Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
C^C* cyclometalated platinum(II) N-heterocyclic carbene complexes with a sterically demanding β-diketonato ligand – synthesis, characterization and photophysical properties†
M.
Tenne
a,
S.
Metz
b,
G.
Wagenblast
b,
Ingo
Münster
b and
T.
Strassner
*a
aPhysikalische Organische Chemie, Technische Universität Dresden, 01069 Dresden, Germany. E-mail: thomas.strassner@chemie.tu-dresden.de
bBASF SE, 67056 Ludwigshafen, Germany
First published on 17th April 2015
Abstract
Neutral cyclometalated platinum(II) N-heterocyclic carbene complexes [Pt(C^C*)(O^O)] with C^C* ligands based on 1-phenyl-1,2,4-triazol-5-ylidene and 4-phenyl-1,2,4-triazol-5-ylidene, as well as acetylacetonato (O^O = acac) and 1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato (O^O = mesacac) ancillary ligands were synthesized and characterized. All complexes are emissive at room temperature in a poly(methyl methacrylate) (PMMA) matrix with emission maxima in the blue region of the spectrum. High quantum efficiencies and short decay times were observed for all complexes with mesacac ancillary ligands. The sterically demanding mesityl groups of the mesacac ligand effectively prevent molecular stacking. The emission behavior of these emitters is in general independent of the position of the nitrogen in the backbone of the N-heterocyclic carbene (NHC) unit and a variety of substituents in 4-position of the phenyl unit, meta to the cyclometalating bond.
Introduction
Square-planar platinum(II) complexes have proven to be strong phosphorescent compounds, and have been extensively explored with respect to a broad field of promising applications, such as chemosensors,1–6 non-linear optical (NLO) materials,7–9 photocatalysts,10–14 optical power limiting materials (OPL),15 photovoltaic devices,16,17 and organic light emitting devices (OLEDs).18–25 Concerning the field of OLED technology especially blue emitting compounds are required to develop full-color displays and efficient lighting devices. Cyclometalated platinum(II) complexes represent a promising class for this purpose, as shown from several reports.26–37 The remarkable phosphorescence of platinum(II) complexes originates from the metal ion, which promotes a strong spin–orbit coupling, and thereby allows for efficient intersystem crossing to the triplet state.38 Cyclometalating ligands also allow for an efficient emission behavior as their strong ligand field ensures a high stability of the complexes and raises the energy of the metal centered (MC) states in the platinum(II) complexes to avoid non-radiative decay.21 In particular platinum(II) complexes with cyclometalating C^N ligands attract a lot of scientific interest. Especially those with the general structure [Pt(C^N)(O^O)] (O^O = β-diketonato) turned out to be a stable and strongly phosphorescent class of emitter molecules. They allow to cover a broad range of the visual spectrum by variation of the C^N cyclometalating ligands and β-diketonato ancillary ligands39–52 Low-energy emissions are also accessible from dimeric or oligomeric complex species. As a consequence of their planar structure, cyclometalated platinum(II) complexes often show molecular stacking by Pt–Pt bonds and π–π interactions, which promotes the formation of broad emission bands at higher wavelengths when compared to the monomer emissions.53–57 Thus planar cyclometalated platinum(II) complexes are often discussed as emitters for single dopant white-light emitting diodes.19,34,58–61After the first report of a C^C* cyclometalated platinum(II) complex in 200662 C^C* cyclometalating ligands have also been used for the development of new phosphorescent platinum(II) emitters of the general structure [Pt(C^C*)(O^O)] (O^O = β-diketonato).63–68 This class of complexes reveals an extraordinary photoluminescent behavior, which strongly depends on the structure of the C^C* cyclometalating ligand (C: carbene carbon atom; C*: cyclometalated carbon atom), as well as on the O^O chelating ligand. Recently, several promising [Pt(C^C*)(O^O)] emitters have been reported, which emit in the green-blue region of the spectrum with quantum efficiencies of more than 90%.66,69,70 It also has been demonstrated that exceptionally high quantum yields can be achieved by using mesityl substituted β-diketonato ligands (mesacac = 1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato).71
Herein we report the synthesis and the photoluminescent properties of a series of new phenyl-1,2,4-triazol-5-ylidene complexes to discuss the influence of the mesityl substituted β-diketonato ligand 1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato (mesacac) and several modifications of the C^C* cyclometalating ligand on the emission behavior.
Results and discussion
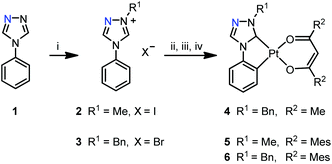
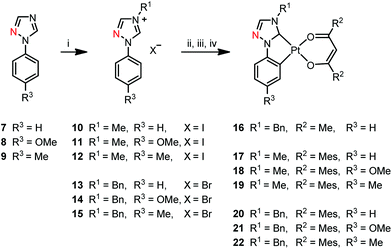
Synthesis
The synthesis of the triazoles 1 and 7–9, and the methyl substituted triazolium salts 2 and 10–12 (Schemes 1 and 2) has been reported previously.68 The triazolium salts 3 and 13–15 were prepared by a quaternisation reaction using benzyl bromide (BnBr), 2 and 10–12 using iodomethane in tetrahydrofuran (THF). All complexes were synthesized in moderate to good yields by a multistep-reaction.66,68 The triazolium salts were reacted with silver(I) oxide in 1,4-dioxane to generate a silver(I) carbene complex in situ, which was then transmetalated with dichloro(1,5-cyclooctadiene)platinum(II) (Pt(COD)Cl2) in a 1,4-dioxane/butanone solvent mixture and cyclometalated at the C2 carbon atom of the phenyl ring at higher temperatures. The reported complexes were obtained after removal of the volatiles by reaction of the intermediate with the respective β-diketone and potassium tert-butoxide (KOtBu) in dimethylformamide (DMF).Although this method was successful with imidazolium-based ligand precursors, the synthesis of platinum(II) triazol-5-ylidene complexes with mesacac ancillary ligands suffered from unsatisfying yields. Optimization of the reaction conditions leads to improved yields by using DMF already for the generation of the silver(I)carbene as well as for the transmetalation step. In comparison to the previously used 1,4-dioxane/butanone solvent mixture, we could more than double the yield of complex 17 (see exp. details, compound 17, method B).
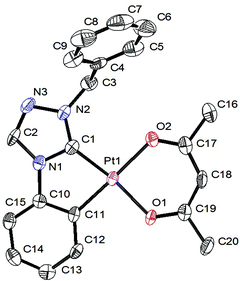
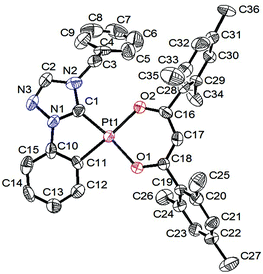
Solid state structure determination
Single crystals suitable for X-ray diffraction analysis were grown from 4 by slow vapor diffusion of diethyl ether into a highly concentrated solution of the respective complex in dichloromethane and from 20 by slow evaporation of the solvent from the solution of the complex in dichloromethane. All details of the solid state structure determinations are summarized in the ESI.† The complexes 4 (Fig. 1 and 2) and 20 (Fig. 3 and 4) crystallize in the monoclinic space group P21/c and are devoid of any solvent molecules. X-ray diffraction analysis confirms a quasi-square planar coordination environment of the platinum(II) center with bond lengths of the coordinative bonds similar to those of the previously reported [Pt(C^C*)(O^O)] complexes.68,71,72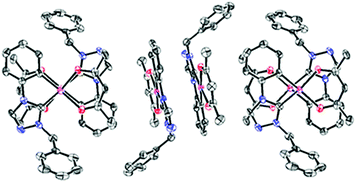 | ||
| Fig. 2 Intermolecular arrangement of 4 in the solid state. All hydrogen atoms are omitted for clarity. | ||
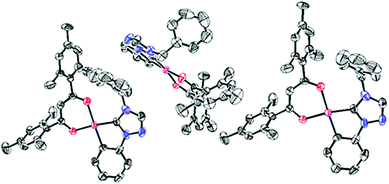 | ||
| Fig. 4 Intermolecular arrangement of 20 in the solid state. All hydrogen atoms are omitted for clarity. | ||
The Pt(1)–O(2) bond lengths of both complexes are slightly elongated when compared to the Pt(1)–O(1) bond lengths, due to the trans effect of the cyclometalated phenyl ring.
The planar structure with coplanar arranged ligands allows 4 to stack with short intermolecular distances. In the solid state two of the molecules form dimers with a Pt–Pt contact shorter than twice the van-der-Waals radius of the platinum atom. However, in the case of 20 the sterically demanding mesityl groups of the β-diketonato ancillary ligand are twisted out of the coordination plane. This efficiently prevents molecular stacking of these complexes so that they are orthogonally arranged to each other.
Photoluminescence properties
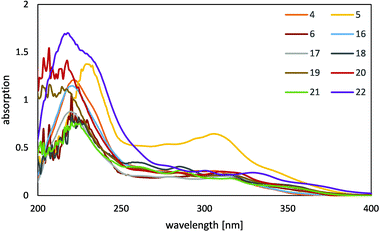
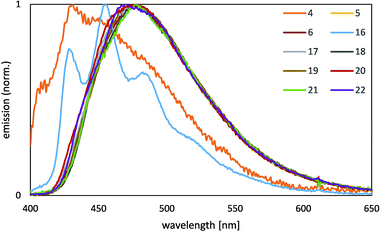
This class of complexes shows very weak emissions from a solution in dichloromethane (see ESI†), but are significantly stronger emissive in a poly(methyl methacrylate) (PMMA) matrix. Therefore, the photophysical properties of all complexes were investigated from polymer films with 2% of the complexes in a PMMA matrix at room temperature. Emission spectra of 5–6 and 16–21 from neat complex films were also recorded to get an insight into the emission behavior of agglomerated complexes. Neat emitter films of 4 and 22 could not be obtained due to rapid crystallization of the complexes during the film preparation. The absorption spectra of all complexes in a PMMA matrix (Fig. 5) display strong bands below 270 nm, which arise from spin allowed 1π–π transitions. In this region the absorption maximum of the complex with a 1-benzyl-4-phenyl triazol-5-ylidene unit and mesacac ancillary ligand 5 is located at around 230 nm, whereas the absorption maxima of all other complexes are detected at around 220 nm. Further bands of moderate intensity up to 350 nm arise from transitions involving the metal center, such as a metal-to-ligand charge transfer 1MLCT transition. Here complex 5 exhibits another absorption maximum at around 310 nm.All complexes are strongly emissive in a PMMA matrix at room temperature. The emission spectra (Fig. 6) and the PL data (Table 1) of 4 and 16 correspond well to those of analogous platinum(II) imidazol-2-ylidene complexes containing an acetylacetonato ancillary ligand and a methyl group instead of a benzyl group bound to the imidazol-N3 atom.68 This clearly indicates that the emission behavior of these complexes is independent of the benzyl group (R1). Both emitters display a structured emission band with the maximum located in the blue region of the spectrum, which indicates strong contributions of a ligand centered state (3LC) to the emission process. The well-structured emission band of 16 reveals a vibronical progression of about 1300 cm−1 which corresponds to vibrations of the NHC ligand as determined by the frequency analysis from the density functional theory (DFT) calculations. However, complexes 5–6 and 17–22 reveal an essentially different emission behavior, which is caused by the mesacac ancillary ligand. In contrast to the vibronic structure of the 3LC emission profiles of the emitters containing an acac ancillary ligand (4 and 16), the complexes 5–6 and 17–22 exhibit unstructured emission bands with only one maximum located between 471 and 478 nm. Therefore, another transition contributes essentially to the emission process of the complexes with a mesacac ligand. This transition is supposed to be a charge transfer transition involving the metal center, as the complexes with a mesacac ligand also show significantly enhanced quantum efficiencies (70–83%) and reduced decay times below 8 μs (Table 1). Due to the change in the emission process to a transition with reduced 3LC character, different substituents at the cyclometalated ligand do not significantly change the emission properties, like quantum yield and emission wavelength. All complexes, even the emitters based on 4-phenyl triazole 5 and 6 reveal high quantum yields, whereas very low efficiencies have been previously reported for several 4-phenyl triazole complexes with acac ancillary ligands in comparison to the 1-phenyl triazole complexes with acac ancillary ligands.68 While in the case of 1-phenyl and 4-phenyl triazole complexes with acac ligands, substitution in 4-position of the phenyl ring of the cyclometalating ligand had a significant effect on the emission wavelengths, this was not observed for the complexes with mesacac ligands. Although the emissions of the respective acac complexes are shifted slightly to lower energies by several substituents, this effect is relatively small for the mesacac complexes 18, 19, 21 and 22.
λ
exc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [nm] a [nm] |
CIE x; yb |
λ
em![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c [nm] c [nm] |
Φ |
τ
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e [μs] e [μs] |
|
|---|---|---|---|---|---|
| a Excitation wavelength. b CIE coordinates. c Max. emission wave-length. d Quantum yield at λexc, N2 atmosphere. e Decay lifetimes (excited by laser pulses (355 nm, 1 ns)) given as τo = τv/ϕ. | |||||
| 4 | 330 | 0.159; 0.157 | 455 | 0.35 | 15 |
| 5 | 345 | 0.183; 0.283 | 477 | 0.82 | 4 |
| 6 | 355 | 0.183; 0.287 | 477 | 0.83 | 4 |
| 16 | 330 | 0.166; 0.165 | 432 | 0.13 | 15 |
| 17 | 340 | 0.185; 0.284 | 478 | 0.82 | 4 |
| 18 | 355 | 0.185; 0.295 | 477 | 0.82 | 5 |
| 19 | 355 | 0.186; 0.294 | 475 | 0.82 | 3 |
| 20 | 370 | 0.182; 0.271 | 477 | 0.72 | 5 |
| 21 | 370 | 0.188; 0.304 | 478 | 0.70 | 7 |
| 22 | 340 | 0.184; 0.279 | 471 | 0.81 | 5 |
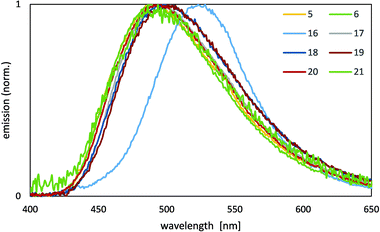
For the acac complexes 4 and 16 we could only get the neat film emission for 16 as 4 shows early crystallization. These are the only two complexes prone for stacking as the mesacac prevents short intermolecular contacts as shown in the solid state structure of 20. The PL spectrum of the neat film of 16 (Fig. 7) reveals a low energy emission in the green region of the spectrum, similar to other acac complexes [Pt(C^C*)(acac)].68 This emission is probably caused by a multi-molecular species, such as a stack of emitter molecules. The planar geometry of the complexes with acac ligands supports aggregation in the solid state with a short intermolecular separation and Pt–Pt distances lower than twice the van-der-Waals radius of platinum. The quantum efficiency of the neat film of 16 (Table 2) is significantly higher than that of the complex in a 2% PMMA matrix. In contrast to 16, the emissions from the neat films of the mesacac complexes 5, 6 and 17–21 correspond well to the results of the measurements of 2% complex in a PMMA matrix, although the emission maxima are slightly shifted to higher wavelengths by 7–22 nm. With respect to the results of the molecular structure determination, which shows no stacking behaviour of 20 in the solid state, the emissions from the neat films of 5–6 and 17–21 are expected to be mono-molecular emissions.
λ
exc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [nm] a [nm] |
CIE x; yb |
λ
em![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c [nm] c [nm] |
Φ |
τ
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e [μs] e [μs] |
|
|---|---|---|---|---|---|
| a Excitation wavelength. b CIE coordinates. c Max. emission wave-length. d Quantum yield at λexc, N2 atmosphere. e Decay lifetimes (excited by laser pulses (355 nm, 1 ns)) given as τo = τv/ϕ. | |||||
| 5 | 345 | 0.222; 0.385 | 489 | 0.28 | 5 |
| 6 | 355 | 0.215; 0.384 | 490 | 0.36 | 6 |
| 16 | 330 | 0.159; 0.157 | 523 | 0.23 | 4 |
| 17 | 340 | 0.232; 0.419 | 495 | 0.42 | 4 |
| 18 | 355 | 0.245; 0.433 | 499 | 0.23 | 5 |
| 19 | 355 | 0.247; 0.441 | 502 | 0.21 | 6 |
| 20 | 370 | 0.225; 0.398 | 494 | 0.46 | 4 |
| 21 | 370 | 0.233; 0.397 | 485 | 0.09 | 8 |
DFT calculations
We recently published a methodology which allows us to predict the emission wavelengths of the platinum complexes.73 Also for the new complexes reported in this study we could successfully use this methodology, where we optimize the singlet and triplet state geometries of all complexes in the gas phase using DFT methods, e.g. BP86 together with a 6-31G(d) basis set and Hay-Wadt-ECP for platinum. The difference between the calculated and the measured wavelength (see Table S2, ESI†) turned out to be ≤10 nm for all complexes reported here with the exception of 4 (21 nm).The geometries of the optimized complexes 4 and 20 are also in good agreement with the data of the solid state structures obtained by X-ray diffraction analysis, which confirms that the DFT functionals used are capable of describing the bonding situation in these complexes.
Conclusion
We synthesized ten new platinum(II) complexes with C^C* cyclometalating ligands based on 1-phenyl-1,2,4-triazol-5-ylidene and 4-phenyl-1,2,4-triazol-5-ylidene, as well as acetylacetonato (O^O = acac) and 1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato) (O^O = mesacac) ancillary ligands. The complexes were thoroughly characterized and investigated in terms of their photophysical properties for a potential application as emitter molecules for OLEDs. The solid-state structure determination of two complexes reveals the steric effect of the O^O ancillary ligands on the stacking behavior of the complexes, which consequently has an impact on the emissions of the neat emitter films. The emission properties of the complexes strongly depend on the O^O ligand, but less on the substituents in 4-position of the cyclometalating phenyl ring. All complexes containing 1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato as O^O ligand are strongly emissive at lower energies and reveal shorter decay times in comparison to the emitters with an acetylacetonato ancillary ligand.Experimental details
The syntheses of the complexes were performed under an argon atmosphere, using common Schlenk techniques and dry solvents. DMF and 1,4-dioxane were dried according to standard techniques and stored over molecular sieve 4 Å. All other chemicals were used as purchased. 4-Phenyl-4H-1,2,4-triazole 1,74 1-phenyl-1H-1,2,4-triazole 7,75 1-(4-methoxyphenyl)-1H-1,2,4-triazole 8, 1-(4-methylphenyl)-1H-1,2,4-triazole 9, 1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato and dichloro(1,5-cyclooctadiene)platinum(II)76 were synthesized according to published procedures.66,68,77,78 Potassium tetrachloroplatinate(II) was purchased from Pressure Chemicals Co. NMR spectra were recorded on a Bruker NMR spectrometer and referenced to the internal resonances of the solvents (1H: 7.26, 13C: 77.0 for CDCl3; 1H: 2.50, 13C: 39.43 for DMSO-d6). 195Pt spectra were referenced to potassium tetrachloroplatinate(II) in D2O (–1617.2 (PtCl42−), −2654.1 (PtCl2)) as external standard. Shifts are given in ppm, coupling constants J in Hz.Elemental analyses were performed by the analytical laboratory of the department using an Eurovektor Hekatech EA-3000 elemental analyzer. Melting points are not corrected. Photoluminescence measurements have been performed in thin PMMA films doped with 2 wt% of the emitter or from the neat emitter films. The films were prepared by doctor blading a solution of emitter (2 mg mL−1) in a 10 wt% PMMA solution in dichloromethane on a substrate with a 60 μm doctor blade. The film was dried and the emission was measured under nitrogen. The excitation was carried out at a wavelength of 325–370 nm (Xe-lamp with monochromator) and the emission was detected with a calibrated quantum-yield detection system (Hamamatsu, model C9920-02). The phosphorescence decay was measured by excitation with pulses of a THG-NdYAG laser (355 nm, 1 ns) and time-resolved photon counting by multichannel scaling (MCS) technique.
General procedure for the synthesis of platinum(II) complexes
A flame-dried schlenk tube was charged with triazolium salt and silver(I) oxide. The reactants were dried in vacuo and the tube was refilled with argon. 1,4-Dioxane was added and the mixture was stirred for 21 h at room temperature under a positive pressure of argon. Afterwards, dichloro(1,5-cyclooctadiene)platinum(II) and butanone were added, and the mixture was slowly heated and stirred for 21 h at 115 °C. The solvent was removed under reduced pressure and the remaining solid was dissolved in DMF. Potassium tert-butoxide and β-diketone were added, and the mixture was stirred for 21 h at room temperature and another 6 h at 100 °C. Afterwards the solvent was removed under reduced pressure and the residue was washed with water and purified by flash chromatography.Synthesis of 1-benzyl-4-phenyl-1,2,4-triazolium bromide 3
In a pressure tube 581 mg 4-phenyl-4H-1,2,4-triazole 1 (4 mmol) was dissolved in 3 mL of THF. 0.96 mL of benzyl bromide (8 mmol, 1.37 g, 2 eq.) were added, the pressure tube was sealed and the mixture was stirred for 22 h at 100 °C. After cooling to room temperature, the solid was filtered, washed with THF, and dried in vacuo to yield a white powder (1.21 g, 3.81 mmol, 95%). Mp: 155 °C. 1H-NMR (500.13 MHz, DMSO-d6): δ 10.98 (s, 1 H, NCHN); 9.79 (s, 1 H, NCHN); 7.86 (d, 3JHH = 7.2 Hz, 2 H, C2Harom and C6Harom of phenyl ring); 7.71 (t, 3JHH = 7.7 Hz, 2 H, C3Harom and C5Harom of phenyl ring); 7.65 (t, 3JHH = 7.4 Hz, 1 H, C4Harom of phenyl ring); 7.55 (d, 3JHH = 7.7 Hz, 2 H, C2Harom and C6Harom of benzyl group); 7.50–7.36 (m, 3 H, C3Harom–C5Harom of benzyl group); 5.71 (s, 2 H, CH2) ppm. 13C-NMR (125.75 MHz, DMSO-d6): δ 143.4 (NCHN); 141.9 (NCHN); 133.0 (C1arom); 132.2 (C1arom); 130.5 (C4Harom of phenyl ring); 130.2 (C3Harom and C5Harom of phenyl ring); 129.1 (C2Harom and C6Harom of benzyl group); 129.0 (C4Harom of benzyl group); 128.8 (C3Harom and C5Harom of benzyl group); 122.6 (C2Harom and C6Harom of phenyl ring); 55.0 (CH2) ppm. Anal. Calcd for C15H14BrN3: C, 56.98; H, 4.46; N, 13.29. Found: C, 57.15; H, 4.63; N, 13.26.Synthesis of (pentan-2,4-dionato-κ2O,O′)(1-benzyl-4-phenyl-κC2-1,2,4-triazol-5-ylidene-κC5)platinum(II) 4
253 mg triazolium bromide 3 (0.8 mmol), 93 mg silver(I) oxide (0.4 mmol, 0.5 eq.), 449 mg dichloro(1,5-cyclooctadiene)platinum(II) (1.2 mmol, 1.5 eq.), 359 mg potassium tert-butoxide (3.2 mmol, 4 eq.), and 0.33 mL of pentane-2,4-dione (3.2 mmol, 320 mg, 4 eq.) were reacted in 20 mL of 1,4-dioxane, 10 mL of butanone, and 20 mL of DMF according to the general procedure. The crude product was purified by flash chromatography (KG60, dichloromethane) to obtain a white powder (130 mg, 0.25 mmol, 31%). Mp: 211 °C. 1H-NMR (300.13 MHz, CDCl3) δ 8.22 (s, 1 H, NCHN); 7.84 (pseudo-t, d, 3JPtH = 26.3 Hz, 3JHH = 7.0 Hz, 1 H, C3Harom of phenyl ring); 7.57 (d, 3JHH = 7.6 Hz, 2 H, C2Harom and C6Harom of benzyl group); 7.34 (m, 3 H, C3Harom–C5Harom of benzyl group); 7.08 (m, 3 H, C4Harom–C6Harom of phenyl ring); 5.85 (s, 2 H; CH2); 5.57 (s, 1 H, CH); 2.11 (s, 3 H, CH3) 1.97 (s, 3 H, CH3) ppm. 13C-NMR (75.48 MHz, CDCl3) δ 185.4 (CO); 185.3 (CO); 143.3 (C1arom of phenyl ring); 136.3 (Cipso); 135.8 (Cipso); 132.4 (C3Harom of phenyl ring); 128.8 (CHarom of benzyl group); 128.7 (CHarom of benzyl group); 128.7 (CHarom of benzyl group); 128.3 (NCHN); 125.4 (CHarom of phenyl ring); 124.4 (C1arom of phenyl ring); 123.9 (C5Harom of phenyl ring); 110.8 (CHarom of phenyl ring); 102.3 (CH); 53.6 (CH2); 28.0 (CH3); 27.8 (CH3) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3393.4 ppm. Anal. Calcd for C20H18N3O2Pt: C, 45.46; H, 3.62; N, 7.95. Found: C, 45.43; H, 3.53; N, 7.97.Synthesis of (1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato-κ2O,O′)(4-phenyl-κC2-1-methyl-1,2,4-triazol-5-ylidene-κC5)platinum(II) 5
459 mg triazolium iodide 2 (1.6 mmol), 185 mg silver(I) oxide (0.8 mmol, 0.5 eq.), 898 mg dichloro(1,5-cyclooctadiene)platinum(II) (2.4 mmol, 1.5 eq.), 718 mg potassium tert-butoxide (6.4 mmol, 4 eq.), and 1970 mg 1,3-bis(2,4,6-trimethylphenyl)-propan-1,3-dione (6.4 mmol, 4 eq.) were reacted in 20 mL of 1,4-dioxane, 10 mL of butanone, and 20 mL of DMF according to the general procedure. The obtained solid was purified by flash chromatography (KG60, dichloromethane/isohexanes solvent mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and the crude product was recrystallized from isohexanes, which in the following turned out to be hard to remove. The product was obtained as a yellow powder (177 mg, 0.27 mmol, 17%). Mp: 79 °C. 1H-NMR (600.16 MHz, CDCl3) δ 8.25 (s, 1 H, NCHN); 7.74 (d, pseudo-t, 3JHH = 7.4 Hz, 3JPtH = 20.9 Hz, 1 H, C3Harom of phenyl ring); 7.09 (d, 3JHH = 7.6 Hz, 1 H, C6Harom of phenyl ring); 7.06 (t, 3JHH = 7.5 Hz, 1 H, C5Harom of phenyl ring); 7.01 (t, 3JHH = 7.4 Hz, 1 H, C4Harom of phenyl ring); 6.87 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.84 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.72 (s, 1 H, (CO)CH(CO)); 4.11 (s, 3 H, NCH3); 2.35 (s, 6 H, o-CH3 of mesityl group); 2.32 (s, 6 H, o-CH3 of mesityl group); 2.31(s, 3 H, p-CH3 of mesityl group); 2.29 (s, 3 H, p-CH3 of mesityl group) ppm. 13C-NMR (150.93 MHz, CDCl3) δ 185.4 (CO); 184.7 (CO); 152.5 (NCN); 143.4 (C1arom of phenyl ring); 139.4 (C1arom of mesityl group); 139.0 (C1arom of mesityl group); 137.8 (C4arom of mesityl group); 137.6 (C4arom of mesityl group); 135.8 (NCHN); 134.2 (C2arom and C6arom of mesityl group); 133.7 (C2arom and C6arom of mesityl group); 132.9 (C3Harom of phenyl ring); 128.2 (C3Harom and C5Harom of mesityl group); 128.1 (C3Harom and C5Harom of mesityl group); 125.5 (C4Harom of phenyl ring); 124.1 (C5Harom of phenyl ring); 123.9 (C2arom of phenyl ring); 110.8 (C6Harom of phenyl ring); 107.2 ((CO)CH(CO)); 37.5 (NCH3); 21.1 (p-CH3 of mesityl group); 21.1 (p-CH3 of mesityl group); 19.9 (o-CH3 of mesityl group); 19.6 (o-CH3 of mesityl group) ppm. 195Pt-NMR (128.56 MHz, CDCl3) δ −3342.3 ppm. Anal Calcd for C30H31N3O2Pt · 0.7 C6H14: C, 57.08; H, 5.52; N, 5.84. Found: C, 57.24; H, 5.38; N, 5.90.
1)) and the crude product was recrystallized from isohexanes, which in the following turned out to be hard to remove. The product was obtained as a yellow powder (177 mg, 0.27 mmol, 17%). Mp: 79 °C. 1H-NMR (600.16 MHz, CDCl3) δ 8.25 (s, 1 H, NCHN); 7.74 (d, pseudo-t, 3JHH = 7.4 Hz, 3JPtH = 20.9 Hz, 1 H, C3Harom of phenyl ring); 7.09 (d, 3JHH = 7.6 Hz, 1 H, C6Harom of phenyl ring); 7.06 (t, 3JHH = 7.5 Hz, 1 H, C5Harom of phenyl ring); 7.01 (t, 3JHH = 7.4 Hz, 1 H, C4Harom of phenyl ring); 6.87 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.84 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.72 (s, 1 H, (CO)CH(CO)); 4.11 (s, 3 H, NCH3); 2.35 (s, 6 H, o-CH3 of mesityl group); 2.32 (s, 6 H, o-CH3 of mesityl group); 2.31(s, 3 H, p-CH3 of mesityl group); 2.29 (s, 3 H, p-CH3 of mesityl group) ppm. 13C-NMR (150.93 MHz, CDCl3) δ 185.4 (CO); 184.7 (CO); 152.5 (NCN); 143.4 (C1arom of phenyl ring); 139.4 (C1arom of mesityl group); 139.0 (C1arom of mesityl group); 137.8 (C4arom of mesityl group); 137.6 (C4arom of mesityl group); 135.8 (NCHN); 134.2 (C2arom and C6arom of mesityl group); 133.7 (C2arom and C6arom of mesityl group); 132.9 (C3Harom of phenyl ring); 128.2 (C3Harom and C5Harom of mesityl group); 128.1 (C3Harom and C5Harom of mesityl group); 125.5 (C4Harom of phenyl ring); 124.1 (C5Harom of phenyl ring); 123.9 (C2arom of phenyl ring); 110.8 (C6Harom of phenyl ring); 107.2 ((CO)CH(CO)); 37.5 (NCH3); 21.1 (p-CH3 of mesityl group); 21.1 (p-CH3 of mesityl group); 19.9 (o-CH3 of mesityl group); 19.6 (o-CH3 of mesityl group) ppm. 195Pt-NMR (128.56 MHz, CDCl3) δ −3342.3 ppm. Anal Calcd for C30H31N3O2Pt · 0.7 C6H14: C, 57.08; H, 5.52; N, 5.84. Found: C, 57.24; H, 5.38; N, 5.90.
Synthesis of (1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato-κ2O,O′)(1-benzyl-4-phenyl-κC2-1,2,4-triazol-5-ylidene-κC5)platinum(II) 6
506 mg triazolium iodide 3 (1.6 mmol), 185 mg silver(I) oxide (0.8 mmol, 0.5 eq.), 599 mg dichloro(1,5-cyclooctadiene)platinum(II) (1.6 mmol, 1 eq.), 359 mg potassium tert-butoxide (3.2 mmol, 2 eq.), and 987 mg 1,3-bis(2,4,6-trimethylphenyl)-propan-1,3-dione (3.2 mmol, 2 eq.) were reacted in 20 mL of 1,4-dioxane, 10 mL of butanone, and 20 mL of DMF according to the general procedure. The remaining solid was purified by flash chromatography (KG60, dichloromethane/isohexanes solvent mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (440 mg, 0.60 mmol, 37%). Mp: 209 °C. 1H-NMR (500.13 MHz, CDCl3): δ 8.26 (s, 1 H, NCHN); 7.76 (d, pseudo-t, 3JHH = 7.7 Hz, 3JPtH = 24.1 Hz, 1 H, C3Harom of phenyl ring); 7.29 (dd, 3JHH = 7.7 Hz, JHH = 1.7 Hz, 2 H, C2Harom and C6Harom of benzyl group); 7.25–7.18 (m, 3 H, C3Harom–C5Harom of benzyl group); 7.08 (dt, JHH = 1.6 Hz, 3JHH = 7.7 Hz, 1 H, C6Harom of phenyl ring); 7.06 (dt, JHH = 1.4 Hz, 3JHH = 7.4 Hz, 1 H, C5Harom of phenyl ring); 7.01 (dt, 3JHH = 7.2 Hz, JHH = 1.8 Hz, 1 H, C4Harom of phenyl ring); 6.87 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.79 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.73 (s, 1 H, (CO)CH(CO)); 5.67 (s, 2 H, CH2); 2.36 (s, 6 H, o-CH3 of mesityl group); 2.31 (s, 3 H, p-CH3 of mesityl group); 2.26 (s, 3 H, p-CH3 of mesityl group); 2.24 (s, 6 H, o-CH3 of mesityl group) ppm. 13C-NMR (125.77 MHz, CDCl3): δ 185.7 (CO); 184.7 (CO); 152.4 (NCN); 143.3 (C1arom of phenyl ring); 139.3 (C1arom of mesityl group); 138.9 (C1arom of mesityl group); 137.8 (C4arom of mesityl group); 137.5 (C4arom of mesityl group); 134.2 (C2arom and C6arom of mesityl group); 133.4 (C2arom and C6arom of mesityl group); 132.8 (C3Harom of phenyl ring); 128.4 (C4Harom of benzyl group); 128.3 (C2Harom and C6Harom of benzyl group); 128.3 (C3Harom and C5Harom of mesityl group); 128.0 (C3Harom and C5Harom of benzyl group); 127.9 (C3Harom and C5Harom of mesityl group); 125.5 (C4Harom of phenyl ring); 124.1 (C2arom of phenyl ring); 124.0 (C5Harom of phenyl ring); 110.8 (C6Harom of phenyl ring); 107.2 ((CO)CH(CO)) 53.6 (CH2), 21.1 (p-CH3 of mesityl group); 21.1 (p-CH3 of mesityl group); 20.0 (o-CH3 of mesityl group); 19.3 (o-CH3 of mesityl group) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3346.4 ppm. Anal. Calcd for C36H35N3O2Pt·0.5 C6H14: C, 60.06; H, 5.43; N, 5.39. Found: C, 60.25; H, 5.21; N, 5.34.
1)) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (440 mg, 0.60 mmol, 37%). Mp: 209 °C. 1H-NMR (500.13 MHz, CDCl3): δ 8.26 (s, 1 H, NCHN); 7.76 (d, pseudo-t, 3JHH = 7.7 Hz, 3JPtH = 24.1 Hz, 1 H, C3Harom of phenyl ring); 7.29 (dd, 3JHH = 7.7 Hz, JHH = 1.7 Hz, 2 H, C2Harom and C6Harom of benzyl group); 7.25–7.18 (m, 3 H, C3Harom–C5Harom of benzyl group); 7.08 (dt, JHH = 1.6 Hz, 3JHH = 7.7 Hz, 1 H, C6Harom of phenyl ring); 7.06 (dt, JHH = 1.4 Hz, 3JHH = 7.4 Hz, 1 H, C5Harom of phenyl ring); 7.01 (dt, 3JHH = 7.2 Hz, JHH = 1.8 Hz, 1 H, C4Harom of phenyl ring); 6.87 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.79 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.73 (s, 1 H, (CO)CH(CO)); 5.67 (s, 2 H, CH2); 2.36 (s, 6 H, o-CH3 of mesityl group); 2.31 (s, 3 H, p-CH3 of mesityl group); 2.26 (s, 3 H, p-CH3 of mesityl group); 2.24 (s, 6 H, o-CH3 of mesityl group) ppm. 13C-NMR (125.77 MHz, CDCl3): δ 185.7 (CO); 184.7 (CO); 152.4 (NCN); 143.3 (C1arom of phenyl ring); 139.3 (C1arom of mesityl group); 138.9 (C1arom of mesityl group); 137.8 (C4arom of mesityl group); 137.5 (C4arom of mesityl group); 134.2 (C2arom and C6arom of mesityl group); 133.4 (C2arom and C6arom of mesityl group); 132.8 (C3Harom of phenyl ring); 128.4 (C4Harom of benzyl group); 128.3 (C2Harom and C6Harom of benzyl group); 128.3 (C3Harom and C5Harom of mesityl group); 128.0 (C3Harom and C5Harom of benzyl group); 127.9 (C3Harom and C5Harom of mesityl group); 125.5 (C4Harom of phenyl ring); 124.1 (C2arom of phenyl ring); 124.0 (C5Harom of phenyl ring); 110.8 (C6Harom of phenyl ring); 107.2 ((CO)CH(CO)) 53.6 (CH2), 21.1 (p-CH3 of mesityl group); 21.1 (p-CH3 of mesityl group); 20.0 (o-CH3 of mesityl group); 19.3 (o-CH3 of mesityl group) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3346.4 ppm. Anal. Calcd for C36H35N3O2Pt·0.5 C6H14: C, 60.06; H, 5.43; N, 5.39. Found: C, 60.25; H, 5.21; N, 5.34.
Synthesis of 4-benzyl-1-phenyl-1,2,4-triazolium bromide 13
In a pressure tube 653 mg 1-phenyl-1H-1,2,4-triazole 7 (4.5 mmol) was dissolved in 4 mL of THF. 1.08 mL of benzyl bromide (9 mmol, 1.54 g, 2 eq.) were added, the pressure tube was sealed and the mixture was stirred for 22 h at 110 °C. After cooling to room temperature, the solid was filtered, washed with THF, and dried in vacuo to yield a white powder (1.22 g, 3.87 mmol, 86%). Mp: 170 °C. 1H-NMR (600.16 MHz, DMSO-d6): δ 11.07 (s, 1 H, NCHN); 9.56 (s, 1 H, NCHN); 7.93 (d, 3JHH = 8.3 Hz, 2 H, C2Harom and C6Harom of phenyl ring); 7.70 (t, 3JHH = 7.8 Hz; 2 H, C3Harom and C5Harom of phenyl ring); 7.65 (t, 3JHH = 7.4 Hz, 1 H, C4Harom of phenyl ring); 7.58 (d, 3JHH = 7.2 Hz, 2 H, C2Harom and C6Harom of benzyl group); 7.50–7.40 (m, 3 H, C3Harom–C5Harom of benzyl group); 5.61 (s, 2 H, CH2) ppm. 13C-NMR (150.93 MHz, DMSO-d6): δ 145.0 (NCHN); 141.8 (NCHN); 135.1 (C1arom of phenyl ring); 133.3 (C1arom of benzyl group); 130.6 (C4Harom of phenyl ring); 130.2 (C3Harom and C5Harom of phenyl ring); 129.1 (C4Harom of benzyl group); 129.1 (C3Harom and C5Harom of benzyl group); 128.9 (C2Harom and C6Harom of benzyl group); 120.9 (C2Harom and C6Harom of phenyl ring); 51.0 (CH2) ppm. Anal. Calcd for C15H14BrN3: C, 56.98; H, 4.46; N, 13.29. Found: C, 57.09; H, 4.46; N, 13.41.Synthesis of 4-benzyl-1-(4-methoxyphenyl)-1,2,4-triazolium bromide 14
In a pressure tube 526 mg 1-(4-methoxyphenyl)-1H-1,2,4-triazole 8 (3 mmol) was dissolved in 2 mL of THF. 0.72 mL of benzyl bromide (6 mmol, 1.03 g, 2 eq.) were added, the pressure tube was sealed and the mixture was stirred for 22 h at 100 °C. After cooling to room temperature, the solid was filtered, washed with THF, and dried in vacuo to yield a white powder (1.01 g, 2.91 mmol, 97%). Mp: 163 °C. 1H-NMR (300.13 MHz, DMSO-d6) δ 10.90 (NCHN); 9.49 (NCHN); 7.84 (d, 3JHH = 9.0 Hz, 2 H, C2Harom and C6Harom of phenyl ring); 7.56 (d, 3JHH = 7.7 Hz, 2 H, C2Harom and C6Harom of phenyl ring); 7.51–7.42 (m, 3 H, C3Harom–C5Harom of benzyl group); 7.23 (d, 3JHH = 9.1 Hz, 2 H, C3Harom and C5Harom of phenyl ring); 5.57 (s, 2 H, CH2); 3.85 (s, 3 H, CH3) ppm. 13C-NMR (75.48 MHz, DMSO-d6) δ 160.5 (C4arom of phenyl ring); 144.8 (NCHN); 141.0 (NCHN); 133.3 (C1arom of benzyl group); 129.0 (C4Harom of benzyl group); 129.0 (C3Harom and C5Harom of benzyl group); 128.8 (C2Harom and C6Harom of benzyl group); 128.1 (C1arom of phenyl ring); 122.6 (C2Harom and C6Harom of phenyl ring); 115.1 (C3Harom and C5Harom of phenyl ring); 55.8 (CCH3); 50.8 (CH2) ppm. Anal. Calcd for C16H16BrN3O: C, 55.51; H, 4.66; N, 12.14. Found: C, 55.55; H, 4.55; N, 12.01.Synthesis of 4-benzyl-1-(4-methylphenyl)-1,2,4-triazolium bromide 15
In a pressure tube 653 mg 1-(4-methylphenyl)-1H-1,2,4-triazole 9 (3 mmol) was dissolved in 2 mL of THF. 0.72 mL of benzyl bromide (6 mmol, 1.03 g, 2 eq.) were added, the pressure tube was sealed and the mixture was stirred for 22 h at 100 °C. After cooling to room temperature, the solid was filtered, washed with THF, and dried in vacuo to yield a white powder (834 mg, 2.53 mmol, 84%). Mp: 162 °C. 1H-NMR (300.13 MHz, DMSO-d6) δ 10.96 (NCHN); 9.51 (NCHN); 7.81 (d, 3JHH = 8.6 Hz, 2 H, C2Harom and C6Harom of phenyl ring); 7.56 (dd, JHH = 1.8 Hz, 3JHH = 7.6 Hz, 2 H, C2Harom and C6Harom of benzyl group); 7.53–7.40 (m, 5 H, C3Harom–C5Harom of benzyl group, C3Harom and C5Harom of phenyl ring); 5.58 (s, 2 H, CH2); 2.41 (s, 3 H, CH3) ppm. 13C-NMR (75.48 MHz, DMSO-d6) δ 144.9 (NCHN); 141.4 (NCHN); 140.5 (C4arom of phenyl ring); 133.3 (C1arom of benzyl group); 132.7 (C1arom of phenyl ring); 130.4 (C2Harom and C6Harom of phenyl ring); 129.1 (C4Harom of benzyl ring); 129.0 (C3Harom and C5Harom of benzyl group); 128.8 (C2Harom and C6Harom of benzyl group); 120.6 (C3Harom and C5Harom of phenyl ring); 50.9 (CH2); 20.7 (CCH3) ppm. Anal. Calcd for C16H16BrN3: C, 58.20; H, 4.88; N, 12.77. Found: C, 58.45; H, 4.70; N, 12.86.Synthesis of (pentan-2,4-dionato-κ2O,O′)(4-benzyl-1-phenyl-κC2-1,2,4-triazol-5-ylidene-κC5)platinum(II) 16
506 mg triazolium bromide 13 (1.6 mmol), 185 mg silver(I) oxide (0.8 mmol, 0.5 eq.), 898 mg dichloro(1,5-cyclooctadiene)-platinum(II) (2.4 mmol, 1.5 eq.), 718 mg potassium tert-but-oxide (6.4 mmol, 4 eq.), and 0.66 mL of pentane-2,4-dione (6.4 mmol, 641 mg, 4 eq.) were reacted in 40 mL of 1,4-dioxane, 20 mL of butanone, and 40 mL of DMF according to the general procedure. The crude product was purified by flash chromatography (KG60, dichloromethane) to obtain a white powder (325 mg, 0.62 mmol 77%). Mp: 224 °C. 1H-NMR (300.13 MHz, CDCl3) δ 7.83 (pseudo-t, d, 3JPtH = 27.4 Hz, 3JHH = 5.4 Hz, 1 H, C3Harom of phenyl ring); 7.78 (s, 1 H, NCHN); 7.47 (d, 3JHH = 7.3 Hz, 2 H, C2Harom and C6Harom of benzyl group); 7.43–7.32 (m, 3 H, C3Harom–C5Harom of benzyl group) 7.30–7.25 (m, 1 H, C6Harom of phenyl ring); 7.12–7.05 (m, 2 H, C4Harom and C5Harom of phenyl ring); 5.74 (s, 2 H, CH2); 5.54 (s, 1 H, (CO)CH(CO)); 2.10 (s, 3 H, CH3); 1.90 (s, 3 H, CH3) ppm. 13C-NMR (75.48 MHz, CDCl3) δ 185.2 (CO); 185.1 (CO); 153.1 (NCN); 145.2 (C1arom of phenyl ring); 140.6 (NCHN); 135.4 (C1arom of benzyl group); 131.2 (C3Harom of phenyl ring); 129.4 (C3Harom and C5Harom of benzyl group); 128.8 (C4Harom of benzyl group); 128.2 (C2Harom and C6Harom of benzyl group); 125.3 (C5Harom of phenyl ring); 124.0 (C4Harom of phenyl ring); 121.6 (C1arom of phenyl ring); 111.6 (C6Harom of phenyl ring); 102.2 ((CO)CH(CO)); 49.5 (CH2); 28.1 (CH3); 27.9 (CH3) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3450.2 ppm. Anal. Calcd for C20H19N3O2Pt: C, 45.46; H, 3.62; N, 7.95. Found: C, 45.21; H, 3.50; N, 7.94.Synthesis of (1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato-κ2O,O′)(1-phenyl-κC2-4-methyl-1,2,4-triazol-5-ylidene-κC5)platinum(II) 17
Method A: 459 mg triazolium iodide 10 (1.6 mmol), 185 mg silver(I) oxide (0.8 mmol, 0.5 eq.), 898 mg dichloro(1,5-cyclo-octadiene)platinum(II) (2.4 mmol, 1.5 eq.), 718 mg potassium tert-butoxide (6.4 mmol, 4 eq.), and 1970 mg 1,3-bis(2,4,6-tri-methylphenyl)propan-1,3-dione (6.4 mmol, 4 eq.) were reacted in 40 mL of 1,4-dioxane, 20 mL of butanone, and 40 mL of DMF according to the general procedure. The obtained solid was purified by flash chromatography (KG60, dichloro-methane/isohexanes solvent mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (28 mg, 0.22 mmol, 13%). Mp: 135 °C. 1H-NMR (300.13 MHz, CDCl3) δ 7.48 (s, 1 H, NCHN); 7.73 (pseudo-t, d, 3JPtH = 28.0 Hz, 3JHH = 7.4 Hz, 1 H, C3Harom of phenyl ring); 7.28 (d, 3JHH = 7.7 Hz, 1 H, C6Harom of phenyl ring); 7.08 (t, 3JHH = 7.5 Hz, 1 H, C5Harom of phenyl ring); 7.00 (t, 3JHH = 7.4 Hz, 1 H, C4Harom of phenyl ring); 6.87 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.84 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.70 (s, 1 H, (CO)CH(CO)); 3.95 (s, 3 H, NCH3); 2.35 (s, 6 H, o-CH3 of mesityl group); 2.31 (s, 6 H, o-CH3 of mesityl group); 2.30 (s, 3 H, p-CH3 of mesityl group); 2.29 (s, 3 H, p-CH3 of mesityl group) ppm. 13C-NMR (125.77 MHz, CDCl3) δ 187.2 (CO); 186.1 (CO); 143.6 (NCHN); 139.9 (C1arom of phenyl ring); 137.9 (C1arom of mesityl group); 137.0 (C1arom of mesityl group); 134.8 (C2arom and C6arom of mesityl group); 134.5 (C2arom and C6arom of mesityl group); 129.5 (C3Harom of phenyl ring); 128.2 (C3Harom and C5Harom of mesityl group); 128.1 (C3Harom and C5Harom of mesityl group); 127.9 (C4Harom of phenyl ring); 127.1 (C5Harom of phenyl ring); 116.09 (C6Harom of phenyl ring); 112.2 ((CO)CH(CO)); 33.8 (NCH3); 21.2 (p-CH3 of mesityl group); 21.1 (p-CH3 of mesityl group); 19.9 (o-CH3 of mesityl group); 19.5 (o-CH3 of mesityl group) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3405.6 ppm. Anal. Calcd for C30H31N3O2Pt · 0.45 C6H14: C, 56.15; H, 5.37; N, 6.01. Found: C, 56.15; H, 5.36; N, 5.95.
1)) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (28 mg, 0.22 mmol, 13%). Mp: 135 °C. 1H-NMR (300.13 MHz, CDCl3) δ 7.48 (s, 1 H, NCHN); 7.73 (pseudo-t, d, 3JPtH = 28.0 Hz, 3JHH = 7.4 Hz, 1 H, C3Harom of phenyl ring); 7.28 (d, 3JHH = 7.7 Hz, 1 H, C6Harom of phenyl ring); 7.08 (t, 3JHH = 7.5 Hz, 1 H, C5Harom of phenyl ring); 7.00 (t, 3JHH = 7.4 Hz, 1 H, C4Harom of phenyl ring); 6.87 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.84 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.70 (s, 1 H, (CO)CH(CO)); 3.95 (s, 3 H, NCH3); 2.35 (s, 6 H, o-CH3 of mesityl group); 2.31 (s, 6 H, o-CH3 of mesityl group); 2.30 (s, 3 H, p-CH3 of mesityl group); 2.29 (s, 3 H, p-CH3 of mesityl group) ppm. 13C-NMR (125.77 MHz, CDCl3) δ 187.2 (CO); 186.1 (CO); 143.6 (NCHN); 139.9 (C1arom of phenyl ring); 137.9 (C1arom of mesityl group); 137.0 (C1arom of mesityl group); 134.8 (C2arom and C6arom of mesityl group); 134.5 (C2arom and C6arom of mesityl group); 129.5 (C3Harom of phenyl ring); 128.2 (C3Harom and C5Harom of mesityl group); 128.1 (C3Harom and C5Harom of mesityl group); 127.9 (C4Harom of phenyl ring); 127.1 (C5Harom of phenyl ring); 116.09 (C6Harom of phenyl ring); 112.2 ((CO)CH(CO)); 33.8 (NCH3); 21.2 (p-CH3 of mesityl group); 21.1 (p-CH3 of mesityl group); 19.9 (o-CH3 of mesityl group); 19.5 (o-CH3 of mesityl group) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3405.6 ppm. Anal. Calcd for C30H31N3O2Pt · 0.45 C6H14: C, 56.15; H, 5.37; N, 6.01. Found: C, 56.15; H, 5.36; N, 5.95.
Method B: A flame-dried schlenk tube was charged with 919 mg triazolium salt 10 (3.2 mmol) and 371 mg silver(I) oxide (1.6 mmol, 0.5 eq.) and the tube was refilled with argon. 40 mL of DMF was added and the mixture was stirred for 21 h at 60 °C under a positive pressure of argon. Afterwards, 1197 mg dichloro(1,5-cyclooctadiene)platinum(II) (3.2 mmol, 1 eq.) were added, and the mixture was stirred for 21 h at room temperature and another 48 h at 130 °C. After cooling to room temperature 718 mg potassium tert-butoxide (6.4 mmol, 2 eq.) and 1974 mg 1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dione (6.4 mmol, 2 eq.) were added, and the mixture was stirred for 21 h at room temperature and another 6 h at 100 °C. Afterwards the solvent is removed under reduced pressure and the remaining residue was washed with water. The obtained solid was purified by flash chromatography (KG60, dichloromethane/isohexanes solvent mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (638 mg, 0.97 mmol, 30%). Anal. Calcd for C30H31N3O2Pt · 0.35 C6H14: C, 55.81; H, 5.24; N, 6.08. Found: C, 55.62; H, 5.40; N, 5.82.
1)) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (638 mg, 0.97 mmol, 30%). Anal. Calcd for C30H31N3O2Pt · 0.35 C6H14: C, 55.81; H, 5.24; N, 6.08. Found: C, 55.62; H, 5.40; N, 5.82.
Synthesis of (1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato-κ2O,O′)(1-(4-methoxyphenyl-κC2)-4-methyl-1,2,4-triazol-5-ylidene-κC5)platinum(II) 18
509 mg triazolium iodide 11 (1.6 mmol), 185 mg silver(I) oxide (0.8 mmol, 0.5 eq.), 599 mg dichloro(1,5-cyclo-octadiene) platinum(II) (1.6 mmol, 1 eq.), 718 mg potassium tert-butoxide (6.4 mmol, 4 eq.), and 1970 mg 1,3-bis(2,4,6-tri-methylphenyl)-propan-1,3-dione (6.4 mmol, 4 eq.) were reacted in 20 mL of 1,4-dioxane, 10 mL of butanone, and 20 mL of DMF according to the general procedure. The obtained solid was purified by flash chromatography (KG60, dichloromethane/isohexanes solvent mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and the crude product was washed with isohexanes to yield a white powder (188 mg, 0.27 mmol, 17%). Mp: 138 °C. 1H-NMR (300.13 MHz, CDCl3) δ 7.81 (s, 1 H, NCHN); 7.29 (d, pseudo-t, JHH = 2.7 Hz, 3JPtH = 31.7 Hz, 1 H, C2Harom of phenyl ring); 7.23 (d, 3JHH = 8.4 Hz, 1 H, C6Harom of phenyl ring); 6.84 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.83 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.62 (d, 3JHH = 8.5 Hz, JHH = 2.7 Hz, 1 H, C5Harom of phenyl ring); 5.70 (s, 1 H, (CO)CH(CO)); 3.93 (s, 3 H, NCH3); 3.76 (s, 3 H, OCH3); 2.34 (s, 6 H, o-CH3 of mesityl group); 2.31 (s, 6 H, o-CH3 of mesityl group); 2.29 (s, 6 H, p-CH3 of mesityl group) ppm. 13C-NMR (125.77 MHz, CDCl3) δ 185.0 (CO); 184.7 (CO); 157.1 (C4arom of phenyl ring); 151.7 (NCN); 141.2 (NCHN); 139.5 (C1arom of mesityl group); 139.1 (C1arom of phenyl ring); 138.8 (C1arom of mesityl group); 137.7 (C4arom of mesityl group); 137.5 (C4arom of mesityl group); 134.1 (C2arom and C5arom of mesityl group); 133.7 (C2arom and C5arom of mesityl group); 128.2 (C3Harom and C5Harom of mesityl group); 128.1 (C3Harom and C5Harom of mesityl group); 123.0 (C2arom of phenyl ring); 117.7 (C3Harom of phenyl ring); 112.2 (C6Harom of phenyl ring); 108.1 (C5Harom of phenyl ring); 107.2 ((CO)CH(CO)); 55.5 (OCH3); 32.8 (NCH3); 21.1 (p-CH3 of mesityl group); 20.0 (o-CH3 of mesityl group); 19.5 (o-CH3 of mesityl group) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3375.8 ppm. Anal. Calcd for C31H33N3O3Pt: C, 53.91; H, 4.82; N, 6.08. Found: C, 54.05; H, 4.96; N, 6.03.
1)) and the crude product was washed with isohexanes to yield a white powder (188 mg, 0.27 mmol, 17%). Mp: 138 °C. 1H-NMR (300.13 MHz, CDCl3) δ 7.81 (s, 1 H, NCHN); 7.29 (d, pseudo-t, JHH = 2.7 Hz, 3JPtH = 31.7 Hz, 1 H, C2Harom of phenyl ring); 7.23 (d, 3JHH = 8.4 Hz, 1 H, C6Harom of phenyl ring); 6.84 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.83 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.62 (d, 3JHH = 8.5 Hz, JHH = 2.7 Hz, 1 H, C5Harom of phenyl ring); 5.70 (s, 1 H, (CO)CH(CO)); 3.93 (s, 3 H, NCH3); 3.76 (s, 3 H, OCH3); 2.34 (s, 6 H, o-CH3 of mesityl group); 2.31 (s, 6 H, o-CH3 of mesityl group); 2.29 (s, 6 H, p-CH3 of mesityl group) ppm. 13C-NMR (125.77 MHz, CDCl3) δ 185.0 (CO); 184.7 (CO); 157.1 (C4arom of phenyl ring); 151.7 (NCN); 141.2 (NCHN); 139.5 (C1arom of mesityl group); 139.1 (C1arom of phenyl ring); 138.8 (C1arom of mesityl group); 137.7 (C4arom of mesityl group); 137.5 (C4arom of mesityl group); 134.1 (C2arom and C5arom of mesityl group); 133.7 (C2arom and C5arom of mesityl group); 128.2 (C3Harom and C5Harom of mesityl group); 128.1 (C3Harom and C5Harom of mesityl group); 123.0 (C2arom of phenyl ring); 117.7 (C3Harom of phenyl ring); 112.2 (C6Harom of phenyl ring); 108.1 (C5Harom of phenyl ring); 107.2 ((CO)CH(CO)); 55.5 (OCH3); 32.8 (NCH3); 21.1 (p-CH3 of mesityl group); 20.0 (o-CH3 of mesityl group); 19.5 (o-CH3 of mesityl group) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3375.8 ppm. Anal. Calcd for C31H33N3O3Pt: C, 53.91; H, 4.82; N, 6.08. Found: C, 54.05; H, 4.96; N, 6.03.
Synthesis of (1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato-κ2O,O′)(1-(4-methylphenyl-κC2)-4-methyl-1,2,4-triazol-5-ylidene-κC5)platinum(II) 19
224 mg triazolium iodide 11 (0.74 mmol), 86 mg silver(I) oxide (0.37 mmol, 0.5 eq.), 278 mg dichloro(1,5-cyclooctadiene)-platinum(II) (0.74 mmol, 1 eq.), 334 mg potassium tert-butoxide (2.98 mmol, 4 eq.), and 918 mg 1,3-bis(2,4,6-trimethylphenyl)-propan-1,3-dione (2.98 mmol, 4 eq.) were reacted in 20 mL of 1,4-dioxane, 10 mL of butanone, and 20 mL of DMF according to the general procedure. The obtained solid was purified by flash chromatography (KG60, dichloromethane/isohexanes solvent mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (133 mg, 0.20 mmol, 27%). Mp: 157 °C. 1H-NMR (600.16 MHz, CDCl3) δ 7.81 (s, 1 H, NCHN); 7.49 (pseudo-t, 3JPtH = 24.0 Hz, 1 H, C3Harom of phenyl ring); 7.15 (d, 3JHH = 7.8 Hz, 1 H, C6Harom of phenyl ring); 6.86 (s, 3 H, C3Harom and C5Harom of mesityl ring, and C5Harom of phenyl ring); 6.82 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.87 (s, 1 H, (CO)CH(CO)); 3.92 (s, 3 H, NCH3); 2.35 (s, 6 H, o-CH3 of mesityl group); 2.30 (s, 12 H, o-CH3 and p-CH3 of mesityl group, and CH3 of phenyl ring); 2.28 (s, 6 H, p-CH3 of mesityl ring) ppm. 13C-NMR (150.93 MHz, CDCl3) δ 185.0 (CO); 184.6 (CO); 152.7 (NCN); 143.0 (C1arom of phenyl ring); 141.3 (NCHN); 139.5 (C1arom of mesityl group); 139.0 (C1arom of phenyl ring); 137.7 (C4arom of mesityl group); 137.5 (C4arom of mesityl group); 135.1 (C4arom of phenyl ring); 134.3 (C2arom and C6arom of mesityl group); 133.7 (C2arom and C6arom of mesityl group); 132.2 (C3Harom of phenyl ring); 128.3 (C3Harom and C5Harom of mesityl group); 128.1 (C3Harom and C5Harom of mesityl group); 124.6 (C5Harom of phenyl ring); 120.7 (C2arom of phenyl ring); 111.4 (C6Harom of phenyl ring); 107.2 ((CO)CH(CO)); 32.8 (NCH3); 21.6 (CH3 of phenyl ring); 21.1 (p-CH3 of mesityl group); 21.1 (p-CH3 of mesityl group); 20.0 (o-CH3 of mesityl group); 19.5 (o-CH3 of mesityl group) ppm. 195Pt-NMR (128.63 MHz, CDCl3) δ = −3385.2 ppm. Anal. Calcd for C31H34N3O2Pt · 0.3 C6H14: C, 56.15; H, 5.49; N, 5.99. Found: C, 56.11; H, 5.46; N, 5.84.
1)) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (133 mg, 0.20 mmol, 27%). Mp: 157 °C. 1H-NMR (600.16 MHz, CDCl3) δ 7.81 (s, 1 H, NCHN); 7.49 (pseudo-t, 3JPtH = 24.0 Hz, 1 H, C3Harom of phenyl ring); 7.15 (d, 3JHH = 7.8 Hz, 1 H, C6Harom of phenyl ring); 6.86 (s, 3 H, C3Harom and C5Harom of mesityl ring, and C5Harom of phenyl ring); 6.82 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.87 (s, 1 H, (CO)CH(CO)); 3.92 (s, 3 H, NCH3); 2.35 (s, 6 H, o-CH3 of mesityl group); 2.30 (s, 12 H, o-CH3 and p-CH3 of mesityl group, and CH3 of phenyl ring); 2.28 (s, 6 H, p-CH3 of mesityl ring) ppm. 13C-NMR (150.93 MHz, CDCl3) δ 185.0 (CO); 184.6 (CO); 152.7 (NCN); 143.0 (C1arom of phenyl ring); 141.3 (NCHN); 139.5 (C1arom of mesityl group); 139.0 (C1arom of phenyl ring); 137.7 (C4arom of mesityl group); 137.5 (C4arom of mesityl group); 135.1 (C4arom of phenyl ring); 134.3 (C2arom and C6arom of mesityl group); 133.7 (C2arom and C6arom of mesityl group); 132.2 (C3Harom of phenyl ring); 128.3 (C3Harom and C5Harom of mesityl group); 128.1 (C3Harom and C5Harom of mesityl group); 124.6 (C5Harom of phenyl ring); 120.7 (C2arom of phenyl ring); 111.4 (C6Harom of phenyl ring); 107.2 ((CO)CH(CO)); 32.8 (NCH3); 21.6 (CH3 of phenyl ring); 21.1 (p-CH3 of mesityl group); 21.1 (p-CH3 of mesityl group); 20.0 (o-CH3 of mesityl group); 19.5 (o-CH3 of mesityl group) ppm. 195Pt-NMR (128.63 MHz, CDCl3) δ = −3385.2 ppm. Anal. Calcd for C31H34N3O2Pt · 0.3 C6H14: C, 56.15; H, 5.49; N, 5.99. Found: C, 56.11; H, 5.46; N, 5.84.
Synthesis of (1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato-κ2O,O′)(4-benzyl-1-phenyl-κC2-1,2,4-triazol-5-ylidene-κC5)platinum(II) 20
506 mg triazolium bromide 13 (1.6 mmol), 185 mg silver(I) oxide (0.8 mmol, 0.5 eq.), 898 mg dichloro(1,5-cyclooctadiene)-platinum(II) (2.4 mmol, 1.5 eq.), 449 mg potassium tert-but-oxide (4 mmol, 2.5 eq.), and 1230 mg 1,3-bis(2,4,6-trimethyl-phenyl)propan-1,3-dione (4 mmol, 2.5 eq.) were reacted in 40 mL of 1,4-dioxane, 20 mL of butanone, and 40 mL of DMF according to the general procedure. The crude product was purified by flash chromatography (KG60, dichloromethane/isohexanes solvent mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) to yield a yellow powder (227 mg, 31 mmol, 19%). Mp: 239 °C. 1H-NMR (300.13 MHz, CDCl3): δ 7.82 (s, 1 H, NCHN); 7.75 (pseudo-t, d, d, 3JPtH = 23.8 Hz, 3JHH = 7.4 Hz, JHH = 1.3 Hz, 1 H, C3Harom of phenyl ring); 7.32–7.22 (m, 4 H, C3Harom–C5Harom of benzyl group and C6Harom of phenyl ring); 7.22–7.15 (m, 2 H, C2Harom and C6Harom of benzyl group); 7.08 (td, 3JHH = 7.6 Hz, JHH = 1.5 Hz, 1 H, C5Harom of phenyl ring); 7.00 (t, 3JHH = 7.4 Hz, JHH = 1.5 Hz, 1 H, C4Harom of phenyl ring); 6.87 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.76 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.70 (s, 1 H, (CO)CH(CO)); 5.57 (s, 2 H, CH2); 2.35 (s, 6 H, o-CH3 of mesityl group); 2.30 (s, 3 H, p-CH3 of mesityl group); 2.24 (s, 3 H, p-CH3 of mesityl group); 2.18 (s, 6 H, o-CH3 of mesityl group) ppm. 13C-NMR (150.93 MHz, CDCl3): δ 185.4 (CO); 184.7 (CO); 153.1 (NCN); 145.2 (C1arom of phenyl ring); 140.9 (NCHN); 139.3 (C1arom of mesityl group); 138.9 (C1arom of mesityl group); 137.8 (C4arom of mesityl group); 137.4 (C4arom of mesityl group); 135.2 (C1arom of benzyl group); 134.2 (C2arom and C6arom of mesityl group); 133.4 (C2arom and C6arom of mesityl group); 131.6 (C3Harom of phenyl ring); 128.9 (C3Harom and C5Harom of benzyl group); 128.4 (C4Harom of benzyl group); 128.2 (C3Harom and C5Harom of mesityl group); 127.9 (C3Harom and C5Harom of mesityl group); 127.8 (C2Harom and C6Harom of benzyl group); 125.4 (C4Harom of phenyl ring); 124.2 (C5Harom of phenyl ring); 121.2 (C2arom of phenyl ring); 111.7 (C6Harom of phenyl ring); 107.2 ((CO)CH(CO)); 49.4 (CH2); 21.1 (p-CH3 of mesityl group); 21.0 (p-CH3 of mesityl group); 19.9 (o-CH3 of mesityl group); 19.3 (o-CH3 of mesityl group) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3405.0 ppm. Anal. Calcd for C36H35N3O2Pt: C, 58.69; H, 4.79; N, 5.70. Found: C, 58.53; H, 4.78; N, 5.65.
1)) to yield a yellow powder (227 mg, 31 mmol, 19%). Mp: 239 °C. 1H-NMR (300.13 MHz, CDCl3): δ 7.82 (s, 1 H, NCHN); 7.75 (pseudo-t, d, d, 3JPtH = 23.8 Hz, 3JHH = 7.4 Hz, JHH = 1.3 Hz, 1 H, C3Harom of phenyl ring); 7.32–7.22 (m, 4 H, C3Harom–C5Harom of benzyl group and C6Harom of phenyl ring); 7.22–7.15 (m, 2 H, C2Harom and C6Harom of benzyl group); 7.08 (td, 3JHH = 7.6 Hz, JHH = 1.5 Hz, 1 H, C5Harom of phenyl ring); 7.00 (t, 3JHH = 7.4 Hz, JHH = 1.5 Hz, 1 H, C4Harom of phenyl ring); 6.87 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.76 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.70 (s, 1 H, (CO)CH(CO)); 5.57 (s, 2 H, CH2); 2.35 (s, 6 H, o-CH3 of mesityl group); 2.30 (s, 3 H, p-CH3 of mesityl group); 2.24 (s, 3 H, p-CH3 of mesityl group); 2.18 (s, 6 H, o-CH3 of mesityl group) ppm. 13C-NMR (150.93 MHz, CDCl3): δ 185.4 (CO); 184.7 (CO); 153.1 (NCN); 145.2 (C1arom of phenyl ring); 140.9 (NCHN); 139.3 (C1arom of mesityl group); 138.9 (C1arom of mesityl group); 137.8 (C4arom of mesityl group); 137.4 (C4arom of mesityl group); 135.2 (C1arom of benzyl group); 134.2 (C2arom and C6arom of mesityl group); 133.4 (C2arom and C6arom of mesityl group); 131.6 (C3Harom of phenyl ring); 128.9 (C3Harom and C5Harom of benzyl group); 128.4 (C4Harom of benzyl group); 128.2 (C3Harom and C5Harom of mesityl group); 127.9 (C3Harom and C5Harom of mesityl group); 127.8 (C2Harom and C6Harom of benzyl group); 125.4 (C4Harom of phenyl ring); 124.2 (C5Harom of phenyl ring); 121.2 (C2arom of phenyl ring); 111.7 (C6Harom of phenyl ring); 107.2 ((CO)CH(CO)); 49.4 (CH2); 21.1 (p-CH3 of mesityl group); 21.0 (p-CH3 of mesityl group); 19.9 (o-CH3 of mesityl group); 19.3 (o-CH3 of mesityl group) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3405.0 ppm. Anal. Calcd for C36H35N3O2Pt: C, 58.69; H, 4.79; N, 5.70. Found: C, 58.53; H, 4.78; N, 5.65.
Synthesis of (1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato-κ2O,O′)(4-benzyl-1-(4-methoxyphenyl-κC2)-1,2,4-triazol-5-ylidene-κC5)platinum(II) 21
277 mg triazolium bromide 14 (0.8 mmol), 93 mg silver(I) oxide (0.4 mmol, 0.5 eq.), 299 mg dichloro(1,5-cyclooctadiene)-platinum(II) (0.8 mmol, 1 eq.), 359 mg potassium tert-butoxide (3.2 mmol, 4 eq.), and 987 mg 1,3-bis(2,4,6-trimethylphenyl)-propan-1,3-dione (3.2 mmol, 4 eq.) were reacted in 20 mL of 1,4-dioxane, 10 mL of butanone, and 20 mL of DMF according to the general procedure. The remaining solid was purified by flash chromatography (KG60, dichloromethane) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (88 mg, 0.12 mmol, 14%). Mp: 238 °C. 1H-NMR (500.13 MHz, CDCl3) δ 7.80 (s, 1 H, NCHN); 7.31 (d, JHH = 2.6 Hz, 1 H, C3Harom of phenyl ring); 7.27–7.25 (s, 3 H, C3Harom–C5Harom of benzyl ring); 7.22 (d, 3JHH = 8.5 Hz, 1 H, C6Harom of phenyl ring); 7.18 (dd, 3JHH = 7.6 Hz, JHH = 1.7 Hz, 1 H, C2Harom and C6Harom of benzyl group); 6.84 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.76 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.63 (dd, 3JHH = 8.5 Hz, JHH = 2.7 Hz, 1 H, C5Harom of phenyl ring); 5.69 (s, 1 H, (CO)CH(CO)); 5.55 (s, 2 H, CH2 of benzyl group); 3.76 (s, 3 H, NCH3); 2.34 (s, 6 H, o-CH3 of mesityl group); 2.29 (s, 3 H, p-CH3 of mesityl group); 2.24 (s, 3 H, p-CH3 of mesityl group); 2.17 (s, 6 H, o-CH3 of mesityl group) ppm. 13C-NMR (125.77 MHz, CDCl3) δ 185.2 (CO); 184.7 (CO); 157.1 (C4arom of phenyl ring); 151.3 (NCN); 140.6 (NCHN); 139.3 (C1arom of mesityl group); 139.0 (C1arom of phenyl ring); 138.8 (C1arom of mesityl group); 137.7 (C4arom of mesityl group); 137.4 (C4arom of mesityl group); 135.3 (C1arom of benzyl group); 134.1 (C2arom and C6arom of mesityl group); 133.4 (C2arom and C6arom of mesityl group); 128.9 (C3Harom and C5Harom of benzyl group); 128.3 (C4Harom of benzyl group);128.2 (C3Harom and C5Harom of mesityl group); 127.9 (C3Harom and C5Harom of mesityl group); 127.7 (C2Harom and C6Harom of benzyl group); 123.1 (C2arom of phenyl ring); 117.6 (C3Harom of phenyl ring); 112.3 (C6Harom of phenyl ring); 108.2 (C5Harom of phenyl ring); 107.3 ((CO)CH(CO)); 55.5 (OCH3); 49.4 (CH2); 21.1 (p-CH3 of mesityl group); 21.0 (p-CH3 of mesityl group); 20.0 (o-CH3 of mesityl group); 19.3 (o-CH3 of mesityl group) ppm. 195Pt-NMR (64.33 MHz, CDCl3) δ −3374.3 ppm. Anal. Calcd for C37H37N3O3Pt · 0.4 C6H14: C, 59.06; H, 5.36; N, 5.24. Found: C, 59.16; H, 5.21; N, 5.29.Synthesis of (1,3-bis(2,4,6-trimethylphenyl)propan-1,3-dionato-κ2O,O′)(4-benzyl-1-(4-methylphenyl-κC2)-1,2,4-triazol-5-ylidene-κC5)platinum(II) 22
528 mg triazolium bromide 15 (1.6 mmol), 185 mg silver(I) oxide (0.8 mmol, 0.5 eq.), 599 mg dichloro(1,5-cyclooctadiene)-platinum(II) (1.6 mmol, 1 eq.), 359 mg potassium tert-butoxide (3.2 mmol, 2 eq.), and 987 mg 1,3-bis(2,4,6-trimethylphenyl)-propan-1,3-dione (3.2 mmol, 2 eq.) were reacted in 40 mL of 1,4-dioxane, 20 mL of butanone, and 40 mL of DMF according to the general procedure. The remaining solid was purified by flash chromatography (KG60, dichloromethane/isohexanes solvent mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (329 mg, 0.44 mmol, 27%). Mp: 241 °C. 1H-NMR (600.16 MHz, CDCl3) δ 7.80 (s, 1 H, NCHN); 7.53 (pseudo-t, 3JPtH = 22.5 Hz, 1 H, C3Harom of phenyl ring); 7.30–7.23 (m, 3 H, C3Harom–C5Harom of benzyl group); 7.19 (d, 3JHH = 5.9 Hz, 2 H, C2Harom and C6Harom of benzyl group); 7.17 (d, JHH = 4.5 Hz, 1 H, C6Harom of phenyl ring); 6.88 (d, 3JHH = 7.1 Hz, 1 H, C5Harom of phenyl ring); 6.87 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.75 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.67 (s, 1 H, (CO)CH(CO)); 5.57 (s, 2 H, CH2 of benzyl group); 2.36 (s, 6 H, o-CH3 of mesityl group); 2.32 (s, 3 H, CH3 of phenyl ring); 2.31 (s, 3 H, p-CH3 of mesityl group); 2.25 (s, 3 H, p-CH3 of mesityl group); 2.17 (s, 6 H, o-CH3 of mesityl group) ppm. 13C-NMR (150.93 MHz, CDCl3) δ 185.2 (CO); 184.6 (CO); 152.3 (NCN); 142.9 (C1arom of phenyl ring); 140.8 (NCHN); 139.3 (C1arom of mesityl group); 139.0 (C1arom of mesityl group); 137.7 (C4arom of mesityl group); 137.3 (C4arom of mesityl group); 135.3 (C1arom of benzyl group); 135.1 (C4arom of phenyl ring); 134.3 (C2arom and C6arom of mesityl group); 133.4 (C2arom and C6arom of mesityl group); 132.2 (C3Harom of phenyl ring); 128.9 (C3Harom and C6Harom of benzyl group); 128.3 (C4Harom of benzyl group); 128.3 (C3Harom and C5Harom of mesityl group); 127.9 (C3Harom and C5Harom of mesityl group); 127.7 (C2Harom and C6Harom of benzyl group); 124.7 (C5Harom of phenyl ring); 120.8 (C2arom of phenyl ring); 111.5 (C6Harom of phenyl ring); 107.2 ((CO)CH(CO)); 49.4 (CH2 of benzyl group); 22.6 (CH3 of phenyl ring); 21.6 (p-CH3 of mesityl group); 21.0 (p-CH3 of mesityl group); 20.0 (o-CH3 of mesityl group); 19.3 (o-CH3 of mesityl group) ppm. 195Pt-NMR (128.56 MHz, CDCl3) δ −3384.7 ppm. Anal. Calcd for C37H37N3O2Pt · 0.7 C6H14: C, 61.01; H, 5.82; N, 5.18. Found: C, 61.11; H, 5.69; N, 5.25.
1)) and the crude product was washed with isohexanes, which in the following turned out to be hard to remove. The product was obtained as a white powder (329 mg, 0.44 mmol, 27%). Mp: 241 °C. 1H-NMR (600.16 MHz, CDCl3) δ 7.80 (s, 1 H, NCHN); 7.53 (pseudo-t, 3JPtH = 22.5 Hz, 1 H, C3Harom of phenyl ring); 7.30–7.23 (m, 3 H, C3Harom–C5Harom of benzyl group); 7.19 (d, 3JHH = 5.9 Hz, 2 H, C2Harom and C6Harom of benzyl group); 7.17 (d, JHH = 4.5 Hz, 1 H, C6Harom of phenyl ring); 6.88 (d, 3JHH = 7.1 Hz, 1 H, C5Harom of phenyl ring); 6.87 (s, 2 H, C3Harom and C5Harom of mesityl group); 6.75 (s, 2 H, C3Harom and C5Harom of mesityl group); 5.67 (s, 1 H, (CO)CH(CO)); 5.57 (s, 2 H, CH2 of benzyl group); 2.36 (s, 6 H, o-CH3 of mesityl group); 2.32 (s, 3 H, CH3 of phenyl ring); 2.31 (s, 3 H, p-CH3 of mesityl group); 2.25 (s, 3 H, p-CH3 of mesityl group); 2.17 (s, 6 H, o-CH3 of mesityl group) ppm. 13C-NMR (150.93 MHz, CDCl3) δ 185.2 (CO); 184.6 (CO); 152.3 (NCN); 142.9 (C1arom of phenyl ring); 140.8 (NCHN); 139.3 (C1arom of mesityl group); 139.0 (C1arom of mesityl group); 137.7 (C4arom of mesityl group); 137.3 (C4arom of mesityl group); 135.3 (C1arom of benzyl group); 135.1 (C4arom of phenyl ring); 134.3 (C2arom and C6arom of mesityl group); 133.4 (C2arom and C6arom of mesityl group); 132.2 (C3Harom of phenyl ring); 128.9 (C3Harom and C6Harom of benzyl group); 128.3 (C4Harom of benzyl group); 128.3 (C3Harom and C5Harom of mesityl group); 127.9 (C3Harom and C5Harom of mesityl group); 127.7 (C2Harom and C6Harom of benzyl group); 124.7 (C5Harom of phenyl ring); 120.8 (C2arom of phenyl ring); 111.5 (C6Harom of phenyl ring); 107.2 ((CO)CH(CO)); 49.4 (CH2 of benzyl group); 22.6 (CH3 of phenyl ring); 21.6 (p-CH3 of mesityl group); 21.0 (p-CH3 of mesityl group); 20.0 (o-CH3 of mesityl group); 19.3 (o-CH3 of mesityl group) ppm. 195Pt-NMR (128.56 MHz, CDCl3) δ −3384.7 ppm. Anal. Calcd for C37H37N3O2Pt · 0.7 C6H14: C, 61.01; H, 5.82; N, 5.18. Found: C, 61.11; H, 5.69; N, 5.25.
Computational details
All calculations were performed with the Gaussian09 package.79 The gradient-corrected density functional BP8680–82 was used together with the 6-31G(d)83–87 basis set. No symmetry or internal coordinate constraints were applied during optimizations. All reported structures were verified as true minima by the absence of negative eigenvalues in the vibrational frequency analysis. In all cases, platinum was described by using a decontracted Hay-Wadt(n + 1) ECP and basis set.88,89 Approximate free energies were obtained through chemical analysis, using the thermal correction to Gibbs free energy as reported by Gaussian09. This takes into account zero-point effects, thermal enthalpy corrections, and entropy. All energies reported in this paper, unless otherwise noted, are free energies at standard conditions (T = 298 K, p = 1 atm), using unscaled frequencies. For visualization CYLview90 was used.Acknowledgements
We are grateful for the support of the project by BMBF (FKZ: 13N10477) and thank all members of the BASF OLED team for helpful discussions and measurements. We also thank the ZIH for computation time at their high-performance computing facility.Notes and references
- M. Albrecht, R. A. Gossage, U. Frey, A. W. Ehlers, E. J. Baerends, A. E. Merbach and G. van Koten, Inorg. Chem., 2001, 40, 850–855 CrossRef CAS.
- M. Albrecht, R. A. Gossage, M. Lutz, A. L. Spek and G. van Koten, Chem. – Eur. J., 2000, 6, 1431–1445 CrossRef CAS.
- S. W. Thomas, K. Venkatesan, P. Müller and T. M. Swager, J. Am. Chem. Soc., 2006, 128, 16641–16648 CrossRef CAS PubMed.
- C.-S. Lee, R. R. Zhuang, S. Sabiah, J.-C. Wang, W.-S. Hwang and I. J. B. Lin, Organometallics, 2011, 30, 3897–3900 CrossRef CAS.
- Z. M. Hudson, C. Sun, K. J. Harris, B. E. G. Lucier, R. W. Schurko and S. N. Wang, Inorg. Chem., 2011, 50, 3447–3457 CrossRef CAS PubMed.
- Q. Z. Yang, L. Z. Wu, H. Zhang, B. Chen, Z. X. Wu, U. P. Zhang and C. H. Tung, Inorg. Chem., 2004, 43, 5195–5197 CrossRef CAS PubMed.
- R. Zieba, C. Desroches, F. Chaput, M. Carlsson, B. Eliasson, C. Lopes, M. Lindgren and S. Parola, Adv. Funct. Mater., 2009, 19, 235–241 CrossRef CAS PubMed.
- C. K. M. Chan, C. H. Tao, H. L. Tam, N. Y. Zhu, V. W. W. Yam and K. W. Cheah, Inorg. Chem., 2009, 48, 2855–2864 CrossRef CAS PubMed.
- R. Liu, A. Azenkeng, D. P. Zhou, Y. H. Li, K. D. Glusac and W. F. Sun, J. Phys. Chem. A, 2013, 117, 1907–1917 CrossRef CAS PubMed.
- G. J. Zhang, X. Gan, Q. Q. Xu, Y. Chen, X. J. Zhao, B. Qin, X. J. Lv, S. W. Lai, W. F. Fu and C. M. Che, Dalton Trans., 2012, 41, 8421–8429 RSC.
- P. W. Du, J. Schneider, P. Jarosz and R. Eisenberg, J. Am. Chem. Soc., 2006, 128, 7726–7727 CrossRef CAS PubMed.
- X. H. Wang, S. Goeb, Z. Q. Ji, N. A. Pogulaichenko and F. N. Castellano, Inorg. Chem., 2011, 50, 705–707 CrossRef CAS PubMed.
- M. N. Roberts, J. K. Nagle, M. B. Majewski, J. G. Finden, N. R. Branda and M. O. Wolf, Inorg. Chem., 2011, 50, 4956–4966 CrossRef CAS PubMed.
- D. Zhang, L. Z. Wu, L. Zhou, X. Han, Q. Z. Yang, L. P. Zhang and C. H. Tung, J. Am. Chem. Soc., 2004, 126, 3440–3441 CrossRef CAS PubMed.
- G. J. Zhou and W. Y. Wong, Chem. Soc. Rev., 2011, 40, 2541–2566 RSC.
- M. J. Currie, J. K. Mapel, T. D. Heidel, S. Goffri and M. A. Baldo, Science, 2008, 321, 226–228 CrossRef CAS PubMed.
- W. J. Wu, J. Zhang, H. B. Yang, B. Jin, Y. Hu, J. L. Hua, C. Jing, Y. T. Long and H. Tian, J. Mater. Chem., 2012, 22, 5382–5389 RSC.
- J. Kalinowski, V. Fattori, M. Cocchi and J. A. G. Williams, Coord. Chem. Rev., 2011, 255, 2401–2425 CrossRef CAS PubMed.
- V. Adamovich, J. Brooks, A. Tamayo, A. M. Alexander, P. I. Djurovich, B. W. D'Andrade, C. Adachi, S. R. Forrest and M. E. Thompson, New J. Chem., 2002, 26, 1171–1178 RSC.
- C. M. Che, C. C. Kwok, S. W. Lai, A. F. Rausch, W. J. Finkenzeller, N. Y. Zhu and H. Yersin, Chem. – Eur. J., 2010, 16, 233–247 CrossRef CAS PubMed.
- Y. Chi and P. T. Chou, Chem. Soc. Rev., 2010, 39, 638–655 RSC.
- X. Yang, C. Yao and G. Zhou, Platinum Met. Rev., 2013, 57, 2–16 CrossRef CAS.
- E. Rossi, A. Colombo, C. Dragonetti, D. Roberto, R. Ugo, A. Valore, L. Falciola, P. Brulatti, M. Cocchi and J. A. G. Williams, J. Mater. Chem., 2012, 22, 10650–10655 RSC.
- A. K. Chan, E. S. Lam, A. Y. Tam, D. P. Tsang, W. H. Lam, M. Y. Chan, W. T. Wong and V. W. Yam, Chem. – Eur. J., 2013, 19, 13910–13924 CrossRef CAS PubMed.
- S.-L. Lai, W.-Y. Tong, S. C. F. Kui, M.-Y. Chan, C.-C. Kwok and C.-M. Che, Adv. Funct. Mater., 2013, 23, 5168–5176 CrossRef CAS PubMed.
- J. A. G. Williams, Photochem. Photophys. Coord. Compd., 2007, 281, 205–268 CAS.
- K. Li, X. Guan, C. W. Ma, W. Lu, Y. Chen and C. M. Che, Chem. Commun., 2011, 47, 9075–9077 RSC.
- X. Zhang, A. M. Wright, N. J. DeYonker, T. K. Hollis, N. I. Hammer, C. E. Webster and E. J. Valente, Organometallics, 2012, 31, 1664–1672 CrossRef CAS.
- M. Maestri, D. Sandrini, V. Balzani, L. Chassot, P. Jolliet and A. von Zelewsky, Chem. Phys. Lett., 1985, 122, 375–379 CrossRef CAS.
- D. Sandrini, M. Maestri, M. Ciano, V. Balzani, R. Lueoend, C. Deuschelcornioley, L. Chassot and A. von Zelewsky, Gazz. Chim. Ital., 1988, 118, 661–665 CAS.
- M. Maestri, C. Deuschel-Cornioley and A. von Zelewsky, J. Photochem. Photobiol., A, 1992, 67, 173–179 CrossRef CAS.
- A. Poloek, C.-T. Chen and C.-T. Chen, J. Mater. Chem. C, 2014, 2, 1376–1380 RSC.
- A. Bossi, A. F. Rausch, M. J. Leitl, R. Czerwieniec, M. T. Whited, P. I. Djurovich, H. Yersin and M. E. Thompson, Inorg. Chem., 2013, 52, 12403–12415 CrossRef CAS PubMed.
- X. Yang, Z. Wang, S. Madakuni, J. Li and G. E. Jabbour, Adv. Mater., 2008, 20, 2405–2409 CrossRef CAS PubMed.
- A. F. Rausch, L. Murphy, J. A. Williams and H. Yersin, Inorg. Chem., 2012, 51, 312–319 CrossRef CAS PubMed.
- S. C. Kui, P. K. Chow, G. Cheng, C. C. Kwok, C. L. Kwong, K. H. Low and C. M. Che, Chem. Commun., 2013, 49, 1497–1499 RSC.
- A. Y. Tam, D. P. Tsang, M. Y. Chan, N. Zhu and V. W. Yam, Chem. Commun., 2011, 47, 3383–3385 RSC.
- H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, Coord. Chem. Rev., 2011, 255, 2622–2652 CrossRef CAS PubMed.
- J. Brooks, Y. Babayan, S. Lamansky, P. I. Djurovich, I. Tsyba, R. Bau and M. E. Thompson, Inorg. Chem., 2002, 41, 3055–3066 CrossRef CAS PubMed.
- G. J. Zhou, Q. Wang, X. Z. Wang, C. L. Ho, W. Y. Wong, D. G. Ma, L. X. Wang and Z. Y. Lin, J. Mater. Chem., 2010, 20, 7472–7484 RSC.
- A. Valore, A. Colombo, C. Dragonetti, S. Righetto, D. Roberto, R. Ugo, F. De Angelis and S. Fantacci, Chem. Commun., 2010, 46, 2414–2416 RSC.
- J. Liu, C. J. Yang, Q. Y. Cao, M. Xu, J. Wang, H. N. Peng, W. F. Tan, X. X. Lu and X. C. Gao, Inorg. Chim. Acta, 2009, 362, 575–579 CrossRef CAS PubMed.
- Z. M. Hudson, C. Sun, M. G. Helander, H. Amarne, Z. H. Lu and S. N. Wang, Adv. Funct. Mater., 2010, 20, 3426–3439 CrossRef CAS PubMed.
- T. Shigehiro, S. Yagi, T. Maeda, H. Nakazumi, H. Fujiwara and Y. Sakurai, J. Phys. Chem. C, 2013, 117, 532–542 CAS.
- S. Culham, P. H. Lanoe, V. L. Whittle, M. C. Durrant, J. A. G. Williams and V. N. Kozhevnikov, Inorg. Chem., 2013, 52, 10992–11003 CrossRef CAS PubMed.
- C. Karakus, L. H. Fischer, S. Schmeding, J. Hummel, N. Risch, M. Schäferling and E. Holder, Dalton Trans., 2012, 41, 9623–9632 RSC.
- D. N. Kozhevnikov, V. N. Kozhevnikov, M. Z. Shafikov, A. M. Prokhorov, D. W. Bruce and J. A. G. Williams, Inorg. Chem., 2011, 50, 3804–3815 CrossRef CAS PubMed.
- J. C. Chan, W. H. Lam, H. L. Wong, N. Zhu, W. T. Wong and V. W. Yam, J. Am. Chem. Soc., 2011, 133, 12690–12705 CrossRef CAS PubMed.
- W. Wu, W. Wu, S. Ji, H. Guo, P. Song, K. Han, L. Chi, J. Shao and J. Zhao, J. Mater. Chem., 2010, 20, 9775 RSC.
- H. Tsujimoto, S. Yagi, Y. Honda, H. Terao, T. Maeda, H. Nakazumi and Y. Sakurai, J. Lumin., 2010, 130, 217–221 CrossRef CAS PubMed.
- T. Fischer, R. Czerwieniec, T. Hofbeck, M. M. Osminina and H. Yersin, Chem. Phys. Lett., 2010, 486, 53–59 CrossRef CAS PubMed.
- D. N. Kozhevnikov, V. N. Kozhevnikov, M. M. Ustinova, A. Santoro, D. W. Bruce, B. Koenig, R. Czerwieniec, T. Fischer, M. Zabel and H. Yersin, Inorg. Chem., 2009, 48, 4179–4189 CrossRef CAS PubMed.
- T. C. Cheung, K. K. Cheung, S. M. Peng and C. M. Che, J. Chem. Soc., Dalton Trans., 1996, 1645–1651 RSC.
- S. W. Lai, M. C. W. Chan, K. K. Cheung, S. M. Peng and C. M. Che, Organometallics, 1999, 18, 3991–3997 CrossRef CAS.
- W. Lu, M. C. W. Chan, K. K. Cheung and C. M. Che, Organometallics, 2001, 20, 2477–2486 CrossRef CAS.
- W. Lu, N. Y. Zhu and C. M. Che, Abstr. Pap. Am. Chem. Soc., 2002, 224, U655–U655 Search PubMed.
- Y. L. Rao, D. Schoenmakers, Y. L. Chang, J. S. Lu, Z. H. Lu, Y. Kang and S. Wang, Chem. – Eur. J., 2012, 18, 11306–11316 CrossRef CAS PubMed.
- B. Ma, P. I. Djurovich, S. Garon, B. Alleyne and M. E. Thompson, Adv. Funct. Mater., 2006, 16, 2438–2446 CrossRef CAS PubMed.
- N. Bakken, J. Photonics Energy, 2012, 2, 021203 CrossRef CAS PubMed.
- M. Cocchi, J. Kalinowski, D. Virgili, V. Fattori, S. Develay and J. A. G. Williams, Appl. Phys. Lett., 2007, 90, 163508 CrossRef PubMed.
- S. C. Kui, P. K. Chow, G. S. Tong, S. L. Lai, G. Cheng, C. C. Kwok, K. H. Low, M. Y. Ko and C. M. Che, Chem. – Eur. J., 2013, 19, 69–73 CrossRef CAS PubMed.
- M. Baete, M. Bold, M. Egen, T. Gessner, H.-H. Johannes, K. Kshle, W. Kowalsky, C. Lennartz, C. Neuber, S. Nord, C. Schildknecht, H.-W. Dchmidt and M. Thelakkar, WO Patent, 2006056418, 2006 Search PubMed.
- T. Strassner, Y. Unger, D. Meyer, O. Molt, I. Muenster and G. Wagenblast, Inorg. Chem. Commun., 2013, 30, 39–41 CrossRef CAS PubMed.
- A. Tronnier, A. Risler, N. Langer, G. Wagenblast, I. Muenster and T. Strassner, Organometallics, 2012, 31, 7447–7452 CrossRef CAS.
- S. Haneder, E. Da Como, J. Feldmann, J. M. Lupton, C. Lennartz, P. Erk, E. Fuchs, O. Molt, I. Munster, C. Schildknecht and G. Wagenblast, Adv. Mater., 2008, 20, 3325–3330 CrossRef CAS PubMed.
- Y. Unger, D. Meyer, O. Molt, C. Schildknecht, I. Munster, G. Wagenblast and T. Strassner, Angew. Chem., Int. Ed., 2010, 49, 10214–10216 CrossRef CAS PubMed.
- Z. M. Hudson, C. Sun, M. G. Helander, Y. L. Chang, Z. H. Lu and S. Wang, J. Am. Chem. Soc., 2012, 134, 13930–13933 CrossRef CAS PubMed.
- M. Tenne, S. Metz, I. Münster, G. Wagenblast and T. Strassner, Organometallics, 2013, 32, 6257–6264 CrossRef CAS.
- A. Tronnier and T. Strassner, Dalton Trans., 2013, 42, 9847–9851 RSC.
- A. Tronnier, N. Nischan, S. Metz, G. Wagenblast, I. Münster and T. Strassner, Eur. J. Inorg. Chem., 2014, 256–264 CrossRef CAS PubMed.
- A. Tronnier, S. Metz, G. Wagenblast, I. Münster and T. Strassner, Dalton Trans., 2014, 43, 3297–3305 RSC.
- M. Tenne, Y. Unger and T. Strassner, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2012, 68, M203–M205 CAS.
- Y. Unger, T. Strassner and C. Lennartz, J. Organomet. Chem., 2013, 748, 63–67 CrossRef CAS PubMed.
- R. K. Bartlett and I. R. Humphrey, J. Chem. Soc. C, 1967, 1664–1666 RSC.
- G. Pellizarri, Gazz. Chim. Ital., 1911, 41, 20–42 Search PubMed.
- D. Drew and J. R. Doyle, Inorg. Synth., 1990, 28, 346–349 CrossRef CAS PubMed.
- C. Zhang, P. Yang, Y. Yang, X. Huang, X.-J. Yang and B. Wu, Synth. Commun., 2008, 38, 2349–2356 CrossRef CAS.
- D. Meyer and T. Strassner, J. Org. Chem., 2011, 76, 305–308 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. C. Tomasi, M. N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian09, Rev. B 0.1, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B: Condens. Matter, 1986, 33, 8822–8824 CrossRef.
- J. P. Perdew, Phys. Rev. B: Condens. Matter, 1986, 34, 7406–7406 CrossRef.
- R. Ditchfield, W. J. Hehre and J. A. Pople, J. Chem. Phys., 1971, 54, 724–728 CrossRef CAS PubMed.
- W. J. Hehre, R. Ditchfield and J. A. Pople, J. Chem. Phys., 1972, 56, 2257–2261 CrossRef CAS PubMed.
- P. C. Hariharan and J. A. Pople, Chem. Phys. Lett., 1972, 16, 217–219 CrossRef CAS.
- P. C. Hariharan and J. A. Pople, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- P. C. Hariharan and J. A. Pople, Mol. Phys., 1974, 27, 209–214 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270–283 CrossRef CAS PubMed.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299–310 CrossRef CAS PubMed.
- C. Y. Legault, CYLview, Université de Sherbrooke, 1.0b edn, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Structure determination, NMR spectra as well as xyz-coordinates and calculated geometries of the DFT-calculations. CCDC 1026181 and 1026238. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4dt03613a |
| This journal is © The Royal Society of Chemistry 2015 |