Cobalt(ii) amido complexes derived from a monodentate arylamido ligand featuring a highly electron-withdrawing C6F5 substituent†
Abstract
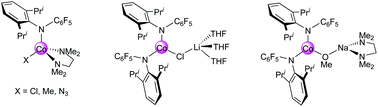
A series of cobalt(II) complexes of a highly electron-withdrawing amido ligand, [N(C6F5)(C6H3Pri2-2,6)]− (L), were synthesized and structurally characterized. Mononuclear [CoL(Cl)(TMEDA)] (3) and heterobimetallic [CoL2(μ-Cl)Li(THF)3] (4) were obtained by direct metathetical reactions of anhydrous CoCl2 with one molar equivalent of [LiL(TMEDA)] (1) (TMEDA = Me2NCH2CH2NMe2) and [LiL(THF)3] (2), respectively. Complex 3 underwent facile ligand substitution reactions with LiMe and NaN3, yielding the corresponding mixed-ligand complexes [CoL(X)(TMEDA)] (X = Me 5, N36). Treatment of 3 with NaOMe led to the heterobimetallic complex [CoL2(μ-OMe)Na(TMEDA)] (7). The solid-state structures of complexes 1–7 were established by X-ray diffraction analysis.
- This article is part of the themed collection: New Talent: Asia-Pacific

 Please wait while we load your content...
Please wait while we load your content...