Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Energy transfer and unusual decay behaviour of BaCa2Si3O9:Eu2+,Mn2+ phosphor†
Matthias
Müller
and
Thomas
Jüstel
*
Department of Chemical Engineering, Münster University of Applied Sciences, Stegerwaldstrasse 39, 48565 Steinfurt, Germany. E-mail: tj@fh-muenster.de
First published on 13th May 2015
Abstract
In this work the photoluminescence (PL) of BaCa2Si3O9:Eu2+,Mn2+ and the energy transfer (ET) between Eu2+ and Mn2+ were examined. A series of powder samples with various Mn2+ concentrations were prepared by high temperature solid state route. Phase purity was investigated using X-ray powder diffractometry. Emission and excitation spectra as well as diffuse reflectance spectra were recorded to elucidate the PL properties of co-doped BaCa2Si3O9:Eu2+,Mn2+. Furthermore, fluorescence lifetime measurements were performed. PL and lifetime measurements were carried out from 100 to 800 K and 100 to 500 K, respectively. Moreover, external quantum efficiencies were determined and colour points were calculated. It turned out that ET from Eu2+ to Mn2+ is of a resonant type and occurs via dipole–quadrupole interactions. Temperature dependent PL measurements indicate high temperature stability of emission intensity in BaCa2Si3O9:Eu2+,Mn2+. Finally, it was found that fluorescence lifetimes of Eu2+ BaCa2Si3O9 show an unusual increase with increasing temperature.
1. Introduction
Even though the invention of bright blue light emitting diodes (LEDs) by Nakamura et al. nearly dates back a quarter of a century, LEDs still have a vast impact on advances in lighting technology.1 Meanwhile, LEDs have replaced more and more conventional light sources such as incandescent and discharge lamps. This is because of the fact that LEDs provide many benefits compared to thermal and gas discharge light sources, such as longer lifetimes, higher wall plug efficiency and higher colour rendering.2 Nowadays, most of the commercially available white light emitting LEDs comprise a blue emitting (In,Ga)N chip pumping a green-yellow emitting phosphor, e.g. (Y,Gd)3Al5O12:Ce3+. This setup provides a high luminous efficacy due to emission in the green spectral range. Unfortunately, these lamps suffer from high colour temperature and low colour rendering index due to the lack of emission in the red spectral range.3 Therefore, these light sources are unpopular for domestic lighting. To overcome these drawbacks, one approach is to use a phosphor blend comprising a blue, green, and red phosphor, which is excited by an ultraviolet emitting LED. Using this concept, cold and warm white emitting LEDs with excellent colour points can be realized.4 However, these packages undergo a loss in blue emission due to re-absorption by the green and red phosphors. For this reason, many research groups are developing single phase white emitting phosphors to convert UV radiation into white light.5One approach to realize a white emitting phosphor is to use the ion couple Eu2+ and Mn2+.6 The broad emission bands in the blue and red spectral range of Eu2+ and Mn2+ in many host structures complement each other to white light due to additive colour mixing. Furthermore, Eu2+ usually exhibits a broad excitation band in the UV range due to the spin and parity allowed [Xe]4f7–[Xe]4f65d1 interconfigurational electric-dipole transition. Therefore, Eu2+ is well appropriated for pumping by UV LEDs. In addition the blue emission band of Eu2+ is also suitable for sensitizing the spin and parity forbidden [Ar]3d5–[Ar]3d5 excitation transitions of Mn2+via energy transfer (ET). To investigate the photoluminescence (PL) properties as well as the ET from Eu2+ to Mn2+, a series of co-doped BaCa2Si3O9:Eu2+,Mn2+ samples with various Mn2+ concentrations were synthesized. BaCa2Si3O9 was described for the first time in the work of Eskola in 1922.7 Later on, in 1965 Alfors et al. investigated the mineral walstromite.8 Based on their results on optical properties and X-ray powder diffraction data they stated that BaCa2Si3O9 is identical to walstromite. Glasser et al. reported on the crystal structure of synthetic BaCa2Si3O9 using photographic X-ray diffraction data in 1968.9 The crystal structure derived from a single crystal analysis of BaCa2Si3O9 was firstly published by Barkley et al. in 2011.10 Shortly afterwards, Yao et al. presented the first report on the blue emission of Eu2+ doped BaCa2Si3O9.11 Moreover, Gaft et al. published an article concerning the luminescence of natural walstromite in 2013.12
BaCa2Si3O9 crystallizes triclinically in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The structure of triclinic BaCa2Si3O9 is built up of layers consisting of alternating Si3O9 rings. Between these layers Ba and Ca atoms are incorporated. BaCa2Si3O9 comprises two nonequivalent Ca sites, namely Ca1 and Ca2. The Ca1 site is coordinated by 8 oxygen atoms with a mean distance of 2.545 Å. The Ca2 site is surrounded by 6 oxygen atoms with an average bond length of 2.370 Å. The Ba atoms are 8-fold coordinated with an average distance of 2.841 Å. The ionic radii of Ca for 8 and 6-fold coordination are 1.12 and 0.96 Å. Ba2+ in 8-fold coordination has an ionic radius of 1.42 Å. The ionic radii of Mn2+ and Eu2+ for 8 and 6-fold coordination are 0.96 and 0.83 as well as 1.25 and 1.17 Å, respectively.13 Based on the ionic radii, it can be assumed that the Mn2+ ions tend to occupy the Ca sites whereas the Eu2+ ions tend to occupy the Ba2+ sites as well as the 8-fold coordinated Ca2+ sites.
. The structure of triclinic BaCa2Si3O9 is built up of layers consisting of alternating Si3O9 rings. Between these layers Ba and Ca atoms are incorporated. BaCa2Si3O9 comprises two nonequivalent Ca sites, namely Ca1 and Ca2. The Ca1 site is coordinated by 8 oxygen atoms with a mean distance of 2.545 Å. The Ca2 site is surrounded by 6 oxygen atoms with an average bond length of 2.370 Å. The Ba atoms are 8-fold coordinated with an average distance of 2.841 Å. The ionic radii of Ca for 8 and 6-fold coordination are 1.12 and 0.96 Å. Ba2+ in 8-fold coordination has an ionic radius of 1.42 Å. The ionic radii of Mn2+ and Eu2+ for 8 and 6-fold coordination are 0.96 and 0.83 as well as 1.25 and 1.17 Å, respectively.13 Based on the ionic radii, it can be assumed that the Mn2+ ions tend to occupy the Ca sites whereas the Eu2+ ions tend to occupy the Ba2+ sites as well as the 8-fold coordinated Ca2+ sites.
2. Experimental
Preparation
Ba0.99Eu0.01(Ca1−xMnx)2Si3O9 samples were synthesized via conventional high temperature solid state reaction. High purity starting materials BaCO3 (Alfa Aesar, 99.8%), CaCO3 (Merck KGaA, Reag. Ph. Eur.), SiO2 (Merck KGaA, Ph. Eur.), Eu2O3 (Treibacher Industrie AG, 99.99%) and MnC2O4·2H2O (Dr Paul Lohmann, chem. pure) were weighed in stoichiometric amounts. 1 wt% H3BO3 (Merck KGaA, Ph. Eur.) was added as a flux. Subsequently, the educts were ground in acetone in an agate mortar. After drying at ambient temperature, the obtained powder blends were heated at 1000 °C for 2 h in air to decompose the carbonates. Afterwards the samples were ground and finally calcined in alumina boats at 1200 °C for 12 h in reducing forming gas flow (Westfalen, 5% H2, 95% N2). After calcination, hard sinter bodies with a slightly yellow body colour were obtained which were subsequently ground to a fine powder.Characterization
Phase purity was investigated using X-ray powder diffractometry (XRD). XRD patterns were recorded on a Rigaku MiniFlex II diffractometer working in Bragg–Brentano geometry using Cu Kα radiation. Step width and integration time were 0.02 and 1 s, respectively.PL spectra and photoluminescence excitation (PLE) spectra were recorded on an Edinburgh Instruments FSL900 spectrometer equipped with a Xe arc lamp (450 W) and a cooled (−20 °C) single-photon counting photomultiplier (Hamamatsu R2658P). PL spectra were corrected by using a correction file obtained from a tungsten incandescent lamp certified by the National Physical Laboratory, UK.
For photoluminescence decay (PLD) measurements on Eu2+, the spectrometer was equipped with a picosecond pulsed laser (5 mW, pulse width = 76.6 ps, λem = 375 nm). For PLD measurements on Mn2+ a microsecond pulsed Xe lamp (100 W, pulse width = 1 μs) was attached to the spectrometer.
For temperature dependent measurements the spectrometer was equipped with a cryostat (Oxford Instruments). Liquid nitrogen was used as a cooling agent. Temperature stabilization time was 60 s and tolerance was set to ±3 K.
Diffuse reflectance (DR) spectra were recorded on an Edinburgh Instruments FS900 spectrometer equipped with a Xe arc lamp (450 W), a cooled (−20 °C) single-photon counting photomultiplier (Hamamatsu R928) as well as a Teflon-coated integration sphere. BaSO4 (99.998%, Sigma-Aldrich) was used as a reflectance standard.
External quantum efficiencies ηext were determined using the approach of Kawamura et al.14 To this end, PL spectra of the sample as well as of the excitation source were recorded in the integration sphere. From this, ηext can be calculated by the following equation:
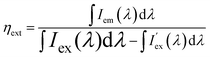 | (1) |
3. Results and discussion
Collected XRD patterns of doped and also of undoped BaCa2Si3O9 samples as well as the ICDD reference cards of BaCa2Si3O9 and SiO2 are presented in Fig. 1. The XRD patterns indicate the formation of the triclinic BaCa2Si3O9 phase. Furthermore, with increasing doping concentrations of Eu2+ and Mn2+, no change in the host structure can be observed. However, samples with x > 0.1 show an additional reflex at about 25.9° which can be assigned to SiO2. This proves the successful synthesis of the investigated BaCa2Si3O9:Eu2+,Mn2+ phosphors with a Mn2+ concentration up to x = 0.1.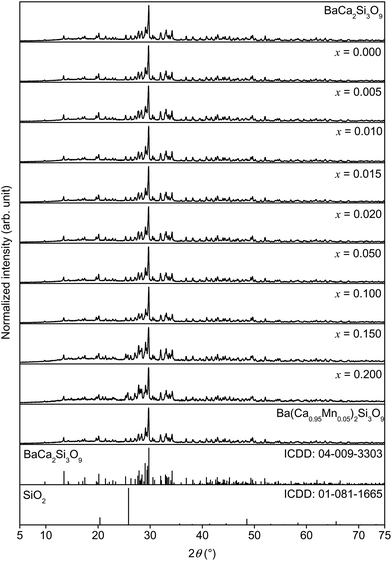 | ||
| Fig. 1 XRD patterns of pure BaCa2Si3O9 as well as of Ba0.99Eu0.01(Ca1−xMnx)2Si3O9 and Ba(Ca0.95Mn0.05)2Si3O9. | ||
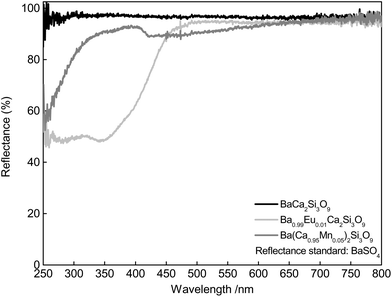
Fig. 2 shows DR spectra of undoped BaCa2Si3O9 host material and also of Ba0.99Eu0.01Ca2Si3O9 and Ba(Ca0.95Mn0.05)2Si3O9. Undoped BaCa2Si3O9 exhibits high reflectance between 250 and 800 nm. Thus, undoped BaCa2Si3O9 possess a white body colour. The DR spectrum of Ba0.99Eu0.01Ca2Si3O9 shows a broad absorption band between 250 and 450 nm due to the allowed [Xe]4f7–[Xe]4f65d1-transition of Eu2+. Because of the absorption band in the blue range of the electromagnetic spectrum, Ba0.99Eu0.01Si3O9 exhibits a slightly yellow body colour. The DR spectrum of Ba(Ca0.95Mn0.05)2Si3O9 shows absorption in the UV region between 250 and 350 nm which can be tentatively assigned to a Mn2+ charge transfer transition.15
The PLE spectrum as well as the PL spectrum of Ba0.99Eu0.01Ca2Si3O9 is depicted in Fig. 3a. The PLE spectrum was recorded monitoring the 460 nm emission of Eu2+ and exhibits a broad band consisting of various unresolved bands. This band is due to excitation from the [Xe]4f7 ground state to the [Xe]5d1 multiplet of Eu2+. The PL spectrum of Ba0.99Eu0.01Ca2Si3O9 (λex = 320 nm) shows an emission band peaking at about 444 nm with FWHM of 47 nm (2333 cm−1). This emission originates from relaxation of the excited [Xe]5d1 state to the [Xe]4f7 ground state. Further, Ba0.99Eu0.01Ca2Si3O9 possesses an emission band in the red region of the spectrum. The PLE spectrum of this emission is shown as a dotted line. Fig. 3b illustrates the PLE and PL spectra of Ba(Ca0.95Mn0.05)2Si3O9. Monitoring the 590 nm emission of Mn2+, the PLE spectrum of Ba(Ca0.95Mn0.05)2Si3O9 shows various bands centred at 326, 348, 359, 405, 432, and 510 nm. These peaks can be assigned to transitions from the 6A1(6S) ground state to 4T1(4P), 4E(4D), 4T2(4D), [4E(4G)4A1(4G)], 4T2(4G), and 4T1(4G), respectively. The PL spectrum of Ba(Ca0.95Mn0.05)2Si3O9 was recorded under an excitation wavelength of λex = 405 nm and exhibits a broad band with a maximum at about 594 nm resulting from a transition from the 4T1(4G) excited state to the 6A1(6S) ground state. This observation is in accordance with the results given by Gaft et al. regarding Mn2+ emission in natural walstromite.12 In addition, the Mn2+ emission band comprises a tailing to the red range of the visible spectrum indicating emission from different crystallographic sites. PLE as well as PL spectra of co-doped Ba0.99Eu0.01(Ca0.95Mn0.05)2Si3O9 are depicted in Fig. 3c. The PL spectrum of Ba0.99Eu0.01(Ca0.95Mn0.05)2Si3O9 shows two bands peaking at about 445 and 590 nm which can be assigned to electronic transitions of Eu2+ ([Xe]5d1 to [Xe]4f7) and Mn2+ (4T1(4G) to 6A1(6S)), respectively. PLE spectra of Ba0.99Eu0.01(Ca0.95Mn0.05)2Si3O9 were recorded, monitoring the 460 nm emission of Eu2+ as well as the 590 nm emission of Mn2+. These PLE spectra appear similar to the PLE of singly doped Ba0.99Eu0.01Ca2Si3O9 monitoring the blue emission of Eu2+. This observation strongly implies the occurrence of ET from Eu2+ to Mn2+.
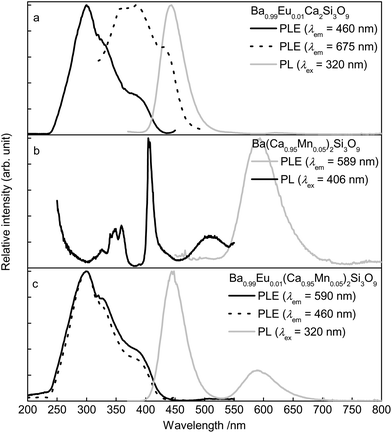 | ||
| Fig. 3 Room temperature PLE and PL spectra of Ba0.99Eu0.01Ca2Si3O9 (a), Ba(Ca0.95Mn0.05)2Si3O9 (b), and Ba0.99Eu0.01(Ca0.95Mn0.05)2Si3O9 (c). | ||
Fig. 4 illustrates the PL spectra of the synthesized Ba0.99Eu0.01(Ca1−xMnx)2Si3O9 series with x = 0, 0.005, 0.010, 0.015, 0.020, 0.050, 0.100, 0.150, and 0.200. As can be concluded from the spectra, with increasing Mn2+ concentration the PL intensity of Eu2+ decreases. This behaviour reflects the assumption provided above regarding ET from Eu2+ to Mn2+. The inset in Fig. 4 depicts the relative PL intensities of Eu2+ and Mn2+. It was found that the PL intensity of Mn2+ increases continuously up to a Mn2+ concentration of x = 0.15 where a saturation effect begins. Concentration quenching of the Mn2+ emission was not observed for the synthesized samples.
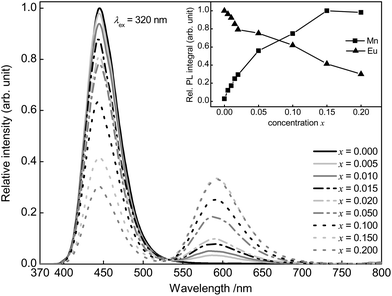 | ||
| Fig. 4 PL spectra of Ba0.99Eu0.01(Ca1−xMnx)2Si3O9 and integrated PL intensity in dependence of the Eu2+ and Mn2+ concentration (inset). | ||
Furthermore, PLD measurements were performed monitoring the Eu2+ emission to investigate the ET from Eu2+ to Mn2+ in more detail. Fluorescence lifetimes τ of Ba0.99Eu0.01(Ca1−xMnx)2Si3O9 samples with increasing Mn2+ content were determined, monitoring the 445 nm emission of Eu2+ under an excitation wavelength of λex = 375 nm. Fig. 5 illustrates the obtained PLD curves. The PLD curves can be best fitted with a bi-exponential function applying the following equation:
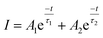 | (2) |
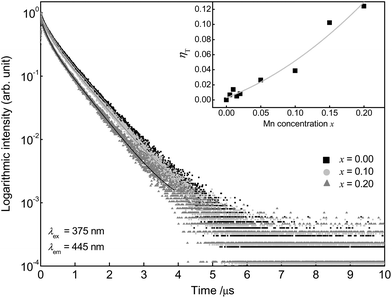 | ||
| Fig. 5 PLD curves of Ba0.99Eu0.01(Ca1−xMnx)2Si3O9 and ET efficiency ηT in dependence of the Mn2+ concentration (inset). | ||
Here, I is the luminescence intensity, A1 and A2 are parameters and t is the time. The terms τ1 and τ2 are the partial lifetimes for the exponential components. The bi-exponential shape of the PLD curves originates from two different emitting centres and suggests Eu2+ emission from two distinct crystallographic sites. This is thoroughly possible since BaCa2Si3O9 provides two different Ca sites and one Ba site, as mentioned above. This assumption is backed by PLD measurements monitoring the emission at 415 and 495 nm which yield different luminescence lifetimes (τ at 415 nm = 602 ns, τ at 415 nm = 702 ns) (Fig. S1 in the ESI†). The average luminescence lifetimes τ of Eu2+ in BaCa2(Si3O9):Eu2+,Mn2+ can be derived using the above mentioned parameters and the following equation:
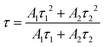 | (3) |
Emission fractions frac1 and frac2, partial lifetimes τ1, and τ2 and also the calculated average lifetimes are summarized in Table 1. The results show a decreasing lifetime τ of Eu2+ with increasing Mn2+ concentration. Since ET processes are usually faster than radiative transitions, this circumstance clearly indicates the occurrence of an ET from Eu2+ to Mn2+. The ET efficiency ηT be calculated using following equation:16
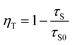 | (4) |
| Sample (x) | frac1 (%) | τ 1 (ns) | frac2 (%) | τ 2 (ns) | τ (ns) |
|---|---|---|---|---|---|
| 0.000 | 13 | 212 | 87 | 698 | 634 |
| 0.005 | 14 | 208 | 86 | 697 | 629 |
| 0.010 | 13 | 206 | 87 | 690 | 625 |
| 0.015 | 13 | 206 | 87 | 693 | 630 |
| 0.020 | 12 | 204 | 88 | 687 | 628 |
| 0.050 | 13 | 201 | 87 | 680 | 617 |
| 0.100 | 13 | 193 | 87 | 673 | 609 |
| 0.150 | 15 | 175 | 85 | 636 | 569 |
| 0.200 | 16 | 176 | 84 | 629 | 555 |
In this equation τS and τS0 are the average lifetimes of Eu2+ in the presence and absence of Mn2+. The inset of Fig. 5 illustrates the calculated values for ηT for the investigated BaCa2Si3O9:Eu2+,Mn2+ samples as a function of the Mn2+ content. This shows that the ET efficiency ηT continuously increases with increasing Mn2+ content. For the highest Mn2+ concentration with x = 0.20, ηT amounts to 12% whereas the PL intensity of Eu2+ decreases around 70%. Due to faster competitive ET processes, e.g. migration to defect sites, a significant amount of emission energy is lost before it can be transferred to the Mn2+ ions. This behaviour increases with increasing Mn2+ concentration.
The average distance between Eu2+ and Mn2+ (REu–Mn) in the host material at which ET occurs can be estimated by applying the following formula published by Blasse:17
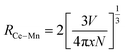 | (5) |
To elucidate the characteristics of the ET process in Ba0.99Eu0.01(Ca1−xMnx)2Si3O9, the obtained decay curves were analysed by applying the Inokuti–Hirayama model. Assuming a uniform distribution of Eu2+ and Mn2+ in the host structure, and neglecting further energy migration processes besides the ET from Eu2+ to Mn2+, the time-dependent course of the PL intensity of Eu2+ can be described by following equation:18
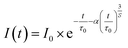 | (6) |
In this equation, t is the time, I(t) is the PL intensity after excitation, I0 is the PL intensity at t = 0, τ0 is the intrinsic luminescence lifetime of the sensitizer in the absence of the activator, α is a parameter for the probability of ET. S is related to the electronic multipole character of the ET. S = 6, 8, and 10 corresponds to dipole–dipole, dipole–quadrupole, and quadrupole–quadrupole interactions, respectively. Modifying eqn (6) leads to following relation:
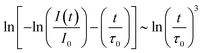 | (7) |
Plotting the PLD curves using this relation yields a straight line with a slope equal to 1/S. The obtained curves for the measured Ba0.99Eu0.01(Ca1−xMnx)2Si3O9 samples with x = 0.005, 0.010, 0.015, 0.020, 0.050, 0.100, 0.150, and 0.200 are depicted in Fig. 6. The calculated S values are roughly coincident with the theoretical value of S = 8. This result suggests dipole–quadrupole interactions as the dominant mechanism of ET from Eu2+ to Mn2+ in co-doped BaCa2Si3O9:Eu2+,Mn2+.
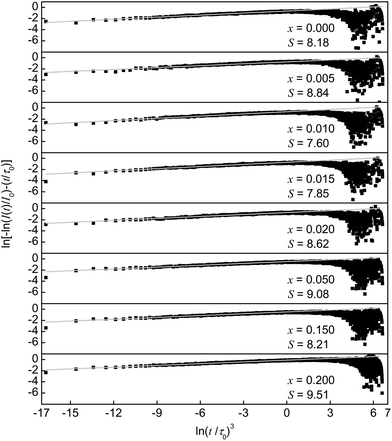 | ||
| Fig. 6 Plots of ln[−ln(I(t)/I0) − (t/τ0)] versus ln(t/τ0)3. Experimental data are represented by the dots. Grey lines represent the fitting functions. | ||
This finding is quite reasonable since the electronic [Xe]4f7–[Xe]4f65d1-transition in Eu2+ is allowed whereas the electronic [Ar]3d5–[Ar]3d5-transition in Mn2+ is forbidden. Therefore, the critical distance Rc for ET from Eu2+ to Mn2+ can be calculated using the spectral overlap method:17,19
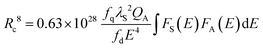 | (8) |
In this formula fq = 10−10 and fd = 10−7 are the oscillator strengths of dipole and quadrupole electronic absorption transitions, respectively, for Mn2+. The absorption cross section QA of Mn2+ can be expressed using the relationship QA = 4.8 × 10−16fd derived by Blasse. λS is the emission wavelength of Eu2+. E is the energy of maximal spectral overlap. From this, the critical distance Rc is calculated to be 8.9 Å. This value is in accordance with the one calculated using Blasse's approach (9.6 Å).
To investigate the temperature dependence of the PL of co-doped Ba0.99Eu0.01(Ca0.90Mn0.10)2Si3O9, PL spectra were recorded from 100 to 800 K. The obtained PL spectra are depicted in Fig. 7. The spectra demonstrate that with increasing temperature PL intensity of Eu2+ and Mn2+ decreases due to thermal quenching. The inset of Fig. 7 illustrates the normalized PL integrals versus their temperature. From this, activation energy EA for thermal quenching can generally be estimated by fitting the data points by a Fermi–Dirac distribution:
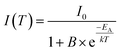 | (9) |
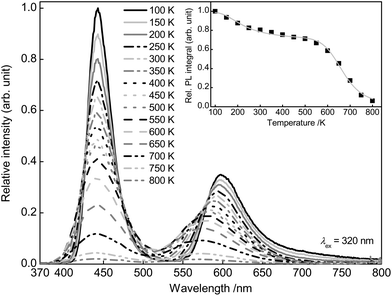 | ||
| Fig. 7 PL spectra of Ba0.99Eu0.01(Ca0.90Mn0.10)2Si3O9 from 100 to 800 K and PL integral in dependence of the temperature (inset). | ||
In this equation, I(T) is the luminescence intensity at a certain temperature and I0 is the luminescence intensity at zero Kelvin. B is the quenching frequency factor, T is the temperature and k is the Boltzmann constant. However, due to an additional turning point, the present data points cannot be fitted by ordinary Fermi–Dirac distribution. Therefore, eqn (9) is expanded to a sum of two weighed Fermi–Dirac distributions:
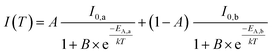 | (10) |
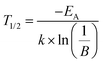 | (11) |
T 1/2 is the temperature at which PL intensity of a luminescent centre is decreased to one-half of its maximum. For Ba0.99Eu0.01(Ca0.90Mn0.10)2Si3O9, T1/2,a and T1/2,b were calculated to be 242 and 666 K, respectively. The PL intensities originating from Eu2+ and Mn2+ were plotted separately (Fig. S2 and S3 in the ESI†). From that it can be seen that the bi-sigmoidal temperature behaviour strongly decreases if investigating the PL intensities of Eu2+ solely. Due to overlapping of the red emission band the bi-sigmoidal shape does not vanish completely. To examine the origin of this double sigmoidal quenching behaviour, PL of singly doped Ba0.99Eu0.01Si3O9 was measured in the temperature range from 100 to 800 K as well (Fig. S4 in the ESI†). At low temperatures an additional emission band extending from about 600 to 750 nm arises. PLD measurements of this emission band revealed a fluorescence lifetime of about 945 ns (Fig. S5 in the ESI†). This value matches well with the decay time of Eu2+. Furthermore, the obtained PLD curve shows mono-exponential decay behaviour, indicating emission from one particular crystallographic site. These Eu2+ ions presumably occupy the 6-fold coordinated Ca2 sites in BaCa2Si3O9. On this small site, the crystal field splitting of the d-orbitals of Eu2+ is rather high because of which the lower edge of the [Xe]5d multiplet is moved towards the ground state. Due to relatively low emission energies in the red spectral range and consequently strong multi-phonon relaxation as well as large Stokes shift, this emission exhibits a lower quenching temperature. Furthermore, with increasing temperature a blue shift of Mn2+ emission can be observed. This behaviour is due to an increasing average distance between the Mn2+ ions and their oxygen ligands with increasing temperatures. As a consequence, the crystal field splitting of the 3d orbitals of Mn2+ decreases resulting in a shift of the 4T1(4G) → 6A1(6S)) transition to higher energies.20
To obtain more detailed information regarding the temperature dependent behaviour of Ba0.99Eu0.01(Ca0.90Mn0.10)2Si3O9, PLD measurements were performed from 100 to 500 K. Fig. 8 depicts the PLD curves of Ba0.99Eu0.01(Ca0.90Mn0.10)2Si3O9 monitoring the 445 nm emission of Eu2+. The curves can be best fitted with a bi-exponential function, suggesting Eu2+ emission from two distinct crystallographic sites as mentioned previously. The derived fluorescence lifetimes are presented in Table 2 and show an unusual increase with increasing temperatures. This phenomenon was only reported by a few authors and is still uncommon.21,22 According to Meijerink and Blasse, this behaviour is presumably caused by thermal population of higher energetic 4f 65d states. Excited states in Eu2+ can either be (spin) octets or sextets. The sextet states are at higher energies than the octet states according to Hund's rule. With increasing temperature the higher energetic sextet states will be populated. For these states, transition to the 8S7/2 ground state is spin-forbidden resulting in lower decay rates and thus longer fluorescence lifetimes.22 Fluorescence lifetimes of Mn2+ emission in dependency of temperature were measured, too. The obtained PLD curves are depicted in Fig. 9 and can be well fitted with a bi-exponential function. This result is consistent with the assumption made above that Mn2+ is located on two different crystallographic sites. The calculated average lifetimes of Mn2+ are summarized in Table 2. With increasing temperature, Mn2+ fluorescence lifetimes decrease continuously due to an increase in non-radiative relaxation to the ground state. Therefore, it can be concluded that thermal quenching of emission in BaCa2Si3O9:Eu2+,Mn2+ is primarily caused by Mn2+ ions. As deduced from PLD curves of Mn2+ emission as well as from ionic radii of Ba2+, Ca2+ and Mn2+, it can be concluded that Mn2+ ions preferably occupy the two Ca sites in BaCa2Si3O9. Since the ionic radii of 6- and 8-fold coordinated Mn2+ are smaller compared to those of Ca2+, the excited states of Mn2+ can strongly be polarized. This leads to an increase in the equilibrium distance between Mn2+ and its oxygen ligands and therefore to a shift of the ground and excited state parabolas in the configurational coordinate diagram. Further, the crossing point of the parabolas is shifted to lower energy, and thus to a lower activation energy for thermal quenching.
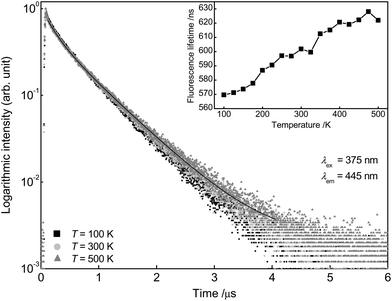 | ||
| Fig. 8 PLD curves of Ba0.99Eu0.01(Ca0.90Mn0.10)2Si3O9 at 100, 300 and 500 K monitoring the emission of Eu2+. Fluorescence lifetimes in dependency of temperature (inset). | ||
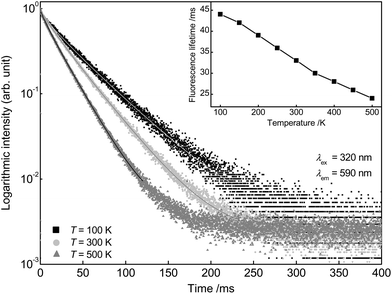 | ||
| Fig. 9 PLD curves of Ba0.99Eu0.01(Ca0.90Mn0.10)2Si3O9 at 100, 300 and 500 K monitoring the emission of Mn2+. Fluorescence lifetimes in dependency of temperature (inset). | ||
| Eu2+ | Mn2+ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T (K) | frac1 (%) | τ 1 (ns) | frac2 (%) | τ 2 (ns) | τ (ns) | frac1 (%) | τ 1 (ms) | frac2 (%) | τ 2 (ms) | τ (ms) |
| 100 | 16 | 214 | 84 | 637 | 570 | 9 | 19 | 91 | 47 | 44 |
| 125 | 15 | 205 | 85 | 637 | 571 | |||||
| 150 | 14 | 190 | 86 | 635 | 574 | 9 | 17 | 91 | 45 | 42 |
| 175 | 13 | 191 | 87 | 638 | 578 | |||||
| 200 | 12 | 184 | 88 | 644 | 587 | 6 | 13 | 94 | 41 | 39 |
| 225 | 12 | 190 | 88 | 645 | 591 | |||||
| 250 | 13 | 205 | 87 | 656 | 597 | 8 | 15 | 92 | 38 | 36 |
| 275 | 13 | 199 | 87 | 656 | 597 | |||||
| 300 | 12 | 199 | 88 | 659 | 602 | 10 | 16 | 90 | 35 | 33 |
| 325 | 13 | 187 | 87 | 663 | 600 | |||||
| 350 | 15 | 215 | 85 | 681 | 612 | 5 | 10 | 96 | 31 | 30 |
| 375 | 15 | 216 | 85 | 684 | 615 | |||||
| 400 | 14 | 216 | 86 | 687 | 621 | 8 | 12 | 92 | 29 | 28 |
| 425 | 14 | 195 | 86 | 689 | 619 | |||||
| 450 | 13 | 199 | 87 | 688 | 622 | 4 | 7 | 96 | 26 | 26 |
| 475 | 15 | 211 | 85 | 701 | 628 | |||||
| 500 | 13 | 201 | 87 | 687 | 622 | 4 | 7 | 96 | 24 | 24 |
To investigate the PL efficiency of the Ba0.99Eu0.01(Ca1−xMnx)2Si3O9 samples, external quantum efficiencies ηext were measured as mentioned above. To the best of our understanding, external quantum efficiency ηext is defined as:
 | (12) |
| Sample | η ext | CIE (x|y) |
|---|---|---|
| x = 0.000 | 0.31 | (0.151|0.043) |
| x = 0.005 | 0.31 | (0.163|0.053) |
| x = 0.010 | 0.30 | (0.170|0.060) |
| x = 0.015 | 0.31 | (0.182|0.073) |
| x = 0.020 | 0.32 | (0.190|0.080) |
| x = 0.050 | 0.34 | (0.224|0.112) |
| x = 0.100 | 0.36 | (0.260|0.146) |
| x = 0.150 | 0.26 | (0.323|0.210) |
| x = 0.200 | 0.30 | (0.354|0.240) |
According to the relevant PL spectra, Commission International de l'Eclairage 1931 (CIE) chromaticity coordinates were calculated. Fig. 10 depicts the CIE chromaticity diagram for Ba0.99Eu0.01(Ca1−xMnx)2Si3O9 with x = 0, 0.005, 0.010, 0.015, 0.020, 0.050, 0.100, 0.150, and 0.200. By increasing the Mn2+ concentration, the chromaticity coordinates can be shifted from blue to magenta colour tones. Due to the lack of emission in the green spectral range, BaCa2Si3O9:Eu2+,Mn2+ has to be combined with an additional green emitting phosphor to generate white light, such as (Ba,Sr)2SiO4:Eu2+, Sr12Al14O32Cl2:Eu2+ or KBaBP2O8:Tb3+.23 Fig. S6† shows a simulated white emission spectrum consisting of Ba0.99Eu0.01Ca(0.80Mn0.20)2Si3O9 and (Ba0.49Sr0.49Eu0.02)2SiO4. The CIE 1931 colour coordinates of the spectrum are x = 0.327 and y = 0.333 which result in a correlated colour temperature (CCT) of 5745 K. The colour rendering index (CRI) Ra of the simulated spectrum is equal to 82. This CCT is similar to that of a phosphor-converted white LED consisting of a blue LED chip and YAG:Ce3+ (CCT = 5600 K) whereas the CRI is higher compared to that of this phosphor-converted LED (Ra = 71).24
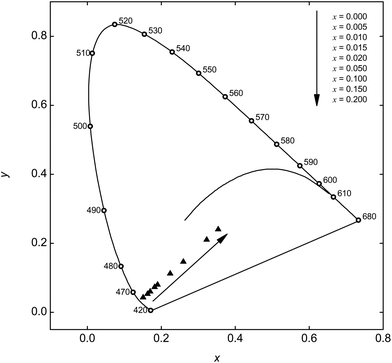 | ||
| Fig. 10 CIE chromaticity diagram of Ba0.99Eu0.01(Ca1−xMnx)2Si3O9 with different Mn2+ concentrations. | ||
4. Conclusions
A series of BaCa2Si3O9:Eu2+,Mn2+ powder samples with various Mn2+ concentrations was prepared via high temperature solid state synthesis. Excited by UV radiation, BaCa2Si3O9:Eu2+,Mn2+ shows two emission bands located at about 444 and 594 nm originating from Eu2+ and Mn2+, respectively. It turned out that Eu2+ ions in BaCa2Si3O9:Eu2+,Mn2+ occupy the 8-fold coordinated Ba and Ca1 sites as well as the 6-fold coordinated Ca2 sites. Mn2+ ions tend to occupy both Ca sites. These results are confirmed by PLD measurements. The highest PL intensity of Mn2+ was found at a Mn2+ concentration of x = 0.15. It was further demonstrated that ET from Eu2+ to Mn2+ in BaCa2Si3O9:Eu2+,Mn2+ is of resonant type and occurs via dipole–quadrupole interaction. The critical distance Rc between Eu2+ and Mn2+ was derived by using Blasse's concentration quenching method as well as the spectral overlap method and was calculated to be 9.6 and 8.9 Å, respectively. Temperature dependent PL measurements prove a “double” sigmoidal decrease of the PL intensity of Eu2+. Therefore, BaCa2Si3O9:Eu2+,Mn2+ has two T1/2 values, T1/2,a and T1/2,b, which were calculated to be 242 and 666 K, respectively. In addition to that, PLD measurements revealed an unusual increase of Eu2+ fluorescence lifetime, which presumably originates from thermal population of higher excited energy levels. Temperature dependent PLD measurements on the Mn2+ emission suggest that thermal quenching in BaCa2Si3O9:Eu2+,Mn2+ is primarily caused by Mn2+ ions. CIE 1931 colour points can be tuned from the blue to the magenta colour range.Acknowledgements
The authors are grateful to Merck KGaA Darmstadt, Germany, for generous financial support.Notes and references
- S. Nakamura, P. Stephen and F. Gerhard, The Blue Laser Diode: The Complete Story, Springer-Verlag, Berlin/Heidelberg, 1997 Search PubMed.
- (a) C. Feldmann, T. Jüstel, C. R. Ronda and P. J. Schmidt, Adv. Funct. Mater., 2003, 13, 511–516 CrossRef CAS PubMed; (b) E. F. Schubert and J. K. Kim, Science, 2005, 308, 1274–1278 CrossRef CAS PubMed.
- H. S. Jang, W. B. Im, D. C. Lee, D. Y. Jeon and S. S. Kim, J. Lumin., 2007, 126, 371–377 CrossRef CAS PubMed.
- Y. Uchida and T. Taguchi, Opt. Eng., 2005, 44, 124003 CrossRef PubMed.
- M. Shang, C. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 1372 RSC.
- (a) W. Lv, M. Jiao, Q. Zhao, B. Shao, W. Lü and H. You, Inorg. Chem., 2014, 53, 11007–11014 CrossRef CAS PubMed; (b) W.-J. Yang and T.-M. Chen, Appl. Phys. Lett., 2006, 88, 101903 CrossRef PubMed.
- P. Eskola, Am. J. Sci., 1922, 4, 331–375 CrossRef CAS.
- J. T. Alfors, M. C. Stinson and R. A. Matthews, Am. Mineral., 1965, 50, 314–340 CAS.
- L. S. Dent Glasser and F. P. Glasser, Am. Mineral., 1968, 53, 9–13 Search PubMed.
- M. C. Barkley, R. T. Downs and H. Yang, Am. Mineral., 2011, 96, 797–801 CrossRef CAS.
- S. Yao, L. Xue and Y. Yan, Opt. Laser Technol., 2011, 43, 1282–1285 CrossRef CAS PubMed.
- M. Gaft, H. Yeates and L. Nagli, Eur. J. Mineral., 2013, 25, 71–77 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Cryst., 1976, 32, 751–767 CrossRef.
- Y. Kawamura, H. Sasabe and C. Adachi, Jpn. J. Appl. Phys., 2004, 43, 7729–7730 CrossRef CAS.
- (a) A. Mehra, Jpn. J. Appl. Phys., 1968, 7, 963–964 CrossRef CAS; (b) E. van der Kolk, P. Dorenbos, C. W. van Eijk, A. P. Vink, M. Weil and J. P. Chaminade, J. Appl. Phys., 2004, 95, 7867 CrossRef CAS PubMed.
- (a) H. C. Kandpal and H. B. Tripathi, Indian J. Pure Appl. Phys., 1979, 17, 587–589 CAS; (b) P. Paulose, G. Jose, V. Thomas, N. Unnikrishnan and M. Warrier, J. Phys. Chem. Solids, 2003, 64, 841–846 CrossRef CAS.
- G. Blasse, Philips Res. Rep., 1969, 24, 131–144 CAS.
- M. Inokuti and F. Hirayama, J. Chem. Phys., 1965, 43, 1978 CrossRef CAS PubMed.
- D. L. Dexter, J. Chem. Phys., 1953, 21, 836 CrossRef CAS PubMed.
- Y. Tanabe and S. Sugano, J. Phys. Soc. Jpn., 1954, 9, 766–779 CrossRef CAS.
- (a) J. P. Spoonhower and M. S. Burberry, J. Lumin., 1989, 43, 221–226 CrossRef CAS; (b) T. Tsuboi and P. Silfsten, J. Phys.: Condens. Matter, 1991, 3, 9163–9167 CrossRef CAS; (c) C. K. Duan, A. Meijerink, R. J. Reeves and M. F. Reid, J. Alloys Compd., 2006, 408–412, 784–787 CrossRef CAS PubMed.
- A. Meijerink and G. Blasse, J. Lumin., 1990, 47, 1–5 CrossRef CAS.
- (a) B. Han, J. Zhang, P. Li and H. Shi, Phys. Status Solidi A, 2014, 211, 2483–2487 CrossRef CAS PubMed; (b) Z. Jiang, Z. Sun, X. Su, L. Duan and X. Yu, J. Alloys Compd., 2013, 577, 683–686 CrossRef CAS PubMed; (c) T. L. Barry, J. Electrochem. Soc., 1968, 115, 1181–1184 CrossRef CAS PubMed.
- M. R. Krames, O. B. Shchekin, R. Mueller-Mach, G. O. Mueller, L. Zhou, G. Harbers and M. G. Craford, J. Disp. Technol., 2007, 3, 160–175 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: PLD curves of Ba0.99Eu0.01Ca2Si3O9 monitored at 415 and 495 nm, PL integrals of Eu2+ and Mn2+ in dependence of temperature, PL spectra of Ba0.99Eu0.01Ca2Si3O9 from 100 to 800 K and PL integral in dependence of temperature, PLD curve of Ba0.99Eu0.01Ca2Si3O9 monitored at λem = 700 nm and T = 77 K, simulated white emission spectrum. See DOI: 10.1039/c5dt00591d |
| This journal is © The Royal Society of Chemistry 2015 |