulongjiang River and winter is the typical low water season. So, another factor contributing to lower concentrations in winter might be much water consumption and the high flow conditions, which might result in a great dilution of the concentrations of antibiotics in WWTP influents.
3.4. Spatial distribution of antibiotics in five WWTPs
Spatial distributions of 31 antibiotics and their metabolites in the current study were also compared among five WWTPs along the Jiulongjiang River Region (Fig. 2).Of all WWTPs, the total level of antibiotics in S1 was relatively higher. For SAs and their metabolites, levels in effluent and influent of S5 were the highest among five WWTPs in both seasons. For QNs, the highest total concentration was found in S1, however, the second highest was found in S5. NOF was the most abundant QN in S1. NOF, which has been proven to be widely used in medical treatments,22 was commonly detected in WWTPs. Two macrolides and two β-Lactams were detected in all WWTP samples. The total levels of macrolides and β-Lactams were the highest in S4 in winter. This is probably because in the S4 region there was higher the population density, while macrolides and β-Lactams were mainly used to treat human disease. TCs were found only in S1–S3. The highest total concentration of TCs and metabolites of TCs was found in S1 in winter. Now TCs are mostly used in treating animal disease in China. S1–S3 are located in the upstream of Jiulongjiang River where there are a lot of swine farms,19 discharging untreated swine breeding wastewaters into the environment.
The concentrations of antibiotics in different WWTP influents and effluents vary significantly, depending on consumption patterns and the types of wastewater treatment processes employed. In S1, there is more population than that in S2–S5. Besides, there is much more livestock breeding in S1. Thus, more population and livestock may result in more usage of antibiotics and more input of water in S1.
3.5. Aqueous removal percentages of antibiotics
The removal efficiency of antibiotics was calculated from the analyte concentration in influent (Cin) and effluent (Cef): [(Cin − Cef)/Cin] × 100%. As shown in Table 6, the average removal efficiencies of SAs ranged from −6.20 to 26.9%. For three metabolites of SAs, the average removal efficiencies were from −19.4 to 47.8%. The average removal efficiencies of SD, SM2 and SMZ in summer were 32.8, 15.6 and 17.8%, respectively. Similar removal efficiency of SD (20–82%) has also been detected in four WWTPs from Taiwan35 and in two WWTPs from the Pearl River Delta.23 The removal efficiency of SMZ (71% in summer and 0–84% in winter) in WWTPs in Italy has also been reported.33 The negative removal efficiency of SMZ in WWTPs may be caused by the presence of metabolites in the influents, which can subsequently be transformed to their parent compounds during biological treatment.36 N4-acetylsulfamethoxazole usually accounts for more than 50% of an administered dose in human excretion and can occur in WWTP influents at concentrations of 2.5–3.5 times higher than concentrations of the parent compound.32 Significant removal efficiencies (81–96% and 68–92%, respectively) of N4-acetylsulfamethoxazole during secondary treatment were reported by Göbel et al.32 and Joss et al.37 N4-acetylsulfamethoxazole can also deconjugate into sulfamethoxazole during wastewater treatment,36 leading to an underestimation of removal efficiency for sulfamethoxazole if this metabolite is not considered. This might be a reason for the highly varying observed elimination rates.| S1 | S2 | S3 | S4 | S5 | Max | Min | Mean | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | ||||
| SD | 21.0 | 11.0 | 22.4 | 11.1 | 36.2 | 33.1 | 59.4 | 32.2 | 24.9 | 17.9 | 59.4 | 11.0 | 26.9 |
| SM2 | −2.80 | −1.80 | 14.3 | 5.70 | 100 | 39.6 | −180 | 26.9 | 10.1 | 100 | −180 | −6.20 | |
| SMZ | −19.7 | 4.60 | −40.4 | 26.3 | 84.6 | 43.0 | 44.8 | 15.6 | 19.9 | 8.70 | 84.6 | −40.4 | 19.3 |
| ASM | 5.40 | −23.4 | 30.9 | −29.0 | −233 | 71.4 | 0.100 | 2.10 | −2.80 | 8.6 | 71.4 | −233 | −17.0 |
| ASD | 60.9 | 19.7 | 100 | 33.8 | 100 | 23.0 | 21.1 | 8.60 | 63.2 | 100 | 8.60 | 47.8 | |
| ASMZ | 28.1 | 15.8 | −141 | 19.1 | 33.6 | 30.3 | −123 | −7.30 | 1.90 | −51.2 | 33.6 | −141 | −19.4 |
| CIP | 10.6 | 20.1 | 100 | 0.600 | 25.0 | 15.8 | 100 | 25.0 | 28.7 | ||||
| NOF | −32.3 | 22.2 | −61.2 | 100 | −1.60 | 2.2 | 15.1 | 100 | −61.2 | 6.40 | |||
| OF | −272 | −14.8 | −20.8 | −137 | −19.3 | −84.8 | 7.10 | 4.90 | −132 | −48.6 | 7.10 | −272 | −71.6 |
| DALA | 10.2 | 10.2 | |||||||||||
| ENX | 0.700 | 6.30 | 5.60 | 3.70 | 9.4 | 9.4 | 0.700 | 5.10 | |||||
| ROX | −20.1 | −63.0 | 48.5 | −224 | 22.9 | −97.8 | 16.2 | −82.1 | 3.10 | −32.7 | 48.5 | −224 | −42.9 |
| ERY | 11.4 | 2.80 | 61.7 | 37.4 | 7.50 | 61.7 | 2.80 | 24.1 | |||||
| CEFM | 32.9 | −79.4 | 33.9 | 15.3 | 22.5 | −18.8 | 66.0 | 70.2 | 16.3 | 20.2 | 66.0 | −79.4 | 17.9 |
| CEFD | 21.1 | −79.4 | 36.4 | 15.3 | 38.4 | −18.8 | 76.8 | 70.2 | 19.8 | 20.2 | 76.8 | −79.4 | 20.0 |
| TC | 46.4 | 18.6 | 88.7 | 1.4 | −20.4 | 88.7 | −20.4 | 26.9 | |||||
| OTC | 50.1 | −6.3 | 8.40 | 8.5 | 100 | −7.00 | 100 | −7.00 | 25.6 | ||||
| CTC | 61.4 | 40.9 | 57.0 | 10.5 | 29.4 | 61.4 | 10.5 | 39.8 | |||||
| DXC | 41.5 | 0.7 | 100 | 47.7 | 100 | 14.5 | 100 | 14.5 | 50.7 | ||||
| ETC | 100 | 6.30 | −8.7 | 100 | −8.70 | 32.5 | |||||||
| EOTC | 7.40 | 2.80 | 0.100 | 5.4 | 100 | 100 | 100 | 100 | 0.100 | 45.1 | |||
| ICTC | 57.6 | 64.2 | 56.1 | 52.8 | 100 | 84.3 | 38.3 | 30.8 | 22.0 | 100 | 22.0 | 56.3 | |
For QNs, the mean removal efficiency (−71.6–28.7%) was lower than those of SAs. The overall removal percentages were very variable. The mean removal efficiency of OF was 50%, which was similar to the value (56%) by the WWTPs in Sweden38 and lower than that (83%) by the WWTPs in Finland.38 For NOF, the mean and maximum removal efficiencies were 6.4 and 100%, respectively. The mean removal efficiency of 28.7% for CIP in our study is lower than Rosal's observation (57.0%)26 and 84% from WWTPs in Finland.39 In Sweden,40 the mean elimination degrees of NOF, NOR and CIP were estimated to be 86, 87 and 87%, respectively. Several studies have also reported that the predominant removal mechanism of quinolones in the WWTPs is adsorption to sludge.41,42 For example, approximately 80% of the total mass of both NOF and CIP are adsorbed to particles in the raw sewage water.43
Compared with the other detected antibiotics, the average removal efficiencies of two macrolides were very lower. The mean removal efficiency of ERY and ROX was 24.2%, which was similar to the reported removal efficiency (26%) of ERY–H2O in four WWTPs in the Pearl River Delta25 and higher than the elimination (4.3%) of ERY in WWTPs in Spain.26 The removal efficiency of ROX ranged from −223 to 48.5% (−42.9% on average) in our study. The negative removal efficiency of macrolides by WWTPs had also been reported in previous work.24,25 One of the possible reasons for these findings is that particles larger than 0.45 μm are not included in the analysis, which may lead to an underestimation of the concentrations of the relevant compounds in the influents. Moreover, the conjugated metabolites in raw influent samples can be de-conjugated during the treatment process, or analyte behavior such as adsorption to particles may be altered by changing physicochemical parameters during the treatment process, thus influencing the removal efficiency.26,40
For four TCs, the mean removal efficiency ranged from 25.62% to 50.74%. In the USA, CTC and DXC have been reported after secondary treatment and chlorination with removal efficiencies of 78% and 67%, respectively.44 Li and Zhang45 reported removals of 24–36% at two plants while higher removals (67.9–100%) were reported by Karthikeyan and Meyer9 and four Taiwanese WWTPs (66–90%) by Lin et al.35 TCs are reported to interact strongly with clay, natural organic matter and metal oxides by cation exchange, surface complexation/cation, bridging hydrophobic partitioning, and electron donor–acceptor interactions.45,46 Besides, other processes such as hydrolysis, epimerization and photolysis may also contribute a certain degree to the degradation of this class of antibiotics in wastewater.47,48 So, the higher removal efficiency of TCs was compared to other antibiotics.
Removal efficiencies for antibiotics appear to vary with wastewater treatment plants, affected by their operations, geographic locations, and environmental factors. The main operational factors that can influence the biological removal of antibiotic residues in wastewater treatment are biochemical oxygen demand (BOD5), existence and size of anoxic and anaerobic compartments, suspended solids (SS) loading, hydraulic retention time (HRT), sludge retention time (SRT), food/microorganism ratio (F/M ratio), mixed liquorsuspended solids (MLSS), pH, temperature of the raw sewage and the plant's configuration.24,32,49,50 In this study, there are differences in treatment processes and operational factors of five WWTPs. So the removal of antibiotics was also different in five studied WWTPs.
4. Conclusion
Out of 31 antibiotics and their metabolites, in summer 16 antibiotics and 6 metabolites were found in the influents and effluents at the five studied WWTPs, while in winter 14 antibiotics and 6 metabolites were detected. The most frequently detected antibiotics were SD, SMZ, ASD, ASMZ, OF, ROX, CEFM and CEFD. The concentrations of antibiotics are higher in winter than those in summer. The highest level of total antibiotics was found in Longyan City WWTPs, where there are more population and swine farms. The removal of antibiotics by the five studied WWTPs is incomplete. TCs were removed relatively more efficiently compared to other studied antibiotics. As for the occurrence and removal of antibiotics from wastewater at the five studied WWTPs, these were found to be similar to reports on other WWTPs. So, antibiotics from studied WWTPs maybe one of the important reasons in the environment of this region.Acknowledgements
This work is financially supported by International Cooperation of Ministry Science and Technology of China (2011DFB91710), Natural Science Foundation of Fujian Province, China (Y1F0511E0) and Fujian Soft Science Research Project (Grant No.2013R01020064).References
- D. Fatta-Kassinos, S. Meric and A. Nikolaou, Anal. Bioanal. Chem., 2011, 399, 251–275 CrossRef CAS PubMed.
- L. Michael, C. S. Rizzo, C. M. McArdell, C. Manaia, T. Merlin, C. Schwartz and D. Dagot, Water Res., 2013, 47, 957–995 CrossRef PubMed.
- N. Czekalski, T. Berthold, S. Caucci, A. Egli and H. Bürgmann, Front Microbiol., 2012, 3, 106–112 Search PubMed.
- P. Servais and J. Passerat, Sci. Total Environ., 2009, 408, 365–372 CrossRef CAS PubMed.
- H. C. Neu, Science, 1992, 257, 1064–1073 CAS.
- A. Pruden, R. Pei, H. Storteboom and K. H. Carlson, Environ. Sci. Technol., 2006, 40, 7445–7450 CrossRef CAS.
- P. M. Verlicchi, A. l. Aukidy and E. Zambello, Sci. Total Environ., 2012, 429, 123–155 CrossRef CAS PubMed.
- A. L. Spongberg and J. D. Witter, Sci. Total Environ., 2008, 397, 148–157 CrossRef CAS PubMed.
- K. Karthikeyan and M. T. Meyer, Sci. Total Environ., 2006, 361, 196–207 CrossRef CAS PubMed.
- J. C. Chee-Sanford, R. I. Aminov, I. J. Krapac, N. Garrigues-Jeanjean and R. I. Mackie, Appl. Environ. Microbiol., 2001, 67, 1494–1502 CrossRef CAS PubMed.
- H. Heuer, A. Focks, M. Lamshöft, K. Smalla, M. Matthies and M. Spiteller, Soil Biol. Biochem., 2008, 40, 1892–1900 CrossRef CAS PubMed.
- C. A. Kan and M. Petz, J. Agric. Food Chem., 2000, 48, 6397–6403 CrossRef CAS PubMed.
- Z. Ye, H. S. Weinberg and M. T. Meyer, Anal. Chem., 2007, 79, 1135–1144 CrossRef CAS PubMed.
- X. G. Hu, Q. X. Zhou and Y. Luo, Environ. Pollut., 2010, 158, 2992–2998 CrossRef CAS PubMed.
- M. J. García-Galán and D. M. Silvi, TrAC, Trends Anal. Chem., 2008, 27, 1008–1022 CrossRef PubMed.
- K. Berger and B. Petersen, Arch. Lebensmittelhyg., 1986, 37, 85–108 Search PubMed.
- B. Halling-Sørensen, G. Sengeløv and J. Tjørnelund, Arch. Environ. Contam. Toxicol., 2002, 42, 263–271 CrossRef PubMed.
- D. D. Zhang, X. Zhang and C. Z. Yan, J. Environ. Monit., 2011, 13, 1593–1601 Search PubMed.
- H. Y. Jiang, D. D. Zhang, S. C. Xiao and X. Zhang, Environ. Sci. Pollut. Res., 2013, 20, 9075–9083 CrossRef CAS PubMed.
- M. J. García-Galán, M. S. Díaz-Cruz and D. Barceló, Environ. Int., 2011, 37, 462–473 CrossRef PubMed.
- W. Li, Y. Shi, L. Gao, J. Liu and Y. Cai, Chemosphere, 2013, 92, 435–444 CrossRef CAS PubMed.
- C. M. Oliphant and G. M. Green, Am. Fam. Physician, 2002, 65, 455–463 Search PubMed.
- R. Andreozzi, M. Raffaele and P. Nicklas, Chemosphere, 2003, 50, 1319–1330 CrossRef CAS.
- A. Gulkowska, H. W. Leung, M. K. So, S. Taniyasu, N. Yamashita, L. W. Y. Yeung, B. J. Richardson, A. P. Lei, J. P. Giesy and P. K. S. Lam, Water Res., 2008, 42, 395–403 CrossRef CAS PubMed.
- W. Xu, G. Zhang, X. Li, S. Zou, P. Li, Z. Hu and J. Li, Water Res., 2007, 41, 4526–4534 CrossRef CAS PubMed.
- R. Rosal, A. Rodríguez, J. A. Perdigón-Melón, A. Petre, E. GarcíaCalvo, M. J. Gómez, A. Aguera and A. R. Fernández-Alba, Water Res., 2010, 44, 578–588 CrossRef CAS PubMed.
- L. Zhou, G. Ying, S. Liu, J. Zhao, B. Yang, Z. Chen and H. Lai, Sci. Total Environ., 2013, 452–453, 365–376 CrossRef CAS PubMed.
- S. Kim, P. Eichhorn, J. N. Jensen, A. S. Weber and D. Aga, Environ. Sci. Technol., 2005, 39, 5816–5823 CrossRef CAS.
- X. S. Miao, F. Bishay, M. Chen and C. D. Metcalfe, Environ. Sci. Technol., 2004, 38, 3533–3541 CrossRef CAS.
- P. Eichhom and D. S. Aga, Anal. Chem., 2004, 76, 6002–6011 CrossRef PubMed.
- G. Brambilla, M. Patrizii, S. P. DeFilippis, G. Bonazzi, P. Mantovi and D. Barchi, Anal. Chim. Acta, 2007, 586, 326–329 CrossRef CAS PubMed.
- A. Göbel, C. McArdell, A. Joss, H. Siegrist and W. Giger, Sci. Total Environ., 2007, 372, 361–371 CrossRef PubMed.
- S. Castiglioni, R. Bagnati, R. Fanelli, F. Pomati, D. Calamari and E. Zuccato, Environ. Sci. Technol., 2006, 40, 357–363 CrossRef CAS.
- Q. Sui, J. Huang, S. Deng, W. Chen and G. Yu, Environ. Sci. Technol., 2011, 45, 3341–3348 CrossRef CAS PubMed.
- A. Y. C. Lin, T. H. Yu and S. K. Lateef, J. Hazard. Mater., 2009, 167, 1163–1169 CrossRef CAS PubMed.
- A. Göbel, A. Thomsen, C. S. Mcardell, A. Joss and W. Giger, Environ. Sci. Technol., 2005, 39, 3981–3989 CrossRef.
- A. Joss, S. Zabczynski, A. Göbel, B. Hoffmann, D. Löffler, C. S. McArdell, T. A. Ternes, A. Thomsen and H. Siegrist, Water Res., 2006, 40, 1686–1696 CrossRef CAS PubMed.
- S. Zorita, L. Mårtensson and L. Mathiasson, Sci. Total Environ., 2009, 407, 2760–2770 CrossRef CAS PubMed.
- N. Vieno, T. Tuhkanen and L. Kronberg, Elimination of pharmaceuticals in sewage treatment plants in Finland, Water Res., 2007, 41, 1001–1012 CrossRef CAS PubMed.
- R. H. Lindberg, P. Wennberg, M. I. Johansson, M. Tysklind and B. A. V. Andersson, Environ. Sci. Technol., 2005, 39, 3421–3429 CrossRef CAS.
- W. Giger, A. C. Alder, E. M. Golet, H. P. E. Kohler, C. S. McArdell, E. Molnar, H. Siegrist and M. J. F. Suter, Chimia, 2003, 57, 485–491 CrossRef CAS.
- A. L. Batt, S. Kim and D. S. Aga, Chemosphere, 2007, 68, 428–435 CrossRef CAS PubMed.
- R. H. Lindberg, K. Bjorklund, P. Rendahl, M. I. Johansson, M. Tysklind and A. V. Andersson, Water Res., 2007, 41, 613–619 CrossRef CAS PubMed.
- S. Yang, J. Cha and K. Carlson, J. Chromatogr. A, 2005, 1097, 40–53 CrossRef CAS PubMed.
- B. Li and T. Zhang, Environ. Sci. Technol., 2010, 44, 3468–3473 CrossRef CAS PubMed.
- L. Aristilde, C. Marichal, J. Miehe-Brendle, B. Lanson and L. Charlet, Environ. Sci. Technol., 2010, 44, 7839–7845 CrossRef CAS PubMed.
- W. Kong, C. Li, J. M. Dolhi, S. Li, J. He and M. Qiao, Chemosphere, 2012, 87, 542–548 CrossRef CAS PubMed.
- K. A. Loftin, C. D. Adams, M. T. Meyer and R. Surampalli, J. Environ. Qual., 2008, 8(37), 378–386 CrossRef PubMed.
- H. Pouliquen, R. Delpepee, M. Larhantec-Verdier, M. L. Morvan and H. Le Bris, Aquaculture, 2007, 262, 23–28 CrossRef CAS PubMed.
- J. E. Drewes, Removal of Pharmaceutical Residues During Wastewater Treatment, in Comprehensive Analytical Chemistry 50, 2008, ch. 4.1 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |