Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mono- and di-cationic hydrido boron compounds†‡
Rajendra S.
Ghadwal
 *ab,
Christian J.
Schürmann
b,
Diego M.
Andrada
c and
Gernot
Frenking
c
*ab,
Christian J.
Schürmann
b,
Diego M.
Andrada
c and
Gernot
Frenking
c
aInstitut für Anorganische Chemie, Universität Bielefeld, Universitätsstrasse 25, 33615 Bielefeld, Germany. E-mail: rghadwal@uni-bielefeld.de
bInstitut für Anorganische Chemie der Georg-August-Universität Göttingen, Tammannstrasse 4, 37077 Göttingen, Germany
cFachbereich Chemie, Philipps-Universität Marburg, Hans-Meerwein-Straße, 35032 Marburg, Germany
First published on 7th July 2015
Abstract
Brønsted acid HNTf2 (Tf = SO2CF3) mediated dehydrogenative hydride abstraction from (L1)BH3 (3) and (L2)BH3 (4) (L1 = IPrCH2 = 1,3-(2,6-di-isopropylphenyl)imidazol-2-methylidene (1); L2 = SIPrCH2 = 1,3-(2,6-di-isopropylphenyl)imidazolidin-2-methylidiene (2)) affords thermally stable hydride bridged mono-cationic hydrido boron compounds [{(L1)BH2}2(μ-H)](NTf2) (5) and [{(L2)BH2}2(μ-H)](NTf2) (6). Furthermore, hydride abstraction yields di-cationic hydrido boron compounds [{(L1)BH}2(μ-H)2](NTf2)2 (7) and [{(L2)BH}2(μ-H)2](NTf2)2 (8). Unique cationic boron compounds with CH2BH2(μ-H)BH2CH2 (5 and 6) and CH2BH(μ-H)2BHCH2 (7 and 8) moieties feature a 3c–2e bond and have been fully characterized. Interesting electronic and structural features of compounds 5–8 are analysed using spectroscopic, crystallographic, and computational methods.
Introduction
Investigation of thermally stable exotic main group compounds with elusive chemical and electronic properties has been a subject of considerable research interest.1 Boron compounds play an important role in organic synthesis and materials science.2 The search for new boron compounds with unique bonding motifs and electronic structures has been a major focus in molecular main group chemistry.3 A variety of mono-cationic boron compounds such as borinium [R2B]+, borenium [(L)R2B]+, and boronium [(L)2R2B]+ species (L = a neutral ligand) have been isolated and characterized.2c,4 Recent studies have demonstrated a remarkable activity of borenium compounds in Lewis acid catalysis as well as in the functionalization of small molecules.2c,5 Interestingly, so far only a few di-cationic boron compounds, in particular with a hydride ligand, have been isolated and adequately characterized (Scheme 1).4c,6Cowley et al. reported the first hydrido boron di-cation (A) stabilized by a pyridine ligand (Scheme 1).6f A direct B–B coupling reaction of a mono-cationic species to afford a di-cationic boron compound (B) stabilized by a cyclic guanidine ligand has been recently described by Himmel and co-workers.4c Singlet carbenes have been recognized as most suitable candidates for taming highly reactive main group species.3n,7 Therefore, boryl radicals,3r,8 borenium ions,4e,5a,d,6b,9 boryl anions,2e,3i,q,s,10 borylenes,3e,o,7b,11 diborene,3g,12 and diboryne3k compounds have been successfully isolated by using N-heterocyclic carbenes (NHCs). Curran et al. employed an NHC and isolated a hydride-bridged boron di-cation (C).6b A carbodicarbene stabilized hydrido boron di-cation (D) has been reported very recently by Ong and co-workers.6a
While the flanking substituents at the imidazole nitrogen atoms of an NHC are well endowed to encapsulate a reactive species to be stabilized, but they may prevent its accessibility for further reactions.3n,13 Moreover, NHC-coordinated main group species sometimes do not exhibit the expected reactivity owing to the diminished electrophilicity. This is due to the strong NHC–element interaction. To circumvent this situation and to expand the scope of reactive main group species to functionalize organic substrates, we became interested in the new class of carbon-donor ligands.14 N-Heterocyclic olefins (NHOs) and their borane adducts were already known as early as in 1993,15 interest in this class of ligands incited us very recently.16 NHOs (1 and 2, Scheme 2) are strong nucleophiles but rather weak electron donors (Lewis bases) than NHCs.17 Therefore, NHOs readily react with a NHC-stabilized dichloro-silylene (IPr)SiCl2 to furnish silyl-functionalized NHOs and liberate free IPr (IPr = 1,3-(2,6-di-isopropylphenyl)imidazol-2-ylidene).18 Similarly, KC8 reduction of (NHO)BRX2 (R = I or Ph; X = Cl or I) compounds led to the insertion of a borylene into a C–N bond to yield boryl-functionalized NHOs.14b Herein, we report on a very facile route to NHO-stabilized mono- and di-cationic hydrido boron compounds featuring [CH2BH2(μ-H)BH2CH2] and [CH2BH(μ-H)2BHCH2] bonding motifs. Synthesis, characterization, reactivity, structure, and computational analysis of these compounds are presented.
Results and discussion
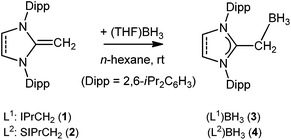
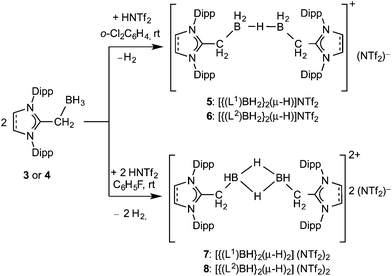
The reaction of an NHO (L1 or L2) with (THF)BH3 quantitatively yields (L1)BH3 (3) and (L2)BH3 (4) as white solids (L1 = IPrCH2 (1) and L2 = SIPrCH2 (2); IPrCH2 = 1,3-(2,6-di-isopropylphenyl)-imidazol-2-methylidene and SIPrCH2 = 1,3-(2,6-di-isopropylphenyl)imidazolidin-2-methylidiene) (Scheme 2). Treatment of compounds 3 and 4 with 0.5 eq. of bis(trifluoromethane)sulfonimide (HNTf2) readily affords mono-cationic hydrido boron compounds [{(L1)BH2}2(μ-H)](NTf2) (5) and [{(L2)BH2}2(μ-H)](NTf2) (6) in a high yield (Scheme 3).Compounds 3–6 have been characterized by 1H, 11B and 13C NMR as well as IR spectroscopic studies. The exocyclic methylene (CH2) group each in 3 and 4 appears as a broad signal in the 1H NMR spectrum at δ 1.60 and δ 1.41 ppm, respectively. Each of compounds 5 (δ −15.4) and 6 (δ −16.4) exhibits a broad 11B NMR signal, which is shifted towards downfield (ca. 14 ppm) when compared with the respective starting compound 3 (δ −29.9) or 4 (δ −29.5). Moreover, the 11B NMR chemical shift, each for 5 and 6, is ca. 10 ppm downfield compared to NHC-analogues.6b This may be due to the lower basicity of L1 and L2 ligands than that of NHCs. Nevertheless, compounds 5 and 6 are stable both in solution (CH2Cl2, CHCl3, C6H5F, and 1,2-Cl2C6H4) and solid phases at room temperature under an inert gas (Ar or N2) atmosphere.
Suitable single crystals for X-ray diffraction study were obtained by a slow diffusion of n-hexane into a saturated dichloromethane solution of 5 at room temperature. The solid-state structure of 5 revealed the formation of a hydride bridged mono-cationic boron compound (Fig. 1). The B–(μ-H)–B bond is apparently derived from a 3c–2e (three-center–two-electrons) interaction. The C(0)–B(0) bond length of 1.630 Å in 5 is slightly shorter than that of 4 (1.68 Å). The C(0)–C(1) bond lengths of 4 (1.452 Å) and 5 (1.467) are comparable with C(sp2)–C(sp3) single bond distances. All hydrogen atoms near the boron atom were located on difference Fourier maps and refined isotropically as independent atoms. Due to experimental restrictions, all B–H distances are underestimated. The electron density of hydrogen is always shifted towards the bonding partner and due to the lack of core electrons, a shortened B–H bond length is obtained in the IAM refinement.19
In order to shed light into the electronic structures, we carried out DFT calculations (M06-2X/def2-SVP)20 for 5 and 6. The optimized bond lengths and angles of 5 are in good agreement with the experimental values (Fig. 1). A comparison of the calculated structures of 5 and 6 indicates (Fig. S2 in the ESI‡) very similar geometries for the H2B–H–BH2 moiety. Compound 6 presents a slightly longer B–μH bond length and more acute C(carbene)–C–B angles than those in 5. Based on the natural population analysis21 (Table 1) the boron fragment B2H5 is negatively charged by −0.23 e and −0.20 e for 5 and 6, respectively. Additionally, natural bond orbital (NBO)22 analysis reveals the presence of a 3c–2e bond (Tables S2 and S3‡) B–μH–B where 47% is at the H atom and roughly 26% on each of the boron atoms. NBO results also point out that the ligands bind boron by C–B σ-bonds which are polarized toward the carbon end (∼70% at C).
| Compound | Q(B) | Q(μH) | Q(C) | Q(Ccarb) | Q(N) | WBO(B–μH) | WBO(C–B) | WBO(B–B) | WBO(C–Ccarb) |
|---|---|---|---|---|---|---|---|---|---|
| 5 | −0.10 | +0.05 | −0.73 | +0.57 | −0.35 | 0.49 | 0.83 | 0.29 | 1.08 |
| 6 | −0.10 | +0.03 | −0.75 | +0.69 | −0.42 | 0.50 | 0.81 | 0.29 | 1.10 |
| 7 | +0.11 | +0.13 | −0.78 | +0.53 | −0.33 | 0.46 | 0.91 | 0.64 | 1.05 |
| 8 | +0.11 | +0.13 | −0.80 | +0.66 | −0.39 | 0.46 | 0.90 | 0.65 | 1.05 |
| 9 | −0.08 | — | −0.74 | +0.69 | −0.42 | — | 0.78 | — | 1.11 |
The reaction of 3 and 4 with HNTf2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio cleanly yields di-cationic hydrido boron compounds 7 and 8 (Scheme 3) as white solids. Compounds 7 and 8 are rather poorly soluble in CH2Cl2 and 1,2-Cl2C6H4 but are freely dissolved in acetonitrile. The 11B NMR spectrum of each of 7 (−19.89) and 8 (−19.78) exhibits a broad resonance, which is ca. 10 ppm downfield compared to that of 3 and 4. The molecular structure of 8 is shown in Fig. 2. The BH2BH2 core features two 3c–2e bonds with the B⋯B distance of 1.755 Å. This is actually similar to B⋯B distances in cationic (NHC)BH2
1 molar ratio cleanly yields di-cationic hydrido boron compounds 7 and 8 (Scheme 3) as white solids. Compounds 7 and 8 are rather poorly soluble in CH2Cl2 and 1,2-Cl2C6H4 but are freely dissolved in acetonitrile. The 11B NMR spectrum of each of 7 (−19.89) and 8 (−19.78) exhibits a broad resonance, which is ca. 10 ppm downfield compared to that of 3 and 4. The molecular structure of 8 is shown in Fig. 2. The BH2BH2 core features two 3c–2e bonds with the B⋯B distance of 1.755 Å. This is actually similar to B⋯B distances in cationic (NHC)BH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6b as well as in neutral RBH2 dimers.23 Similarly, the B⋯B distance in 8 is consistent with that of the parent B2H6 determined by electron diffraction (1.77 Å)24 or X-ray methods (1.78–1.79 Å).25
6b as well as in neutral RBH2 dimers.23 Similarly, the B⋯B distance in 8 is consistent with that of the parent B2H6 determined by electron diffraction (1.77 Å)24 or X-ray methods (1.78–1.79 Å).25
The exact location of the B–H hydrogen is uncertain due to the experimental restrictions. Nevertheless, DFT(M06-2X/def2-SVP) optimized structures present a reasonable agreement with the experimental structure (Fig. 2). The theoretically predicted B–H bond lengths are longer than the experimental values, which is a feature commonly observed between solid-state and theoretical structures which refer to isolated molecules.26 The experimentally observed B–B distances are well represented by DFT calculations. The short B⋯B distances possibly indicate a weak bond. In fact, the B–B Wiberg Bond Indices (WBO in Table 1) are 0.64 au and 0.65 au for compounds 7 and 8, respectively. The increase in the bond order comes from the significantly shorter B–B distances in the di-cations. In this case, NBO calculations revealed the occurrence of two B–H–B 3c–2e bonds in the HB(μ-H)2BH moiety where 43% is located at the H bridges and ∼28% at each boron atom (Tables S4 and S5‡). The NHO ligands have a C–B σ-bond where the polarization towards carbon is slightly lower (65% for 7 and 8) than that in 5 and 6. The calculated charge distribution for the latter di-cationic species suggests that a positive charge of roughly +1.5 e resides at the NHO ligands. The somewhat counter intuitive charge at the boron atom can be rationalized in terms of the donor–acceptor bonding model27 which has successfully been used to explain the structure of boron compounds3m,28 and to predict new boron molecules with unusual bonding situations.3k,3o,29 Compounds 5–8 may be formally considered as complexes where a charged central boron fragment (B2H5+ in the mono-cations and B2H42+ in the di-cations) serves as an acceptor and the NHOs serve as donors, NHO → B2Hnq ← NHO. The strong charge donation leads to negative charges at boron in the cations 5 and 6 and to rather small positive charges in the di-cations 7 and 8. A negative partial charge was previously found at the BH2+ fragment in the cations (L → BH2 ← L]+.3m
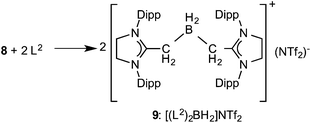
Treatment of 8 with two equivalents of the NHO (L2) leads to the clean formation of a boronium compound [(L2)2BH2][NTf2] (9) (Scheme 4). The 11B NMR spectrum of 9 shows a broad signal at δ −23.8 ppm. While hydrogen atoms of the BH2 group could not be located, the 1H NMR spectrum of compound 9 exhibits a remarkably up-field signal (δ 0.34 ppm) for methylene (CH2BH2) protons, which has been confirmed by a 1H–13C-HSQC (heteronuclear single quantum coherence) experiment. Colourless crystals of 9 were obtained from a solution of dichloromethane/n-hexane (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature. The molecular structure of 9 features a CH2BH2CH2 moiety with the C–B–C angle of 109.22° (Fig. 3). The C–B bond distance of 1.69 Å is comparable to that of 8.
1) at room temperature. The molecular structure of 9 features a CH2BH2CH2 moiety with the C–B–C angle of 109.22° (Fig. 3). The C–B bond distance of 1.69 Å is comparable to that of 8.
Compounds 3–9 exhibit characteristic absorption bands for the terminal ν(B–H) stretching vibrations from 2230 to 2463 cm−1. IR absorption bands in the 1561–1594 cm−1 region may be assigned for the bridging ν(B–H) vibrations.30
Experimental
All syntheses and manipulations were carried out under an inert atmosphere of dry argon or nitrogen gas using Schlenk line techniques and a glove box. CD2Cl2, CH2Cl2, o-Cl2C6D4 and o-Cl2C6H4 (over CaH2), C6D6 and THF (over K-benzophenone ketyl) were dried and distilled under a dry argon atmosphere prior to use. All other solvents were dried and purified by using a MBRAUN solvent purification system (MB SPS 800). 1H, 11B and 13C NMR spectra were recorded using a Bruker Avance III 300 or a Bruker Avance DRX 500 spectrometer. ESI mass spectra were recorded with a Bruker micrOTOF or a Bruker maXis spectrometer. Melting points were measured with a Büchi Melting Point B-540 apparatus. Elemental analyses were performed at the Institute for Inorganic Chemistry, Universität Göttingen. (THF)BH3 (Aldrich) and HNTf2 (Aldrich) were used without further purification. SIPrCH2 (L1) (1) and IPrCH2 (L2) (2) were prepared by adopting the reported methods.14b,31Synthesis and characterization of compounds 3–9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. Mp.: 201 °C. MS (ESI, m/z [M]): 415.33 [M − H]+, 416.33 [M]+. Elemental analysis for C28H41N2B (416): C 80.75, H 9.92, N 6.73; found C 80.44, H 9.85, N 6.68. IR (cm−1): 2960, 2871, 2322, 2258, 2230, 1560. 1H NMR (300 MHz, CD2Cl2, 25 °C): δ 1.16 (d, 12H, J = 6.8 Hz, HCMe2); 1.36 (d, 12H, J = 6.8 Hz, HCMe2); 1.60 (br, 2H, CCH2); 2.70 (sept, 4H, J = 6.8 Hz, HCMe2); 6.98 (s, 2H, NCH); 7.36 (d, 4H, J = 7.9 Hz, m-C6H3); 7.56 (t, 2H, J = 7.7 Hz, p-C6H3). 13C{1H} NMR (75 MHz, CD2Cl2, 25 °C): δ 16.55 (CCH2); 22.97, 26.02 (HCMe2); 29.49 (HCMe2); 121.55 (NCH); 125.13 (m-C6H3); 131.50, 131.98 (p-C6H3, o-C6H3); 146.66 (ipso-C6H3); 165.41 (CCH2) ppm. B{1H}NMR (96 MHz, CD2Cl2, 25 °C): δ −29.89 ppm. 11B NMR (160 MHz, CD2Cl2, 25 °C): δ −29.89 (q, JB–H = 85.43 Hz) ppm.
1) solution. Mp.: 201 °C. MS (ESI, m/z [M]): 415.33 [M − H]+, 416.33 [M]+. Elemental analysis for C28H41N2B (416): C 80.75, H 9.92, N 6.73; found C 80.44, H 9.85, N 6.68. IR (cm−1): 2960, 2871, 2322, 2258, 2230, 1560. 1H NMR (300 MHz, CD2Cl2, 25 °C): δ 1.16 (d, 12H, J = 6.8 Hz, HCMe2); 1.36 (d, 12H, J = 6.8 Hz, HCMe2); 1.60 (br, 2H, CCH2); 2.70 (sept, 4H, J = 6.8 Hz, HCMe2); 6.98 (s, 2H, NCH); 7.36 (d, 4H, J = 7.9 Hz, m-C6H3); 7.56 (t, 2H, J = 7.7 Hz, p-C6H3). 13C{1H} NMR (75 MHz, CD2Cl2, 25 °C): δ 16.55 (CCH2); 22.97, 26.02 (HCMe2); 29.49 (HCMe2); 121.55 (NCH); 125.13 (m-C6H3); 131.50, 131.98 (p-C6H3, o-C6H3); 146.66 (ipso-C6H3); 165.41 (CCH2) ppm. B{1H}NMR (96 MHz, CD2Cl2, 25 °C): δ −29.89 ppm. 11B NMR (160 MHz, CD2Cl2, 25 °C): δ −29.89 (q, JB–H = 85.43 Hz) ppm.
Crystallographic details
Suitable single crystals were selected from the mother liquor under Schlenk conditions and covered with perfluorinated polyether oil on a microscope slide, which was cooled under a nitrogen gas flow using the X-Temp2 device.32 The diffraction data of compounds 5, 8 and 9 were collected at 100 K on a Bruker D8 three circle diffractometer, equipped with a SMART APEX II CCD detector and an INCOATEC microfocus source (Ag Kα radiation) with INCOATEC Quazar mirror optics (Table 2). The diffraction data of compound 4 were collected at 100 K on a Bruker D8 three-circle diffractometer, equipped with a SMART APEX II CCD detector and an INCOATEC microfocus source (Mo Kα radiation) with INCOATEC Quazar mirror optics. The data were integrated with SAINT33 and a multi-scan absorption correction with SADABS34 was applied. The structure solution was performed with SHELXT35 and structure refinement was performed with SHELXL,36 using the graphical user interface SHELXLE.37 All non-hydrogen atoms were refined with anisotropic displacement parameters. All hydrogen atoms, except those bound to boron atoms, were assigned to ideal positions and refined using a riding model with Uiso constrained to 1.2 (1.5) times the Ueq value of the parent carbon atom. The positions of boron bound hydrogen atoms were found by difference Fourier analysis and the positions were refined.| Compound | 4 | 5 | 8 | 9 |
|---|---|---|---|---|
| CCDC number | 1401734 | 1060035 | 1060034 | 1060033 |
| Empirical formula | C28H43BN2 | C58H81B2F6N5O4S2 | C60.66H85.32B2Cl1.32F12N6O8S4 | C67H89.50BF7.50N5O4S2 |
| Formula weight [g mol−1] | 418.45 | 1112.01 | 1451.24 | 1246.36 |
| Temperature [K] | 100(2) | 100(2) | 100(2) | 100(2) |
| Wavelength [Å] | 0.71073 | 0.56086 | 0.56086 | 0.56086 |
| Crystal system, space group | Monoclinic, P21/c | Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| Unit cell dimensions [Å] | a = 11.624(2) | a = 10.754(2) | a = 12.306(2) | a = 13.061(3) |
| b = 15.921(3) | b = 16.407(2) | b = 12.691(2) | b = 15.613(4) | |
| c = 14.028(2) | c = 18.510(2) | c = 13.389(2) | c = 16.727(4) | |
| α [°] | 90 | 83.78(2) | 95.14(2) | 96.88(2) |
| β [°] | 99.23(2) | 73.47(2) | 101.53(2) | 101.45(2) |
| γ [°] | 90 | 83.41(2) | 118.07(2) | 98.29(2) |
| Volume [Å3] | 2562.5(8) | 3100.4(4) | 1766.6(6) | 3269.2(14) |
| Z | 4 | 2 | 1 | 2 |
| Absorption coefficient [mm−1] | 0.062 | 0.085 | 0.146 | 0.088 |
| F(000) | 920 | 1184 | 760 | 1326 |
| Crystal size [mm3] | 0.100 × 0.100 × 0.100 | 0.195 × 0.194 × 0.104 | 0.317 × 0.301 × 0.110 | 0.180 × 0.153 × 0.079 |
| Theta range for data collection [°] | 1.775 to 27.887 | 1.289 to 22.073 | 2.508 to 20.125 | 1.739 to 19.601 |
| Reflections collected/unique | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 574/6095 574/6095 |
238![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 549/15 549/15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 767/6769 767/6769 |
161![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683/11 683/11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 676 676 |
| R int | 0.0369 | 0.0605 | 0.0554 | 0.0810 |
| Completeness | 99.9 | 100.0 | 99.8 | 99.6 |
| Max. and min. transmission | 0.7456 and 0.7110 | 0.7447 and 0.7196 | 0.4251 and 0.3948 | 0.7444 and 0.6314 |
| Data/restraints/parameters | 6095/3/300 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500/513/768 500/513/768 |
6769/1636/863 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 676/111/808 676/111/808 |
| Goodness-of-fit on F2 | 1.039 | 1.024 | 1.044 | 1.044 |
| Final R indices [I > 2σ(I)] | R 1 = 0.0446, wR2 = 0.1078 | R 1 = 0.0383, wR2 = 0.0901 | R 1 = 0.0745, wR2 = 0.2054 | R 1 = 0.0544, wR2 = 0.1442 |
| R indices (all data) | R 1 = 0.0631, wR2 = 0.1167 | R 1 = 0.0550, wR2 = 0.0999 | R 1 = 0.0956, wR2 = 0.2259 | R 1 = 0.0737, wR2 = 0.1589 |
| Largest diff. peak and hole (e A−3) | 0.280 and −0.214 | 0.334 and −0.440 | 0.785 and −0.449 | 0.746 and −0.565 |
Computational details
The geometries of compounds 5–9 have been optimized using the functional M06-2X20a combined with the def2-SVP basis set.20b Stationary points were located with the Berny algorithm38 using redundant coordinates. Analytical Hessians were computed to determinate the nature of the stationary points.39 All geometry optimizations were performed using the Gaussian 09 suite of programs.40 The NBO21,22,41 analyses have been carried out with the GENNBO 5.942 program at the M06-2X/def2-TZVPP level of theory.Conclusions
In conclusion, mono-cationic [{(L1)BH2}2(μ-H)](NTf2) (5) and [{(L2)BH2}2(μ-H)](NTf2) (6) and di-cationic [{(L1)BH}2(μ-H)2](NTf2)2 (7) and [{(L2)BH}2(μ-H)2](NTf2)2 (8) hydrido boron compounds are readily accessible by a hydride abstraction reaction of 3 and 4 with a commercially available Brønsted acid. Structure and bonding of these compounds featuring CH2BH2(μ-H)BH2CH2 (5 and 6) and CH2BH(μ-H)2BHCH2 (7 and 8) scaffolds have been analysed using experimental and theoretical methods. The NHO ligand forms a C–B σ-bond, where the polarization towards carbon is slightly lower (65% for 7 and 8) than that for 5 and 6. NBO calculations revealed the occurrence of two B–H–B 3c–2e bonds in the HB(μ-H)2BH moiety where 43% is located at the H bridges and ∼28% at each boron atom.Crystallographic data of compounds 4, 5, 8 and 9 have been deposited with the Cambridge Crystallographic Data Centre.
Acknowledgements
We are thankful to the Deutsche Forschungsgemeinschaft (DFG) (GH 129/4-1) for financial support.Notes and references
- (a) B. D. Rekken, T. M. Brown, J. C. Fettinger, H. M. Tuononen and P. P. Power, J. Am. Chem. Soc., 2012, 134, 6504–6507 CrossRef CAS PubMed; (b) P. P. Power, Nature, 2010, 463, 171–177 CrossRef CAS PubMed; (c) R. C. Fischer and P. P. Power, Chem. Rev., 2010, 110, 3877–3923 CrossRef CAS PubMed.
- (a) A. J. Lennox and G. C. Lloyd-Jones, Chem. Soc. Rev., 2014, 43, 412–443 RSC; (b) T. Banu, K. Sen, D. Ghosh, T. Debnath and A. K. Das, RSC Adv., 2014, 4, 1352–1361 RSC; (c) T. S. De Vries, A. Prokofjevs and E. Vedejs, Chem. Rev., 2012, 112, 4246–4282 CrossRef CAS PubMed; (d) M. Yamashita and K. Nozaki, in Synthesis and Application of Organoboron Compounds, ed. E. Fernández and A. Whiting, Springer International Publishing, 2015, vol. 49, ch. 1, pp. 1–37 Search PubMed; (e) Y. Segawa, M. Yamashita and K. Nozaki, Science, 2006, 314, 113–115 CrossRef CAS PubMed; (f) Contemporary Boron Chemistry, ed. M. G. Davidson, K. Wade, T. B. Marder and A. K. Hughes, The Royal Society of Chemistry, Cambridge, UK, 2000 Search PubMed.
- (a) H. Braunschweig, W. C. Ewing, K. Geetharani and M. Schafer, Angew. Chem., Int. Ed., 2015, 54, 1662–1665 CrossRef CAS PubMed; (b) P. Q. Huang and C. H. Lai, Comput. Theor. Chem., 2015, 1051, 17–23 CrossRef CAS PubMed; (c) R. Koppe and H. Schnockel, Chem. Sci., 2015, 6, 1199–1205 RSC; (d) F. Dahcheh, D. W. Stephan and G. Bertrand, Chem. – Eur. J., 2015, 21, 199–204 CrossRef CAS PubMed; (e) D. A. Ruiz, M. Melaimi and G. Bertrand, Chem. Commun., 2014, 50, 7837–7839 RSC; (f) P. Bissinger, H. Braunschweig, A. Damme, I. Krummenacher, A. K. Phukan, K. Radacki and S. Sugawara, Angew. Chem., Int. Ed., 2014, 53, 7360–7363 CrossRef CAS PubMed; (g) P. Bissinger, H. Braunschweig, A. Damme, T. Kupfer, I. Krummenacher and A. Vargas, Angew. Chem., Int. Ed., 2014, 53, 5689–5693 CrossRef CAS PubMed; (h) T. B. Tai and M. T. Nguyen, Angew. Chem., Int. Ed., 2013, 52, 4554–4557 CrossRef CAS PubMed; (i) D. A. Ruiz, G. Ung, M. Melaimi and G. Bertrand, Angew. Chem., Int. Ed., 2013, 52, 7590–7592 CrossRef CAS PubMed; (j) H. Braunschweig, T. Herbst, K. Radacki, C. W. Tate and A. Vargas, Chem. Commun., 2013, 49, 1702–1704 RSC; (k) H. Braunschweig, R. D. Dewhurst, K. Hammond, J. Mies, K. Radacki and A. Vargas, Science, 2012, 336, 1420–1422 CrossRef CAS PubMed; (l) G. Frenking and N. Holzmann, Science, 2012, 336, 1394–1395 CrossRef CAS PubMed; (m) M. A. Celik, R. Sure, S. Klein, R. Kinjo, G. Bertrand and G. Frenking, Chem. – Eur. J., 2012, 18, 5676–5692 CrossRef CAS PubMed; (n) H. Braunschweig, R. D. Dewhurst, K. Hammond, J. Mies, K. Radacki and A. Vargas, Science, 2012, 336, 1420–1422 CrossRef CAS PubMed; (o) R. Kinjo, B. Donnadieu, M. A. Celik, G. Frenking and G. Bertrand, Science, 2011, 333, 610–613 CrossRef CAS PubMed; (p) Z. Liu, J. Chem. Phys., 2010, 132 Search PubMed; (q) H. Braunschweig, C. W. Chiu, K. Radacki and T. Kupfer, Angew. Chem., Int. Ed., 2010, 49, 2041–2044 CrossRef CAS PubMed; (r) S. H. Ueng, A. Solovyev, X. Yuan, S. J. Geib, L. Fensterbank, E. Lacote, M. Malacria, M. Newcomb, J. C. Walton and D. P. Curran, J. Am. Chem. Soc., 2009, 131, 11256–11262 CrossRef CAS PubMed; (s) H. Braunschweig, Angew. Chem., Int. Ed., 2007, 46, 1946–1948 CrossRef CAS PubMed.
- (a) Y. Shoji, N. Tanaka, K. Mikami, M. Uchiyama and T. Fukushima, Nat. Chem., 2014, 6, 498–503 CrossRef CAS PubMed; (b) C. Reus and M. Wagner, Nat. Chem., 2014, 6, 466–467 CrossRef CAS PubMed; (c) S. Litters, E. Kaifer, M. Enders and H. J. Himmel, Nat. Chem., 2013, 5, 1029–1034 CrossRef CAS PubMed; (d) C. I. Someya, S. Inoue, C. Prasang, E. Irran and M. Driess, Chem. Commun., 2011, 47, 6599–6601 RSC; (e) D. P. Curran, A. Solovyev, M. Makhlouf Brahmi, L. Fensterbank, M. Malacria and E. Lacote, Angew. Chem., Int. Ed., 2011, 50, 10294–10317 CrossRef CAS PubMed; (f) W. E. Piers, S. C. Bourke and K. D. Conroy, Angew. Chem., Int. Ed., 2005, 44, 5016–5036 CrossRef CAS PubMed; (g) A. Prokofjevs and E. Vedejs, J. Am. Chem. Soc., 2011, 133, 20056–20059 CrossRef CAS PubMed; (h) B. Inés, M. Patil, J. Carreras, R. Goddard, W. Thiel and M. Alcarazo, Angew. Chem., Int. Ed., 2011, 50, 8400–8403 CrossRef PubMed; (i) J.-H. Tsai, S.-T. Lin, R. B.-G. Yang, G. P. A. Yap and T.-G. Ong, Organometallics, 2010, 29, 4004–4006 CrossRef CAS; (j) C. Bonnier, W. E. Piers, M. Parvez and T. S. Sorensen, Chem. Commun., 2008, 4593–4595 RSC.
- (a) J. M. Farrell, R. T. Posaratnanathan and D. W. Stephan, Chem. Sci., 2015, 6, 2010–2015 RSC; (b) M. Ingleson, in Synthesis and Application of Organoboron Compounds, ed. E. Fernández and A. Whiting, Springer International Publishing, 2015, vol. 49, ch. 2, pp. 39–71 Search PubMed; (c) Z. X. Wang, L. L. Zhao, G. Lu, H. X. Li and F. Huang, in Frustrated Lewis Pairs I: Uncovering and Understanding, 2013, vol. 332, pp. 231–266 Search PubMed; (d) A. Prokofjevs, A. Boussonniere, L. Li, H. Bonin, E. Lacote, D. P. Curran and E. Vedejs, J. Am. Chem. Soc., 2012, 134, 12281–12288 CrossRef CAS PubMed; (e) Y. Y. Xu, Z. Li, M. Borzov and W. L. Nie, Prog. Chem., 2012, 24, 1526–1532 CAS.
- (a) W. C. Chen, C. Y. Lee, B. C. Lin, Y. C. Hsu, J. S. Shen, C. P. Hsu, G. P. Yap and T. G. Ong, J. Am. Chem. Soc., 2014, 136, 914–917 CrossRef CAS PubMed; (b) A. Prokofjevs, J. W. Kampf, A. Solovyev, D. P. Curran and E. Vedejs, J. Am. Chem. Soc., 2013, 135, 15686–15689 CrossRef CAS PubMed; (c) H. Braunschweig, M. Kaupp, C. Lambert, D. Nowak, K. Radacki, S. Schinzel and K. Uttinger, Inorg. Chem., 2008, 47, 7456–7458 CrossRef CAS PubMed; (d) D. Vidovic, M. Findlater and A. H. Cowley, J. Am. Chem. Soc., 2007, 129, 8436–8437 CrossRef CAS PubMed; (e) R. Dinda, O. Ciobanu, H. Wadepohl, O. Hubner, R. Acharyya and H. J. Himmel, Angew. Chem., Int. Ed., 2007, 46, 9110–9113 CrossRef CAS PubMed; (f) I. Vargas-Baca, M. Findlater, A. Powell, K. V. Vasudevan and A. H. Cowley, Dalton Trans., 2008, 6421–6426 RSC.
- (a) M. Soleilhavoup and G. Bertrand, Acc. Chem. Res., 2015, 48, 256–266 CrossRef CAS PubMed; (b) F. Dahcheh, D. Martin, D. W. Stephan and G. Bertrand, Angew. Chem., Int. Ed., 2014, 53, 13159–13163 CrossRef CAS PubMed; (c) Y. Wang and G. H. Robinson, Inorg. Chem., 2014, 53, 11815–11832 CrossRef CAS PubMed; (d) J. Bohnke, H. Braunschweig, W. C. Ewing, C. Horl, T. Kramer, I. Krummenacher, J. Mies and A. Vargas, Angew. Chem., Int. Ed., 2014, 53, 9082–9085 CrossRef PubMed; (e) R. S. Ghadwal, R. Azhakar and H. W. Roesky, Acc. Chem. Res., 2013, 46, 444–456 CrossRef CAS PubMed; (f) Y. Xiong, S. L. Yao, S. Inoue, J. D. Epping and M. Driess, Angew. Chem., Int. Ed., 2013, 52, 7147–7150 CrossRef CAS PubMed; (g) Y. Xiong, S. L. Yao and M. Driess, Z. Naturforsch., B: Chem. Sci., 2013, 68, 445–452 CrossRef CAS PubMed; (h) C. D. Martin, M. Soleilhavoup and G. Bertrand, Chem. Sci., 2013, 4, 3020–3030 RSC; (i) T. Rovis and S. P. Nolan, Synlett, 2013, 1188–1189 CrossRef CAS; (j) D. Martin, M. Soleilhavoup and G. Bertrand, Chem. Sci., 2011, 2, 389–399 RSC; (k) Y. Xiong, S. Yao and M. Driess, J. Am. Chem. Soc., 2009, 131, 7562–7563 CrossRef CAS PubMed; (l) P. de Fremont, N. Marion and S. P. Nolan, Coord. Chem. Rev., 2009, 253, 862–892 CrossRef CAS PubMed; (m) C. A. Dyker and G. Bertrand, Science, 2008, 321, 1050–1051 CrossRef CAS PubMed; (n) S. Diez-Gonzalez and S. P. Nolan, Coord. Chem. Rev., 2007, 251, 874–883 CrossRef CAS PubMed; (o) R. S. Ghadwal, R. Azhakar, H. W. Roesky, K. Pröpper, B. Dittrich, C. Goedecke and G. Frenking, Chem. Commun., 2012, 48, 8186–8188 RSC; (p) R. S. Ghadwal, H. W. Roesky, C. Schulzke and M. Granitzka, Organometallics, 2010, 29, 6329–6333 CrossRef CAS; (q) R. S. Ghadwal, S. S. Sen, H. W. Roesky, M. Granitzka, D. Kratzert, S. Merkel and D. Stalke, Angew. Chem., Int. Ed., 2010, 49, 3952–3955 CrossRef CAS PubMed; (r) R. S. Ghadwal, H. W. Roesky, S. Merkel, J. Henn and D. Stalke, Angew. Chem., Int. Ed., 2009, 48, 5683–5686 CrossRef CAS PubMed.
- (a) J. C. Walton, M. M. Brahmi, J. Monot, L. Fensterbank, M. Malacria, D. P. Curran and E. Lacote, J. Am. Chem. Soc., 2011, 133, 10312–10321 CAS; (b) J. C. Walton, M. M. Brahmi, L. Fensterbank, E. Lacote, M. Malacria, Q. Chu, S. H. Ueng, A. Solovyev and D. P. Curran, J. Am. Chem. Soc., 2010, 132, 2350–2358 CrossRef CAS PubMed; (c) S. H. Ueng, M. M. Brahmi, E. Derat, L. Fensterbank, E. Lacote, M. Malacria and D. P. Curran, J. Am. Chem. Soc., 2008, 130, 10082–10083 CrossRef CAS PubMed.
- (a) P. Eisenberger, B. P. Bestvater, E. C. Keske and C. M. Crudden, Angew. Chem., Int. Ed., 2015, 54, 2467–2471 CrossRef CAS PubMed; (b) T. Matsumoto and F. P. Gabbaï, Organometallics, 2009, 28, 4252–4253 CrossRef CAS.
- (a) J. Monot, A. Solovyev, H. Bonin-Dubarle, E. Derat, D. P. Curran, M. Robert, L. Fensterbank, M. Malacria and E. Lacote, Angew. Chem., Int. Ed., 2010, 49, 9166–9169 CrossRef CAS PubMed; (b) H. Braunschweig, C. W. Chiu, K. Radacki and T. Kupfer, Angew. Chem., Int. Ed., 2010, 49, 2041–2044 CrossRef CAS PubMed.
- L. Kong, Y. Li, R. Ganguly, D. Vidovic and R. Kinjo, Angew. Chem., Int. Ed., 2014, 53, 9280–9283 CrossRef CAS PubMed.
- (a) H. Braunschweig and C. Horl, Chem. Commun., 2014, 50, 10983–10985 RSC; (b) P. Bissinger, H. Braunschweig, A. Damme, T. Kupfer and A. Vargas, Angew. Chem., Int. Ed., 2012, 51, 9931–9934 CrossRef CAS PubMed; (c) Y. Wang, B. Quillian, P. Wei, C. S. Wannere, Y. Xie, R. B. King, H. F. Schaefer III, P. v. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2007, 129, 12412–12413 CrossRef CAS PubMed.
- Y. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schaefer III, P. v. R. Schleyer and G. H. Robinson, Science, 2008, 321, 1069–1071 CrossRef CAS PubMed.
- (a) R. S. Ghadwal, S. O. Reichmann and R. Herbst-Irmer, Chem. – Eur. J., 2015, 21, 4247–4251 CrossRef CAS PubMed; (b) R. S. Ghadwal, C. J. Schürmann, F. Engelhardt and C. Steinmetzger, Eur. J. Inorg. Chem., 2014, 2014, 4921–4926 CrossRef CAS PubMed.
- (a) N. Kuhn, H. Bohnen, J. Kreutzberg, D. Blaser and R. Boese, Chem. Commun., 1993, 1136–1137 RSC; (b) N. Kuhn, H. Bohnen, G. Henkel and J. Kreutzberg, Z. Naturforsch., B: Chem. Sci., 1996, 51, 1267–1278 CrossRef.
- (a) E. Rivard, Dalton Trans., 2014, 43, 8577–8586 RSC; (b) S. Kronig, P. G. Jones and M. Tamm, Eur. J. Inorg. Chem., 2013, 2013, 2301–2314 CrossRef CAS PubMed; (c) C. J. Berger, G. He, C. Merten, R. McDonald, M. J. Ferguson and E. Rivard, Inorg. Chem., 2014, 53, 1475–1486 CrossRef CAS PubMed; (d) Y. Wang, M. Y. Abraham, R. J. Gilliard, Jr., D. R. Sexton, P. Wei and G. H. Robinson, Organometallics, 2013, 32, 6639–6642 CrossRef CAS; (e) A. C. Malcolm, K. J. Sabourin, R. McDonald, M. J. Ferguson and E. Rivard, Inorg. Chem., 2012, 51, 12905–12916 CrossRef CAS PubMed.
- (a) B. Maji, M. Breugst and H. Mayr, Angew. Chem., Int. Ed., 2011, 50, 6915–6919 CrossRef CAS PubMed; (b) B. Maji and H. Mayr, Angew. Chem., Int. Ed., 2012, 51, 10408–10412 CrossRef CAS PubMed; (c) B. Maji, M. Horn and H. Mayr, Angew. Chem., Int. Ed., 2012, 51, 6231–6235 CrossRef CAS PubMed.
- R. S. Ghadwal, S. O. Reichmann, F. Engelhardt, D. M. Andrada and G. Frenking, Chem. Commun., 2013, 49, 9440–9442 RSC.
- A. O. Madsen, H. O. Sorensen, C. Flensburg, R. F. Stewart and S. Larsen, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2004, 60, 550–561 Search PubMed.
- (a) Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS; (b) F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS PubMed.
- (a) A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS; (b) A. E. Reed and F. Weinhold, J. Chem. Phys., 1985, 83, 1736–1740 CrossRef CAS PubMed.
- (a) R. J. Wehmschulte, A. A. Diaz and M. A. Khan, Organometallics, 2003, 22, 83–92 CrossRef CAS; (b) U. D. Eckensberger, M. Weber, J. Wildt, M. Bolte, H.-W. Lerner and M. Wagner, Organometallics, 2010, 29, 5301–5309 CrossRef CAS; (c) Y. Shoji, T. Matsuo, D. Hashizume, H. Fueno, K. Tanaka and K. Tamao, J. Am. Chem. Soc., 2010, 132, 8258–8260 CrossRef CAS PubMed.
- K. Hedberg and V. Schomaker, J. Am. Chem. Soc., 1951, 73, 1482–1487 CrossRef CAS.
- H. W. Smith and W. N. Lipscomb, J. Chem. Phys., 1965, 43, 1060–1064 CrossRef CAS PubMed.
- V. Jonas, G. Frenking and M. T. Reetz, J. Am. Chem. Soc., 1994, 116, 8741–8753 CrossRef CAS.
- G. Frenking, Angew. Chem., Int. Ed., 2014, 53, 6040–6046 CrossRef CAS PubMed.
- N. Holzmann, M. Hermann and G. Frenking, Chem. Sci., 2015, 6, 4089–4094 RSC.
- (a) N. Holzmann, A. Stasch, C. Jones and G. Frenking, Chem. – Eur. J., 2011, 17, 13517–13525 CrossRef CAS PubMed; (b) G. Frenking, Nature, 2015, 522, 297–298 CrossRef CAS PubMed; (c) H. Braunschweig, R. D. Dewhurst, F. Hupp, M. Nutz, K. Radacki, C. W. Tate, A. Vargas and Q. Ye, Nature, 2015, 522, 327–330 CrossRef CAS PubMed.
- O. Thomas, Organoborane Chemistry, Academic Press Inc. (London) Ltd, New York, 1975 Search PubMed.
- S. M. I. Al-Rafia, A. C. Malcolm, S. K. Liew, M. J. Ferguson, R. McDonald and E. Rivard, Chem. Commun., 2011, 47, 6987–6989 RSC.
- (a) D. Stalke, Chem. Soc. Rev., 1998, 27, 171–178 RSC; (b) T. Kottke and D. Stalke, J. Appl. Crystallogr., 1993, 26, 615–619 CrossRef.
- Bruker AXS Inc., Bruker Apex CCD, SAINT v8.30C, Bruker AXS Inst. Inc., Madison, WI, USA, 2013 Search PubMed.
- L. Krause, R. Herbst-Irmer, G. M. Sheldrick and D. Stalke, J. Appl. Crystallogr., 2015, 48, 3–10 CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2015, 71, 3–8 CrossRef PubMed.
- C. B. Huebschle, G. M. Sheldrick and B. Dittrich, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2011, 67, C592 Search PubMed.
- C. Y. Peng, P. Y. Ayala, H. B. Schlegel and M. J. Frisch, J. Comput. Chem., 1996, 17, 49–56 CrossRef CAS.
- J. W. McIver and A. Komornicki, J. Am. Chem. Soc., 1972, 94, 2625–2633 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- J. P. Foster and F. Weinhold, J. Am. Chem. Soc., 1980, 102, 7211–7218 CrossRef CAS.
- E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales and F. Weinhold, GENNBO 5.9 ver, Theoretical Chemistry Institute, University of Wisconsin, Madison, WI, 2009 Search PubMed.
Footnotes |
| † This paper is dedicated to Professor Manfred Scheer on the occasion of his 60th birthday. |
| ‡ Electronic supplementary information (ESI) available: Crystallographic data and computational details. CCDC 1401734 (4), 1060035 (5), 1060034 (8) and 1060033 (9). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt02237a |
| This journal is © The Royal Society of Chemistry 2015 |