Reducing zirconium(iv) phthalocyanines and the structure of a Pc4−Zr complex†
Abstract
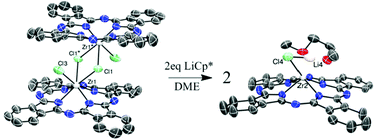
The synthesis and characterization of the ring-unsubstituted zirconium phthalocyanine PcZrCl2 (1; Pcx− = phthalocyaninatox−) and its reduction products are described. X-ray analysis of 1 (crystallized from hot 1-chloronaphthalene) reveals that 1 is a chloride-bridged dimer [PcZrCl]2(μ-Cl)2 in the solid-state; 1 was also characterized by UV-vis/MCD spectroscopy and cyclic voltammetry, which indicated reduction potentials at −0.55, −0.95 and −1.28 V. Although attempts to access these Pc-ring reduced species with KC8 led to mixtures of reduced products due to the insolubility of both starting materials, one equivalent of the reducing agent KEt3BH reacted with 1 to generate Pc3−-containing species, as indicated by visible Q-band spectral changes (from λmax = 686 for 1 to 589/611 nm), a single ESR peak (g = 2.001) and paramagnetically shifted 1H NMR resonances consistent with the presence of a Pc-radical anion. Addition of two equivalents of KEt3BH to 1 generated Pc4−-containing species, confirmed by a shift in λmax to 522 nm and upfield-shifted 1H NMR peaks relative to 1. Reaction of 1 with one and two equivalents of LiCp* did not generate Cp*-substituted products but also effected reduction to analogous Pc3− and Pc4− species. This latter material, the air-sensitive ring di-reduced “ate”-complex Pc4−Zr(LiCl)1.5(DME)3, of the form [LiCl(DME)4]0.5[Pc4−ZrClLi(DME)] was structurally characterized, illustrating partial bond localization in the Pc4− ring, which also adopts a saddle-shape vs. the more typical dome-configuration found in 1. This represents a rare example of an isolated and structurally characterized Pc4− complex.


 Please wait while we load your content...
Please wait while we load your content...