Stepwise assembly of mixed-metal coordination cages containing both kinetically inert and kinetically labile metal ions: introduction of metal-centred redox and photophysical activity at specific sites†
Abstract
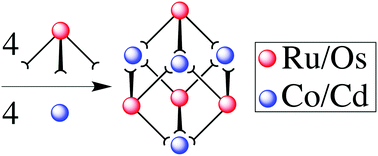
Stepwise preparation of the heterometallic octanuclear coordination cages [(Ma)4(Mb)4L12]16+ is reported, in which Ma = Ru or Os and Mb = Cd or Co (all in their +2 oxidation state). This requires initial preparation of the kinetically inert mononuclear complexes [(Ma)L3]2+ in which L is a ditopic ligand with two bidentate chelating pyrazolyl-pyridine units: in the complexes [(Ma)L3]2+ one terminus of each ligand is bound to the metal ion, such that the complex has three pendant bidentate sites at which cage assembly can propagate by coordination to additional labile ions Mb in a separate step. Thus, combination of four [(Ma)L3]2+ units and four [Mb]2+ ions results in assembly of the complete cages [(Ma)4(Mb)4L12]16+ in which a metal ion lies at each of the eight vertices, and a bridging ligand spans each of the twelve edges, of a cube. The different types of metal ion necessarily alternate around the periphery with each bridging ligand bound to one metal ion of each type. All four cages have been structurally characterised: in the Ru(II)/Cd(II) cage (reported in a recent communication) the Ru(II) and Cd(II) ions are crystallographically distinct; in the other three cages [Ru(II)/Co(II), Os(II)/Cd(II) and Os(II)/Co(II), reported here] the ions are disordered around the periphery such that every metal site refines as a 50 : 50 mixture of the two metal atom types. The incorporation of Os(II) units into the cages results in both redox activity [a reversible Os(II)/Os(III) couple for all four metal ions simultaneously, at a modest potential] and luminescence [the Os(II) units have luminescent 3MLCT excited states which will be good photo-electron donors] being incorporated into the cage superstructure.

 Please wait while we load your content...
Please wait while we load your content...