Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Utilization of mixtures of aromatic N-donor ligands of different coordination ability for the solvothermal synthesis of thiostannate containing molecules†
J.
Hilbert
,
C.
Näther
and
W.
Bensch
*
Institute of Inorganic Chemistry, Christian-Albrechts-University of Kiel, Max-Eyth-Str. 2, 24118 Kiel, Germany. E-mail: wbensch@ac.uni-kiel.de; Fax: +49 431 880–1520; Tel: +49 431 880–2419
First published on 26th May 2015
Abstract
Utilization of mixtures of differently coordinating aromatic N-donor ligands leads to the formation of the two new compounds {[Ni(phen)2]2Sn2S6}·4,4′-bipy·½H2O I and {[Ni(phen)2]2Sn2S6}·2,2′-bipy II that could be prepared under solvothermal conditions (4,4′-bipy = 4,4′-bipyridine, C10H8N2; phen = 1,10-phenanthroline, C12H8N2; 2,2′-bipy = 2,2′-bipyridine, C10H8N2). In the structures of both compounds Ni–S bond formation is observed which is highly unusual when only bidentate N-donor ligands are applied in the reaction mixture. The detailed analysis of the crystal structure indicates that the presence of 4,4′-bipy and 2,2′-bipy molecules are essential for the stabilization of the arrangement of the constituents. The main structural motif {[Ni(phen)2]2Sn2S6} is arranged generating off center parallel stacking of the phen ligands. The empty spaces between the {[Ni(phen)2]2Sn2S6} moieties are occupied by either 2,2′-bipy (I) or 4,4′-bipy (II) molecules which are oriented towards the phen ligands to form intermolecular π–π interactions.
1 Introduction
Compounds containing thiostannate building blocks may be classified according to different criteria. One group of thiostannates are pure inorganic compounds containing e.g. metal cations and thiostannate anions like in Na4Sn2S6·14H2O,1 Rb6Sn2S7,2 Cs2Sn4S9,3 Cs2Sn2S6 or K2Sn2S5,4 (Rb4(H2O)4)[SnS4],5 K10M4Sn4S17 (M = Mn, Fe, Co, Zn)6 and (NH4)4[Sn2S6]·3H2O7 to mention just a few. In these compounds the cations and anions are held together by mainly electrostatic interactions. The examples demonstrate the structural variability with thiostannate ions displaying different Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratios and therefore different structural motifs. Another group of compounds is composed of organic cations and thiostannate anions, like e.g. (TMA)[Sn3S7]·2H2O (TMA = tetramethylammonium),8 (DABCOH)2[Sn3S7]·H2O (DABCO = 1,4-diazabicyclo[2.2.2]octane),9 (enH)4[Sn2S6] (en = ethylenediamine),10 or (tmdpH2)[Sn3S7] (tmdp = 4,4′-trimethylenedipiperidine).11 Charge compensation may be also achieved by transition metal or lanthanoide cation centered complexes like in (M(en)3)2[Sn2S6] (M = Mn, Co, Ni, Zn),12–14 (M(dien)2)2[Sn2S6] (M = Mn, Co, Ni; dien = diethylentriamine),14–16 (Nd(dien)3)2[Sn2S6]Cl2, (Nd(dien)3)2[Sn2S6](SH)2,17 (Y2(dien)2(OH)2)[Sn2S6],18 (Ni(1,2-dap)3)2[Sn2S6]·2H2O (1,2-dap = 1,2-diaminopropane),12 (Ni(1,2-dach)3)2[Sn2S6]·4H2O (1,2-dach = 1,2-diaminocyclohexane), (Ni(peha))2[Sn2S6]·H2O (peha = pentaethylenehexamine) or (Ni(aepa)2)2[Sn2S6] (aepa = N-2-aminoethyl-1,3-propandiamine).16
S ratios and therefore different structural motifs. Another group of compounds is composed of organic cations and thiostannate anions, like e.g. (TMA)[Sn3S7]·2H2O (TMA = tetramethylammonium),8 (DABCOH)2[Sn3S7]·H2O (DABCO = 1,4-diazabicyclo[2.2.2]octane),9 (enH)4[Sn2S6] (en = ethylenediamine),10 or (tmdpH2)[Sn3S7] (tmdp = 4,4′-trimethylenedipiperidine).11 Charge compensation may be also achieved by transition metal or lanthanoide cation centered complexes like in (M(en)3)2[Sn2S6] (M = Mn, Co, Ni, Zn),12–14 (M(dien)2)2[Sn2S6] (M = Mn, Co, Ni; dien = diethylentriamine),14–16 (Nd(dien)3)2[Sn2S6]Cl2, (Nd(dien)3)2[Sn2S6](SH)2,17 (Y2(dien)2(OH)2)[Sn2S6],18 (Ni(1,2-dap)3)2[Sn2S6]·2H2O (1,2-dap = 1,2-diaminopropane),12 (Ni(1,2-dach)3)2[Sn2S6]·4H2O (1,2-dach = 1,2-diaminocyclohexane), (Ni(peha))2[Sn2S6]·H2O (peha = pentaethylenehexamine) or (Ni(aepa)2)2[Sn2S6] (aepa = N-2-aminoethyl-1,3-propandiamine).16
Another class of compounds is characterized by M–S bonds between the thiostannate ion and transition metal cations which are further surrounded by N donor atoms from amine ligands. In such compounds the [Sn2S6]4− and [SnS4]4− anion, respectively, acts as bidentate ligand like in {[Mn(1,2-dach)2(H2O)]2[Sn2S6]},19 {[Mn(en)2]2[Sn2S6]}n,20 {[Mn(1,2-dap)2]2[Sn2S6]}n,21 {[M(tepa)]2[Sn2S6]} (M = Mn, Fe, Co, Ni; tepa = tetraethylenepentamine),22,23 {[Mn(trien)]2[SnS4]}n·4nH2O (trien = triethylenetetramine),24 {[M(tren)]2[Sn2S6]}12,25 (M = Mn, Co; tren = tris(2-aminoethyl-)amine), {[Co(cyclam)]2[Sn2S6]}n·2nH2O26 (cyclam = 1,4,8,11-tetraaza-cyclotetradecane), o-{[Ni(tepa)]2[Sn2S6]}, {[Mn(trien)]2[SnS4]}n16 or in a tetradentate fashion as observed for e.g. {[Mn(phen)2]2[Sn2S6]},27 {[TM(phen)2]2[Sn2S6]}28 (M = Fe, Co) or {[Mn(phen)]2[SnS4]}n·nH2O.29
Finally metal cations may be integrated into the thiostannate unit as observed for (1,4-dabH)2MnSnS4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (1,4-dab = 1,4-diaminobutane), (DBUH)CuSnS3 (DBU = 1,8-diaza-bicyclo[5.4.0]undec-7-ene) and (1,4-dabH2)Cu2SnS4,31 (1,4-dabH2)Ag2SnS4,32 (enH)3Cu7Sn4S12,33 (DBNH)2Cu6Sn2S8
30 (1,4-dab = 1,4-diaminobutane), (DBUH)CuSnS3 (DBU = 1,8-diaza-bicyclo[5.4.0]undec-7-ene) and (1,4-dabH2)Cu2SnS4,31 (1,4-dabH2)Ag2SnS4,32 (enH)3Cu7Sn4S12,33 (DBNH)2Cu6Sn2S8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 (DBN = 1,5-diazabicyclo[4.3.0]non-5-ene), (dienH2)Cu2Sn2S6,35 (NH4)2Ag6Sn3S10,36 (enH2)Ag2SnS4,37 (enH2)HgSnS4,38 (enH2)2Cu8Sn3S12
34 (DBN = 1,5-diazabicyclo[4.3.0]non-5-ene), (dienH2)Cu2Sn2S6,35 (NH4)2Ag6Sn3S10,36 (enH2)Ag2SnS4,37 (enH2)HgSnS4,38 (enH2)2Cu8Sn3S12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 and [Mn(dien)2]MnSnS4.21
39 and [Mn(dien)2]MnSnS4.21
Many of the above-mentioned compounds were obtained under solvothermal conditions and state-of-art of the chemistry of thiostannates and other thiometallates was reviewed in several articles highlighting the synthetic approaches, the structural variability and flexibility of the anions.40 Analyzing the structures of thiometallate compounds with transition metal cations having bonds to S atoms of the anions, the Mn2+ ion can be easily integrated in the anions independent from the amine applied, whereas Fe2+, Co2+, Ni2+, or Zn2+ prefer bond formation to the N atoms of the amine molecules and M–S bond formation must be forced by applying multidentate amine molecules (tetra- or pentadentate) in the synthesis mixture which do not satisfy the coordination requirements of the cations. Mn2+ seems to have a comparable affinity to both S and N atoms as can be seen from the thiostannate examples presented above but a similar observation was also made for e.g. thioantimonates.41
During a systematic study of the Mn/phen/Sn/S system we prepared {[Mn(phen)2]2(μ2-Sn2S6)}·phen and {[Mn(phen)2]2(μ2-Sn2S6)}·phen·H2O containing co-crystallized phen molecules which are arranged with respect to phen ligands of the Mn2+ centered complexes to achieve attractive so-called π–π interactions.27 The energy involved in these interactions is between ca. 10 and 13 kcal mol−1.42–45 While the interaction energy between stacked phen molecules is low compared to that of covalent or ionic bonds, it seems to be large enough to stabilize frequently observed arrangements of aromatic molecules with respect to each other, like e.g. face-to-face, off-center, slipped or edge-to-face. Modifying the synthesis conditions originally applied for the preparation of {[Mn(phen)2]2(μ2-Sn2S6)}·phen and {[Mn(phen)2]2(μ2-Sn2S6)}·phen·H2O and using Co, Fe instead of Mn we were able to crystallize {[TM(phen)2]2Sn2S6}·phen·H2O (TM = Co, Fe) with similar arrangements of the phen molecules found for the analogous Mn compound.28 This was a surprising result because Co2+/Fe2+ have a strong preference for N donor atoms yielding normally isolated [TM(L)n]2+ (L = bidentate or tridentate ligands) complexes and thiometallate anions.13,16,46–49 All thiometallate compounds displaying a Co–S/Fe–S bond were obtained in the presence of a tetradentate or pentadentate ligand like tren,12,48–50 tepa23 and cyclam,26 with one exception where 1,2-dach (1,2-dach = 1,2 diaminocyclohexane) was used as amine.51
In our ongoing synthetic work all attempts to synthesize the analogous Ni2+ containing compounds failed despite varying the reaction conditions reported in.27–29 One possible reason is the high stability of the [Ni(phen)3]2+ complex, which is in situ formed under the solvothermal reaction conditions. Hence, we developed a new synthetic strategy applying mixtures of aromatic N-donor ligands which are either strong (phen), medium (2,2′-bipyridine), weak/monodentate coordinating (4,4′-bipyridine) which should lead to the solely coordination of Ni2+ by phen and the other molecule-donor ligands might act as structural stabilizers via intermolecular π–π-interactions.
During the experiments we were able to prepare and characterize the two new compounds {[Ni(phen)2]2Sn2S6}·4,4′-bipy·½H2O (I) and {[Ni(phen)2]2Sn2S6}·2,2′-bipy (II). Here we report the syntheses, crystal structures and spectroscopic data of these compounds.
2 Results and discussion
Synthetic aspects
For the synthesis of thiometallates applying elemental Sn and S a very weak coordinating amine like methylamine is necessary which generates the basic conditions for production of polysulfide anions which attack Sn to form the thiostannate ion.In our previous investigations of the TM/Sn/S/phen systems (TM = Mn, Fe, Co, Ni) we demonstrated that Mn2+ easily forms bonds to thiostannate anions and five compounds with compositions {[Mn(phen)2]2Sn2S6}, {[Mn(phen)2]2Sn2S6}·phen, {[Mn(phen)2]2Sn2S6}·phen·H2O and {[Mn(phen)2]2[SnS4]2[Mn(phen)]2}·H2O could be prepared of which four were accessible even under stirring conditions.27 The syntheses with Fe and Co yielded only two compounds ({[M(phen)2]2Sn2S6} and {[M(phen)2]2Sn2S6}·phen·H2O, M = Fe, Co) and only under static conditions.28 Applying the synthesis conditions used for TM = Mn, Fe, and Co no compounds were accessible with Ni and the reaction products only contained the crystallized [Ni(phen)3]2+ complex (counter ion: Cl−) and X-ray amorphous sulfides. One reason may be the differing stabilities of the [TM(phen)3]2+ complexes with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β (TM): Mn: 10.5, Fe: 21.2, Co: 19.9, Ni: 24.3.52 To force bond formation between Ni2+ and the thiostannate anion syntheses were performed with mixtures of aromatic bidentate N-donor ligands which have different coordination abilities. The bidentate amine phen is a strong coordinating ligand,52 while 2,2′-bipy has a weak/medium coordination tendency52 and 4,4′-bipy can only act as a monodentate ligand to one Ni2+ center. Both 4,4′-bipy and 2,2′-bipy may act as stabilizing molecules via π–π interactions and both are not strong competitors for phen. During the explorative synthetic work we observed that the ratio phen
β (TM): Mn: 10.5, Fe: 21.2, Co: 19.9, Ni: 24.3.52 To force bond formation between Ni2+ and the thiostannate anion syntheses were performed with mixtures of aromatic bidentate N-donor ligands which have different coordination abilities. The bidentate amine phen is a strong coordinating ligand,52 while 2,2′-bipy has a weak/medium coordination tendency52 and 4,4′-bipy can only act as a monodentate ligand to one Ni2+ center. Both 4,4′-bipy and 2,2′-bipy may act as stabilizing molecules via π–π interactions and both are not strong competitors for phen. During the explorative synthetic work we observed that the ratio phen![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bipy is an important parameter. Compound I containing co-crystallized 4,4′-bipy was only obtained when the amount of 4,4′-bipy was between 20–65% of the total amount of amine. The highest yield was observed for a Ni
bipy is an important parameter. Compound I containing co-crystallized 4,4′-bipy was only obtained when the amount of 4,4′-bipy was between 20–65% of the total amount of amine. The highest yield was observed for a Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phen
phen![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4,4′-bipy molar ratio of 1
4,4′-bipy molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Applying mixtures of phen and 2,2′-bipy compound II crystallized only applying 20–50% 2,2′-bipy of the total amount of amine. For compound II the highest yield was obtained at a molar ratio of 1
1. Applying mixtures of phen and 2,2′-bipy compound II crystallized only applying 20–50% 2,2′-bipy of the total amount of amine. For compound II the highest yield was obtained at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for Ni
1 for Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phen
phen![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2′-bipy. When the syntheses were carried out using the analogue cobalt and iron salts (CoCl2·6H2O or FeCl2·4H2O) under the conditions mentioned above, the known compounds {[Co(phen)2]2Sn2S6}28 and {[Fe(phen)2]2Sn2S6},28 were obtained.
2.2′-bipy. When the syntheses were carried out using the analogue cobalt and iron salts (CoCl2·6H2O or FeCl2·4H2O) under the conditions mentioned above, the known compounds {[Co(phen)2]2Sn2S6}28 and {[Fe(phen)2]2Sn2S6},28 were obtained.
Crystal structures
The compound {[Ni(phen)2]2Sn2S6}·4,4′-bipy·½H2O (I) crystallizes in the monoclinic space group C2/c with four formula units in the unit cell with all atoms on general positions except the two unique Ni and Sn atoms. {[Ni(phen)2]2Sn2S6}·2,2′-bipy (II) crystallizes in the monoclinic space group P21/n with two formula units in the unit cell and all atoms are located on general sites.Both structures feature the [Sn2S6]4− anion and charge compensating Ni2+ centered complexes. The Ni2+ cations of the [Ni(phen)2]2+ complexes have two Ni–S bonds leading to the formation of discrete molecules (Fig. 1).
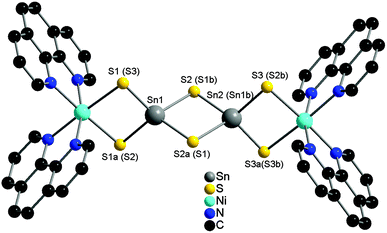 | ||
| Fig. 1 Structure of the {[Ni[phen)2]2Sn2S6} moieties in I as a representative. Labelling of the atoms for II are given in parentheses. The hydrogen atoms are omitted for clarity. | ||
The Ni–S bond lengths (2.4960 Å–2.5047 Å) as well as the Ni–N bonds (2.083 Å–2.124 Å) are within the range reported in literature.12–14,16,23 (Table 1).
| I | II | ||
|---|---|---|---|
| a Symmetry transformations used to generate equivalent atoms: a: −x + 1, y, −z + 1/2; b: −x + 1, −y + 1, −z. | |||
| Sn1–S1 | 2.3366(10) | Sn–S3 | 2.3296(9) |
| Sn1–S1a | 2.3382(10) | Sn–S2 | 2.3475(9) |
| Sn1–S2 | 2.4532(9) | Sn–S1b | 2.4488(9) |
| Sn1–S2a | 2.4630(9) | Sn–S1 | 2.4524(9) |
| S1–Sn–S1a | 100.59(5) | S2–Sn–S3 | 100.43(3) |
| S2–Sn–S2a | 92.78(4) | S1–Sn–S1b | 92.46(3) |
| S1–Sn–S2a | 117.27(4) | S1–Sn–S3 | 120.84(3) |
| S1a–Sn–S2a | 115.02(3) | S1–Sn–S2 | 113.00(3) |
| S2–Sn–S3 | 117.28(4) | S2–Sn–S1b | 118.10(3) |
| S2a–Sn–S3 | 114.57(3) | S3–Sn–S1b | 113.30(3) |
| Sn1–S1–Sn2 | 87.46(3) | Sn–S1–Snb | 87.54(3) |
| Ni1–N1 | 2.103(3) | Ni1–N1 | 2.120(3) |
| Ni1–N2 | 2.122(3) | Ni1–N2 | 2.161(3) |
| Ni1–N21 | 2.083(3) | Ni1–N21 | 2.106(3) |
| Ni1–N22 | 2.124(3) | Ni1–N22 | 2.112(3) |
| Ni1–S1 | 2.4960(11) | Ni1–S2 | 2.4946(10) |
| Ni1–S3 | 2.5047(11) | Ni1–S3 | 2.4984(10) |
The angles around Ni2+ scatter between 78.64(13)° and 175.86(9)° (I) and between 77.38(11)° and 172.28(9)° (II), respectively, indicative for a severe distortion of the octahedra, but still in the range of literature data.16 The distortion is caused by the fixed position of the N-atoms in the phen-molecule leading to acute angles around the Ni2+ cations (I: N1–Ni1–N2: 78.64(13), N21–Ni1–N22: 79.03(13); II: N1–Ni1–N2: 77.38(11), N21–Ni1–N22: 78.25(12)) (Table S1†). The values of the dihedral angles between the phen ligands are around 90° ± 10° (Table S2†) and they are comparable with literature data.27,28 The [Sn2S6]4− ion which is generated by edge-sharing of two SnS4 tetrahedra exhibits the typical Sn–S bonding pattern of short Sn–St (t = terminal) and longer Sn–Sb (b = bridging) bonds. Comparing the geometric parameters of the thiostannate ion of the title compounds with those of the discrete anion [Sn2S6]4−(ref. 1,12–16,46) the Sn–S bond lengths do not differ significantly (Table 1). Different, however, are the bond angles which cover a wide range from 87.46 to 117.27° in I and they are between 87.54° and 120.84° in II evidencing a strong deviation from ideal tetrahedral geometry, a phenomenon also observed previously.12,23,26–28,53
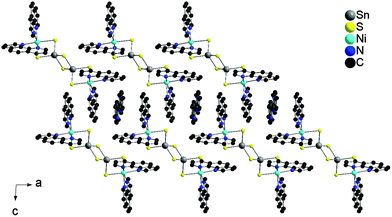
Besides the {[Ni(phen)2]2Sn2S6} moiety, the structure of I contains an additional 4,4′-bipy and a water molecule, which is disordered over two half occupied positions. The {[Ni(phen)2]2Sn2S6} units are arranged as rods in all three crystallographic directions (Fig. 2). The 4,4′-bipy molecules are assembled in a chain-like fashion along [100] and [001] (Fig. 2). The water molecule is located between adjacent 4,4′-bipy molecules and the N⋯O separation of 2.922 Å indicates hydrogen bonding interaction.
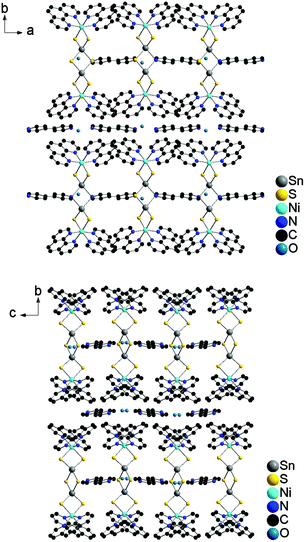 | ||
| Fig. 2 Parallel arrangement of the molecules in I within the ab-plane (top) and bc-plane (bottom), respectively. Hydrogen atoms are omitted for clarity. | ||
The arrangement of the constituents for optimal intermolecular interactions in I becomes clear viewing along [10−1]: The {[Ni(phen)2]2Sn2S6} moieties are interlaced along [101] and the 4,4′-bipy molecules are located in the thus generated voids (Fig. 3).
 | ||
| Fig. 3 Arrangement of the molecules in I viewed along [10−1]. The 4,4′-bipy molecules are located in the free spaces between the {[Ni(phen)2]2Sn2S6} moieties. Hydrogen atoms are omitted for clarity. | ||
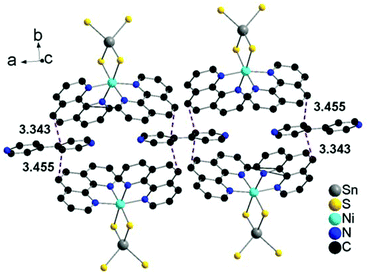
The phen ligands of neighbored complexes exhibit a short intermolecular distance of 3.489 Å and two even shorter separations are observed between phen and co-crystallized 4,4′-bipy molecules at 3.343–3.455 Å. Such short distances between off-centered parallel stacked aromatic molecules indicate π–π interactions (Fig. 4 and 5).42–45,54,55
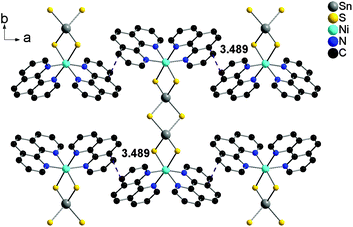 | ||
| Fig. 4 Off-center parallel orientation of the phen ligands in I within the ab-plane. The purple dashed lines indicate the shortest intermolecular distances. Hydrogen atoms are omitted for clarity. | ||
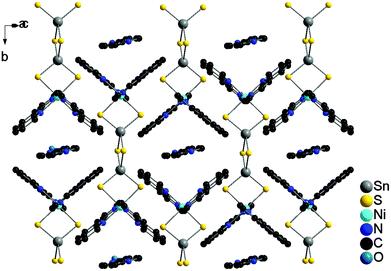
In the structure of II the {[Ni(phen)2]2Sn2S6} moieties are lined-up along a and c, respectively (Fig. 6). The 2,2′-bipy molecules are located between two adjacent {[Ni(phen)2]2Sn2S6} units yielding a sequence AAB along [100] where A stands for the phen Ligand and B for the 2,2′-bipy molecule.
Like in compound I the aromatic components adopt orientations which allow attractive intermolecular interactions. Along [010] all molecules are arranged in rows, while along [101] the phen ligands are staggered. The 2,2′-bipy molecules can now be aligned parallel to the upper and the lower phen ligand of adjacent rows (Fig. 7, top) leading either to a parallel arrangement or a staggered arrangement (Fig. 7, bottom). The intermolecular distances for these orientations between the 2,2′-bipy molecule and the phen ligands are almost identical with 3.279–3.826 Å for the parallel and 3.335–3.921 Å and 3.396–4.059 Å for the staggered orientations. As discussed above and in agreement with literature data such distances can be regarded as π–π interactions.54,55
Physcial properties
The Sn–S modes in the Raman spectra of thiostannates are located in the region of 400 to 100 cm−1. The assignment of the modes for compounds I and II was done on the basis of the data documented for the [Sn2S6]4− anion1,56,57 and NiS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 (Table 2).
58 (Table 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 in cm−1
58 in cm−1
| [Sn2S6]4− | NiS2 | I | II |
|---|---|---|---|
| 391 | 394 | 399 | |
| 341 | 336 | 340 | |
| 301 | 298 | 298 | |
| 281 | 283 | 273 | 276 |
| 222 | 219 | 212 | |
| 190 | 181 | 180 |
The resonance of the symmetric Sn–St stretching mode is found around 390 cm−1 in the discrete anion. In the title compounds the [Sn2S6]4− unit has bonds to Ni2+ resulting in slightly shorter Sn–S bonds leading to a shift of the signal to higher wave numbers. The Sn–S–Sn vibrations are located at lower wave numbers at about 340 cm−1. The mode at 280 cm−1 could be caused by a Sn2S2 ring vibration, the energetic differences between the resonances of I and II and those reported for Na4Sn2S6·14H2O could be generated by slightly differing bond lengths and angles. But since the Ni–S vibrations are located in this region as well, a detailed assignment is not possible.
In the IR spectra, the absorptions can be assigned to phen59–61, the Ni–N stretching vibration at ≈ 420 cm−1 (ref. 27 and 28) and to one of the bipy molecules62–65 (Table 3). The absorptions of the 4,4′-bipy molecule are mostly overlapping with the absorptions of phen, but for example at ca. 800 cm−1 only 4,4′-bipy causes a signal, which therefore can only be observed in compound I. For 2,2′-bipy (II) this absorption is shifted to slightly higher wavenumbers (818/819 cm−1) due the different position of the N-atoms in the aromatic ring.
| 1,10-phen | 4,4′-bipy | 2,2′-bipy | I | II | Assignment |
|---|---|---|---|---|---|
| 3035 | 3023 | — | 3042 | 3035w | ν(C–H) |
| — | — | 2982 | — | 2979w | ν(C–H) |
| — | — | 2916 | — | 2922w | ν(C–H) |
| 1616 | 1604 | — | 1624 | 1620 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| — | — | 1595 | — | 1598 | ν(C–C) + ν(C–N) |
| 1586 | 1585 | — | 1587 | — |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| — | — | 1579 | — | 1578 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| 1560 | — | 1555 | — | 1557 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| 1516 | — | 1517 | 1510 | 1510 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| 1494 | 1491 | — | 1494 | 1491 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| — | — | 1458 | — | 1456 |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
| 1421 | 1416 | — | 1419 | 1416 | Combination band |
| 1344 | — | — | 1342 | 1344 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| — | — | 1253 | — | 1255 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| 1216 | 1218 | — | 1218 | 1222 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| — | — | 1210 | — | 1206 |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
| 1137 | 1133 | 1238 | 1136 | 1134 |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
| 1092 | — | — | 1096 | 1096 |
ν (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| — | 1068 | — | 1065 | — | Ring breathing |
| 1035 | — | 1038 | — | 1036 | ν(C–N) |
| 996 | 992 | — | 991 | — |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
| 987 | — | — | — | 985 |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
| 869 | — | — | 866 | — | ν(ring) |
| 853 | — | — | — | 858 |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| 848 | — | — | 844 | 846 |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| — | — | 818 | — | 819 |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
| — | 800 | — | 802 | — |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
| 779 | — | — | 780 | 779 | δ(C–H) |
| — | — | 758 | — | 753 |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) C–H) |
| 721 | 724 | — | 724 | 722 | δ(C–H) |
| 643 | — | — | 640 | 637 | ν(ring) |
| 625 | — | 616 | — | 620 | δ(C–H) |
| 606 | 607 | — | 609 | — |
δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) C) |
| 478 | — | — | 476 | — | ν(ring) |
| — | — | — | 422 | 422 | ν(Ni–N) |
In the UV/vis spectra of both compounds three bands are observed (see Fig. S4 and S5†). The first two absorptions (I: ∼2.48 eV and ∼3.32 eV; II: ∼2.55 eV and ∼3.16 eV) can be assigned to the Ni2+ d–d transition 3A2g → 3T(3F) and 3A2g → 3T(3P).66 The third band (I: 4.63 eV; II: 4.59 eV) might probably be traced back to the π → π* transition of the aromatic amines.59
The thermal properties of both compounds were investigated by simultaneously differential thermoanalysis and thermogravimetry. On heating compound I at 4 °C min−1 one mass step of 40.8% is observed in the TG curve that is accompanied with an endothermic peak in the DTA curve at about 340 °C (Fig. S6†). On further heating a second much smaller mass step is observed and in the following the samples mass decreases continuously. The experimental mass loss observed in each step are not in agreement with those calculated for a stepwise removal of the organic ligands. Therefore, this reaction seems to be more complex. For compound II several poorly resolved mass steps are observed, all of them accompanied with endothermic events in the DTA curve, indicating that the organic ligands are stepwise removed.
Both compounds exhibit paramagnetic behaviour (see Fig. S8 and S9†). The value for the Weiss constant is near zero demonstrating that the Ni2+ cations are magnetically isolated. The effective magnetic moment for Ni2+ amounts to 2.88 μB which is near the spin only value.
3 Experimental section
Synthesis
Structure determination
The intensity data for the compounds were collected using a STOE IPDS-1 (Imaging Plate Diffraction System) with Mo-Kα radiation. The structures were solved with direct methods using the program SHELXS-97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 and the refinements were done against F2 with SHELXL-97.68 For all non-hydrogen atoms anisotropic displacement parameters were used. The hydrogen atoms were positioned with idealized geometry and were refined using a riding model. In compound I the O–H hydrogen atoms were located in the difference Fourier map, their bond lengths set to ideal values and refined using a riding model. The water molecule is disordered over two half occupied positions and was refined using a split model. The structure contains additional non-coordinating 4,4′-bipy molecules that are located on centres of inversion. In compound II, the non-coordinating 2,2′-bipy molecule is disordered and was refined using a split model with restraints for bond lengths and angles.
67 and the refinements were done against F2 with SHELXL-97.68 For all non-hydrogen atoms anisotropic displacement parameters were used. The hydrogen atoms were positioned with idealized geometry and were refined using a riding model. In compound I the O–H hydrogen atoms were located in the difference Fourier map, their bond lengths set to ideal values and refined using a riding model. The water molecule is disordered over two half occupied positions and was refined using a split model. The structure contains additional non-coordinating 4,4′-bipy molecules that are located on centres of inversion. In compound II, the non-coordinating 2,2′-bipy molecule is disordered and was refined using a split model with restraints for bond lengths and angles.
Selected refinement results are summarized in Table 4. Structural data have been deposited in the Cambridge Crystallographic Data Centre as publication no. CCDC 1054460 (I), CCDC 1054461 (II).
| {[Ni(phen)2]2Sn2S6}·4,4′-bipy·½H2O | {[Ni(phen)2]2Sn2S6}·2,2′-bipy | |
|---|---|---|
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | P21/n |
| M (g mol−1) | 1442.18 | 1424.16 |
| a (Å) | 18.3431(6) | 10.5715(2) |
| b (Å) | 19.4475(6) | 9.9086(2) |
| c (Å) | 15.0835(5) | 24.9960(4) |
| α (°) | 90 | 90 |
| β (°) | 95.556(2) | 92.8000(10) |
| γ (°) | 90 | 90 |
| V (Å3) | 5355.4(3) | 2615.17(8) |
| Temperature (K) | 200(2) | 200(2) |
| Z | 4 | 2 |
| D calculated (g cm−3) | 1.789 | 1.809 |
| μ (mm−1) | 1.9.03 | 1.946 |
| Scan range (°) | 1.53 ≤ θ ≤ 28.00 | 1.63 ≤ θ ≤ 27.93 |
| Reflections collected | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 723 723 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107 107 |
| Independent reflections | 6419 | 6238 |
| Observed reflections | 5042 | 5617 |
| Goodness-of-fit on F2 | 1.084 | 1.059 |
| R values (I > 2σ(I)) | R 1 = 0.0466 | R 1 = 0.0480 |
| wR2 = 0.1038 | wR2 = 0.1274 | |
| R values (all data) | R 1 = 0.0641 | R 1 = 0.0520 |
| wR2 = 0.1113 | wR2 = 0.1307 | |
| Res. elec. dens. (e Å−3) | 0.706 and −0.801 | 1.024 and −0.770 |
X-ray powder diffractometry
The X-ray powder diffraction patterns were recorded on a STOE Stadi-P powder diffractometer (Cu-Kα1 radiation, λ = 1.540598 Å, Ge monochromator) in transmission geometry.SEM and EDX
Scanning electron microscopy investigations and energy dispersive X-ray analyses (EDX) were done with a Philips Environmental Scanning Electron Microscope ESEM XL30 equipped with an EDAX detector.Raman spectroscopy
Raman spectra were recorded with a Bruker IFS 66 Fourier transform Raman spectrometer (wavelength: 541.5 nm) in the region from 100 to 3500 cm−1.Infrared spectroscopy
MIR spectra (400–4000 cm−1) were recorded with a Bruker Alpha P spectrometer.UV/visible spectroscopy
UV/vis spectra were recorded with an UV-vis-NIR two channel spectrometer Cary 5 from Varian Techtron Pty., Darnstadt at room temperature of powdered samples with BaSO4 powder as reference material. The absorption data were calculated applying the Kubelka–Munk relation for diffuse reflectance data.Thermal properties
DTA-TG measurements were performed using a Netzsch STA 409 CD under a nitrogen flow of 75 mL min−1 and at a heating rate of 4 K min−1. The instrument was calibrated using standard reference materials.Magnetic properties
The magnetic properties were investigated using a physical properties measurement system (PPMS) Model 600 from Quantum Design at H = 100 Oe in the temperature range 1.9–325 K.4 Conclusions
In the manuscript we presented a new synthesis strategy based on the observation of different coordination abilities of aromatic N-donor ligands towards Ni2+. Applying suitable mixtures of the aromatic amine molecules with the proper ratio between Ni2+ and the strong coordinating phen ligand, products crystallized where the medium/weak or non-resp. monodentate aromatic amine molecule acts as stabilizer of the structures via π–π-interactions. Currently we experimentally investigate whether this is a new general concept, which can be applied to other transition metals opening new opportunities for the generation of hitherto not accessible thiostannates.Acknowledgements
Financial support by the State of Schleswig-Holstein and the DFG is gratefully acknowledged.Notes and references
- B. Krebs, S. Pohl and W. Schiwy, Angew. Chem., Int. Ed. Engl., 1970, 9, 897–898 CrossRef CAS PubMed.
- (a) K. O. Klepp and F. Fabian, Z. Naturforsch., B: Chem. Sci., 1999, 54, 1505–1509 CAS; (b) Klepp, O. Kurt and F. Fabian, Z. Naturforsch., B: Chem. Sci., 1999, 54, 1505–1509 CAS.
- G. A. Marking, M. Evain, V. Petricek and M. G. Kanatzidis, J. Solid State Chem., 1998, 141, 17–28 CrossRef CAS.
- J.-H. Liao, C. Varotsis and M. G. Kanatzidis, Inorg. Chem., 1993, 32, 2453–2462 CrossRef CAS.
- E. Ruzin, S. Jakobi and S. Dehnen, Z. Anorg. Allg. Chem., 2008, 634, 995–1001 CrossRef CAS PubMed.
- O. Palchik, R. G. Iyer, J. H. Liao and M. G. Kanatzidis, Inorg. Chem., 2003, 42, 5052–5054 CrossRef CAS PubMed.
- P. Nørby, J. Overgaard, P. S. Christensen, B. Richter, X. Song, M. Dong, A. Han, J. Skibsted, B. B. Iversen and S. Johnsen, Chem. Mater., 2014, 26, 4494–4504 CrossRef.
- J. B. Parise, Y. Ko, J. Rijssenbeek, D. M. Nellis, K. Tan and S. Koch, J. Chem. Soc., Chem. Commun., 1994, 527 RSC.
- T. Jiang, A. Lough and G. A. Ozin, Adv. Mater., 1998, 10, 42–46 CrossRef CAS.
- H. Pada Nayek, Z. Lin and S. Dehnen, Z. Anorg. Allg. Chem., 2009, 635, 1737–1740 CrossRef CAS PubMed.
- X. Wang, T.-L. Sheng, S.-C. Xiang, S.-M. Hu, R.-B. Fu and X.-T. Wu, Chin. J. Struct. Chem., 2010, 29, 260–264 CAS.
- M. Behrens, S. Scherb, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2003, 629, 1367–1373 CrossRef CAS PubMed.
- D.-X. Jia, Y. Zhang, J. Dai, Q.-Y. Zhu and X.-M. Gu, Z. Anorg. Allg. Chem., 2004, 630, 313–318 CrossRef CAS PubMed.
- D.-X. Jia, J. Dai, Q.-Y. Zhu, Y. Zhang and X.-M. Gu, Polyhedron, 2004, 23, 937–942 CrossRef CAS PubMed.
- M.-L. Fu, G.-C. Guo, B. Liu, A.-Q. Wu and J.-S. Huang, Chin. J. Inorg. Chem., 2005, 21, 25–29 CAS.
- N. Pienack, H. Lühmann, B. Seidlhofer, J. Ammermann, C. Zeisler, F. Danker, C. Näther and W. Bensch, Solid State Sci., 2014, 33, 67–72 CrossRef CAS PubMed.
- X.-H. Lu, J.-J. Liang, J. Zhao, Y. Zhang and D.-X. Jia, J. Chem. Crystallogr., 2011, 41, 557–562 CrossRef CAS.
- J. Zhou, X. Liu, L. An, F. Hu, W. Yan and Y. Zhang, Inorg. Chem., 2012, 51, 2283–2290 CrossRef CAS PubMed.
- N. Pienack, C. Näther and W. Bensch, Z. Naturforsch., B: Chem. Sci., 2008, 63, 1243–1251 CAS.
- Z. Wang, G. Xu, Y. Bi and C. Wang, CrystEngComm, 2010, 12, 3703 RSC.
- C.-Y. Yue, X.-W. Lei, L. Yin, X.-R. Zhai, Z.-R. Ba, Y.-Q. Niu and Y.-P. Li, CrystEngComm, 2015, 17, 814–823 RSC.
- J. Zhou, G.-Q. Bian, J. Dai, Y. Zhang, A.-B. Tang and Q.-Y. Zhu, Inorg. Chem., 2007, 46, 1541–1543 CrossRef CAS PubMed.
- N. Pienack, S. Lehmann, H. Lühmann, M. El-Madani, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2008, 634, 2323–2329 CrossRef CAS PubMed.
- N. Pienack, C. Näther and W. Bensch, Eur. J. Inorg. Chem., 2009, 1575–1577 CrossRef CAS PubMed.
- N. Pienack, D. Schinkel, A. Puls, M.-E. Ordolff, H. Lühmann, C. Näther and W. Bensch, Z. Naturforsch., B: Chem. Sci., 2012, 67, 1098–1106 CrossRef CAS.
- C. Zeisler, C. Näther and W. Bensch, CrystEngComm, 2013, 15, 8874 RSC.
- J. Hilbert, C. Näther and W. Bensch, Inorg. Chem., 2014, 53, 5619–5630 CrossRef CAS PubMed.
- J. Hilbert, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2014, 640, 2858–2863 CrossRef CAS PubMed.
- G.-N. Liu, G.-C. Guo, F. Chen, S.-P. Guo, X.-M. Jiang, C. Yang, M.-S. Wang, M.-F. Wu and J.-S. Huang, CrystEngComm, 2010, 12, 4035 RSC.
- N. Pienack, K. Möller, C. Näther and W. Bensch, Solid State Sci., 2007, 9, 1110–1114 CrossRef CAS PubMed.
- N. Pienack, C. Näther and W. Bensch, Solid State Sci., 2007, 9, 100–107 CrossRef CAS PubMed.
- N. Pienack and W. Bensch, Z. Anorg. Allg. Chem., 2006, 632, 1733–1736 CrossRef CAS PubMed.
- M. Behrens, M.-E. Ordolff, C. Näther, W. Bensch, K.-D. Becker, C. Guillot-Deudon, A. Lafond and J. A. Cody, Inorg. Chem., 2010, 49, 8305–8309 CrossRef CAS PubMed.
- N. Pienack, C. Näther and W. Bensch, Eur. J. Inorg. Chem., 2009, 937–946 CrossRef CAS PubMed.
- N. Pienack, A. Puls, C. Näther and W. Bensch, Inorg. Chem., 2008, 47, 9606–9611 CrossRef CAS PubMed.
- M. BaiYin, L. Ye, Y. An, X. Liu, C. Jia and G. Ning, Bull. Chem. Soc. Jpn., 2005, 78, 1283–1284 CrossRef CAS.
- Y. An, B. Menghe, L. Ye, M. Ji, X. Liu and G. Ning, Inorg. Chem. Commun., 2005, 8, 301–303 CrossRef CAS PubMed.
- Y. Wang, M. BaiYin, S. Ji, X. Liu, Y. An and G. Ning, Chem. Res. Chin. Univ., 2006, 22, 411–414 CrossRef CAS.
- R.-C. Zhang, H.-G. Yao, S.-H. Ji, M.-C. Liu, M. Ji and Y.-L. An, Chem. Commun., 2010, 46, 4550–4552 RSC.
- (a) A. Rabenau, Angew. Chem., Int. Ed. Engl., 1985, 97, 1017–1032 CrossRef CAS PubMed; (b) W. S. Sheldrick and M. Wachhold, Angew. Chem., Int. Ed. Engl., 1997, 36, 206–224 CrossRef CAS PubMed; (c) W. S. Sheldrick and M. Wachhold, Coord. Chem. Rev., 1998, 176, 211–322 CrossRef CAS; (d) S. Dehnen and M. Melullis, Coord. Chem. Rev., 2007, 251, 1259–1280 CrossRef CAS PubMed; (e) G. Demazeau, J. Mater. Sci., 2008, 43, 2104–2114 CrossRef CAS PubMed; (f) W. S. Sheldrick, J. Chem. Soc., Dalton Trans., 2000, 3041–3052 RSC; (g) X. Bu, N. Zheng and P. Feng, Chem. – Eur. J., 2004, 10, 3356–3362 CrossRef CAS PubMed; (h) B. Seidlhofer, N. Pienack and W. Bensch, Z. Naturforsch., B: Chem. Sci., 2010, 65, 937–975 CrossRef CAS; (i) W.-W. Xiong, G. Zhang and Q. Zhang, Inorg. Chem. Front., 2014, 1, 292 RSC.
- (a) W. Bensch and M. Schur, Eur. J. Solid State Inorg. Chem., 1996, 33, 1149–1160 CAS; (b) W. Bensch and M. Schur, Z. Naturforsch., B: Chem. Sci., 1997, 52, 405–409 CrossRef CAS; (c) M. Schur, C. Näther and W. Bensch, Z. Naturforsch., B: Chem. Sci., 2001, 56, 79–84 CrossRef CAS; (d) A. Puls, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2006, 632, 1239–1243 CrossRef CAS PubMed.
- L. Goerigk, H. Kruse and S. Grimme, ChemPhysChem, 2011, 12, 3421–3433 CrossRef CAS PubMed.
- S. Grimme, Angew. Chem., Int. Ed., 2008, 47, 3430–3434 CrossRef CAS PubMed.
- S. Grimme, WIREs Comput. Mol. Sci., 2011, 1, 211–228 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- J. Zhou, X. Liu, G.-Q. Chen, F. Zhang and L.-R. Li, Z. Naturforsch., B: Chem. Sci., 2010, 65, 1229–1234 CAS.
- (a) C.-Y. Yue, X.-W. Lei, Y.-X. Ma, N. Sheng, Y.-D. Yang, G.-D. Liu and X.-R. Zhai, Cryst. Growth Des., 2014, 14, 101–109 CrossRef CAS; (b) R. J. Lees, A. V. Powell and A. M. Chippindale, Polyhedron, 2005, 24, 1941–1948 CrossRef CAS PubMed; (c) H.-O. Stephan and M. G. Kanatzidis, Inorg. Chem., 1997, 36, 6050–6057 CrossRef CAS PubMed; (d) H. Lühmann, Z. Rejai, K. Möller, P. Leisner, M.-E. Ordolff, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2008, 634, 1687–1695 CrossRef PubMed; (e) R. Stähler, C. Näther and W. Bensch, Eur. J. Inorg. Chem., 2001, 1835–1840 CrossRef; (f) R. Kiebach, W. Bensch, R.-D. Hoffmann and R. Pöttgen, Z. Anorg. Allg. Chem., 2003, 629, 532–538 CrossRef CAS PubMed; (g) C. Anderer, N. Delwa de Alarcon, C. Näther and W. Bensch, Chem. – Eur. J., 2014, 20, 16953–16959 CrossRef CAS PubMed; (h) J. Zhou and L. An, CrystEngComm, 2011, 13, 5924–5928 RSC; (i) J. Zhou, L. An, X. Liu, L. Huang and X. Huang, Dalton Trans., 2011, 40, 11419–11424 RSC; (j) M.-L. Feng, W.-W. Xiong, D. Ye, J.-R. Li and X.-Y. Huang, Chem. – Asian J., 2010, 5, 1817–1823 CrossRef CAS PubMed; (k) J. Zhou, L. An and F. Zhang, Inorg. Chem., 2011, 50, 415–417 CrossRef CAS PubMed; (l) X. Liu, Inorg. Chem. Commun., 2011, 14, 437–439 CrossRef CAS PubMed; (m) W. Tang, C. Tang, F. Wang, R. Chen, Y. Zhang and D. Jia, J. Solid State Chem., 2013, 199, 287–294 CrossRef CAS PubMed; (n) P. Vaqueiro, A. M. Chippindale and A. V. Powell, Inorg. Chem., 2004, 43, 7963–7965 CrossRef CAS PubMed; (o) H.-O. Stephan and M. G. Kanatzidis, J. Am. Chem. Soc., 1996, 118, 12226–12227 CrossRef CAS; (p) X. Liu and J. Zhou, Inorg. Chem. Commun., 2011, 14, 1286–1289 CrossRef CAS PubMed; (q) M.-F. Wang, C.-Y. Yue, Z.-D. Yuan and X.-W. Lei, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2013, 69, 855–858 CAS; (r) P. Vaqueiro, D. P. Darlow, A. V. Powell and A. M. Chippindale, Solid State Ionics, 2004, 172, 601–605 CrossRef CAS PubMed; (s) R. J. Lees, A. V. Powell and A. M. Chippindale, J. Phys. Chem. Solids, 2007, 68, 1215–1219 CrossRef CAS PubMed; (t) L. Engelke, C. Näther, P. Leisner and W. Bensch, Z. Anorg. Allg. Chem., 2008, 634, 2959–2965 CrossRef CAS PubMed.
- C.-Y. Yue, X.-W. Lei, R.-Q. Liu, H.-P. Zhang, X.-R. Zhai, W.-P. Li, M. Zhou, Z.-F. Zhao, Y.-X. Ma and Y.-D. Yang, Cryst. Growth Des., 2014, 14, 2411–2421 CAS.
- J. Zhou, G.-Q. Bian, Y. Zhang, J. Dai and N. Cheng, Z. Anorg. Allg. Chem., 2007, 633, 2701–2705 CrossRef CAS PubMed.
- (a) R. Stähler and W. Bensch, J. Chem. Soc., Dalton Trans., 2001, 2518–2522 RSC; (b) M. Schaefer, R. Stähler, W.-R. Kiebach, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2004, 630, 1816–1822 CrossRef CAS PubMed; (c) J. Lichte, H. Lühmann, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2009, 635, 2021–2026 CrossRef CAS PubMed; (d) J. Zhou, X. Liu, L. An, F. Hu, Y. Kan, R. Li and Z. Shen, Dalton Trans., 2013, 42, 1735–1742 RSC.
- R. Kiebach, R. Warratz, C. Näther and W. Bensch, Z. Anorg. Allg. Chem., 2009, 635, 988–994 CrossRef CAS PubMed.
- H. Irving and J. Mellor, J. Chem. Soc., 1962, 5222–5237 RSC.
- (a) Y. Zhang, J. Zhou, A.-B. Tang, G.-Q. Bian and J. Dai, J. Chem. Crystallogr., 2010, 40, 496–500 CrossRef CAS; (b) X.-M. Gu, J. Dai, D.-X. Jia, Y. Zhang and Q.-Y. Zhu, Cryst. Growth Des., 2005, 5, 1845–1848 CrossRef CAS.
- E. C. Lee, D. Kim, P. Jurecka, P. Tarakeshwar, P. Hobza and K. S. Kim, J. Phys. Chem. A, 2007, 111, 3446–3457 CrossRef CAS PubMed.
- C. R. Martinez and B. L. Iverson, Chem. Sci., 2012, 3, 2191 RSC.
- B. Krebs and W. Schiwy, Z. Anorg. Allg. Chem., 1973, 398, 63–71 CrossRef CAS PubMed.
- W. Schiwy, C. Blutau, D. Gäthje and B. Krebs, Z. Anorg. Allg. Chem., 1975, 412, 1–10 CrossRef CAS PubMed.
- D. W. Bishop, P. S. Thomas and A. S. Ray, Mater. Res. Bull., 1998, 33, 1303–1306 CrossRef CAS.
- M. S. Atanassova and G. D. Dimitrov, Spectrochim. Acta, Part A, 2003, 59, 1655–1662 CrossRef.
- M. Reiher, G. Brehm and S. Schneider, J. Phys. Chem. A, 2004, 108, 734–742 CrossRef CAS.
- D. A. Thornton and G. M. Watkins, J. Coord. Chem., 1992, 25, 299–315 CrossRef CAS PubMed.
- R. Podgajny, M. Bałanda, M. Sikora, M. Borowiec, L. Spałek, C. Kapusta and B. Sieklucka, Dalton Trans., 2006, 2801–2809 RSC.
- J. G. Contreras and C. J. Díz, J. Coord. Chem., 2007, 16, 245–249 CrossRef PubMed.
- E. Castellucci, L. Angeloni, N. Neto and G. Sbrana, Chem. Phys., 1979, 43, 365–373 CrossRef CAS.
- M. L. Niven and G. C. Percy, Transition Met. Chem., 1978, 3, 267–271 CrossRef CAS.
- S. P. Roe, J. O. Hill and R. J. Magee, Monatsh. Chem., 1991, 122, 467–478 CrossRef CAS.
- G. M. Sheldrick, SHELXS-97, Program for the Solution of Crystal Structures, University of Göttingen, Göttingen (Germany), 1997 Search PubMed.
- G. M. Sheldrick, SHELXS-97, Program for the Solution of Crystal Structures, University of Göttingen, Göttingen (Germany), 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD pattern, selected angles of the octahedral Ni2+ enivironment, IR- and Raman spectra. CCDC 1054460 and 1054461. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt01145k |
| This journal is © The Royal Society of Chemistry 2015 |