Efficient and recyclable heterogeneous zinc alkyl carboxylate catalyst for the synthesis of N-phenyl carbamate from aniline and dimethylcarbonate†
Yi
Wang
* and
Bo
Liu
Key Laboratory of Green Chemical Engineering and Technology of College of Heilongjiang Province, College of Chemical and Environmental Engineering, Harbin University of Science and Technology, Harbin 150040, PR China. E-mail: wydicp@aliyun.com; Tel: +86 451 86392712
First published on 9th October 2014
Abstract
Zinc alkyl carboxylate molecules were chemically bonded on a silica surface through an elaborately designed precursor agent and special grafting method. The heterogeneous salt catalyst possessing a cyclic single active site was highly active and recyclable in the reaction between dimethylcarbonate and aniline to produce methyl N-phenyl carbamate.
The demand for sustainable catalysts and green chemical processes has stimulated great progress in the immobilization of homogeneous transition metal ions on porous substrates.1 Conventional impregnation and sol–gel process results in dispersed metal oxides with a low atomic efficiency for surface functionalized catalytic utilizations because quite a number of the ions are embedded in the bulk phase, even if the size of the oxides is on the nanoscale.2 Incorporation of the ions into a zeolitic framework leads to a highly dispersed active site. However, the hydrothermal process is complicated and the type and loading of ions are limited by the number of appropriate crystallographic positions in the zeolite, and considerable active sites may not be well accessible to reactants due to steric hindrance or diffusion obstacles, especially for microporous zeolite and in liquid phase reactions.3 Catalysts prepared by anchoring the ions on polymers may suffer from inferior thermal, mechanical and chemical stabilities of the substrates.4 The metal ions can also be fixed in a metal–organic framework (MOF) reported recently, however, the thermal stability of a MOF is still questionable in some cases and leaching of organic ligands may occur.5 In recent years, grafting of special ligands onto robust inorganic porous matrices such as silica has resulted in highly dispersed metal complexes with catalytic activities comparable to, or better than, those of their homogeneous counterparts, while the preparation process often involves complicated ligands and tedious steps, and most catalysts can only be used under mild conditions.6
In fact, simple organic salts of various transition metal ions, especially metal alkyl carboxylates, without additional ligands, can act as efficient thermally stable catalysts in many reactions. For example, zinc acetate/activated carbon was well used as a commercial catalyst for gas phase synthesis of vinyl acetate.7 Esterification and transesterification reactions concerning biodiesel production could be well catalyzed by zinc stearate and zinc salts of other long-chain fatty acids.8 Zinc glutarate was reported by Ree et al. to be catalytically active in the chemical fixation of CO2 into polycarbonates.9 Cobalt carboxylates, such as cobalt naphthenate and cobalt acetate, could be used for catalyzing selective oxidation of hydrocarbons.10 Dodecyl sulfonates of various metals are reported to be highly efficient catalysts for a special esterification reaction.11 The catalysts mentioned above are difficult to be recovered when they are used homogeneously. Although some of them can be used as either supported catalysts or insoluble solid catalysts, leaching or dissolving of the active component more or less into reaction mixture occurs inevitably in liquid phase reactions.7,8 In addition, pure insoluble solid salt crystals are not in a highly porous structure and the atomic efficiency of the catalyst is rather low.8 All the drawbacks can be anticipated to be overcome by chemically fixing the organic salt molecule precisely on the surface of an inorganic porous support, which may create a new class of catalysts with high activity, atomic efficiency and recyclability. However, no report has been published on this subject and it is still a significant technological challenge to be addressed.
Here we report a new approach for covalently bonding the zinc alkyl carboxylate molecules on silica through an elaborately designed procedure, and the heterogeneous salt catalyst was evaluated to be highly efficient and recyclable in the liquid phase reaction between aniline and dimethylcarbonate to synthesize methyl N-phenyl carbamate (MPC). MPC is a key intermediate for the synthesis of many useful chemicals, such as pesticides, pharmaceuticals, and especially for the green synthesis of an important polyurethane monomer, i.e. diphenylmethane-4,4′-diisocyanate (MDI), without involving toxic phosgene as feedstock (Scheme S1†).12 Previously reported homogeneous catalysts include zinc carboxylate, Zn4O(OAc)6, compounds of Pb, Sn and Zr, triflate salts of Sc, La and Yb, L-proline/TBAB, special N-containing organic compounds, etc.,13 which are difficult to recover. ZnO–TiO2 and immobilized complexes of Co2+ such as Co(salen) were reported as heterogeneous catalysts, however, the selectivity of MPC is rather low (<70%) on the former catalyst and both the catalysts deactivate obviously upon recycling.14 Recently, basic zinc carbonate was reported to be a recyclable catalyst,15 while the atomic efficiency was not discussed. Among all the catalysts, zinc alkyl carboxylate (e.g. zinc acetate) is the most frequently reported and is the best catalyst in terms of both activity and environmental friendliness. However, few efforts have been made to render it recyclable by immobilization. Activated carbon and silica supported zinc acetate prepared by impregnation were not reported to be good recyclable catalysts, although the initial activities are high.16 Guo et al. reported a zinc carboxylate functionalized SBA-15 catalyst for MDC synthesis, while the preparation procedure is rather complicated.17
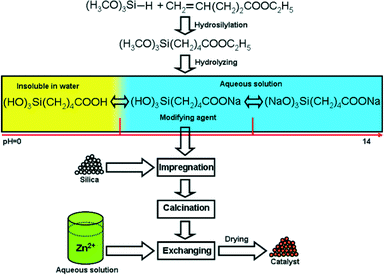
In our strategy, a special organosilicon compound was synthesized firstly as a precursor agent through the well documented hydrosilylation process.18 As shown in Fig. 1, trimethoxysilane reacts with pent-4-enoate to produce a compound comprising an alkyl chain connecting with both a Si atom and an ester group, which can be transformed into a carboxylate group by hydrolysis without affecting the Si–C bond. The 1H NMR and 13C NMR spectra are shown in Fig. S1 and S2,† respectively. After hydrolysis and adjustment of the pH to a weak alkaline value, a soluble modifying agent was then well distributed on silica by impregnation and immobilized in the following calcination step, through forming Si–O–Si bonds with surface hydroxyls by condensation. Zn2+ was introduced by ion-exchange. Every step is simple and finished in an aqueous media except the synthesis of the precursor agent. In previous reports, grafting of organosilicon species on silica often involved a tedious refluxing procedure in toluene and the metal ions were often immobilized through coordination with complicated and bulky ligands previously grafted on the support by successive steps.19
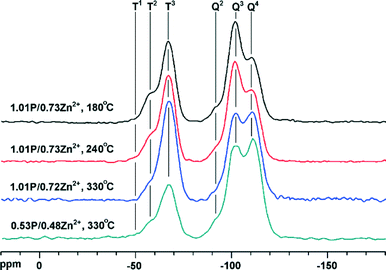
Fig. 2 shows the 1H → 29Si CP/MAS NMR spectra of various catalysts. Q2, Q3 and Q4 are assigned to silicon atoms in the bulk silica connected with two, one and zero surface hydroxyls, respectively.20 Peaks T1, T2 and T3 are ascribed to organosilicon compounds (bearing three silanol groups) grafted to the silica surface through forming one, two, and three Si–O–Si bonds, respectively.21 It is clear that the modifying agent is covalently bonded to the silica surface through forming three or two Si–O–Si bonds and that the three covalently bonded species dominates. The intensity ratio T3/T2 increases with the calcination temperature and the intensity of Q3 decreases accordingly, implying that a higher temperature favors the condensation between the silanol and surface hydroxyls. The intensity of Q3 of the two catalysts calcined at 330 °C is comparable on the spectra, with rather different intensities of T2 and T3, inferring that the modifying agents bond not only with the silica surface, but also with each other, especially on the catalyst with a higher loading, where the silanols of the neighbouring modifying agent are in close proximity.
1H → 13C CP/MAS NMR spectrum of a typical catalyst (Fig. S3†) confirms the existence of a surface alkyl carboxylate group, since every characteristic peak can be differentiated, and coincides well with the corresponding peaks shown in Fig. S2.† On the other hand, FT-IR results shown in Fig. S4† also reflect the characteristic bands of the alkyl carboxylate group. Bands at ca. 2934 cm−1, 2864 cm−1 and 1453 cm−1 are assigned to the asymmetric stretching vibration (νas), symmetric stretching vibration (νs) and symmetric deformation vibration of the C–H bond in methylene species (–CH2–), respectively.22 The strong band at around 1556 cm−1 and the weaker band at around 1417 cm−1 are due to the νas and νs of the zinc carboxylate group, respectively.23 The small band at 1715 cm−1 is assigned to the carboxyl group.24
The activities of various catalysts in the reaction between aniline and dimethylcarbonate are shown in Table 1. As shown, pure silica exhibits little activity for MPC synthesis, and the surface hydroxyls are the sites dominantly generating the byproducts. After loading the zinc alkyl carboxylate species, the catalytic activity is greatly improved.
| Catalysta | Selectivity of MPC (%) | TOF of MPC (s−1 × 103) |
|---|---|---|
| a The catalyst is marked in terms of the loading of alkyl carboxylate (mmol g−1)/the content of Zn2+ (mmol g−1), calcination temperature. b Exchanged with the solution of zinc nitrate, formate and propionate during preparation, respectively. All the other catalysts underwent ion-exchange with zinc acetate. Reaction conditions: temperature = 180 °C; reaction time = 15 minutes; aniline/dimethylcarbonate = 1/20, molar ratio; Zn2+/aniline = 1/200, molar ratio. | ||
| Silica | 3.8 | |
| 0.53P/0.48Zn2+, 330 °C | 84.6 | 23.6 |
| 1.01P/0.72Zn2+, 330 °C | 94.0 | 42.6 |
| 1.25P/0.73Zn2+, 330 °C | 87.9 | 32.7 |
| 1.01P/0.73Zn2+, 180 °C | 81.8 | 9.3 |
| 1.01P/0.73Zn2+, 240 °C | 84.2 | 14.7 |
| 1.01P/0.62Zn2+, 360 °C | 92.6 | 46.6 |
| 0.50P/0.49Zn2+, 420 °C | 37.3 | 5.8 |
| 1.01P/0.48Zn2+, 330 °Cb | 80.3 | 17.1 |
| 1.01P/0.45Zn2+, 330 °Cb | 81.8 | 17.0 |
| 1.01P/0.57Zn2+, 330 °Cb | 89.6 | 32.4 |
| Zinc acetate (anhydrous) | 96.6 | |
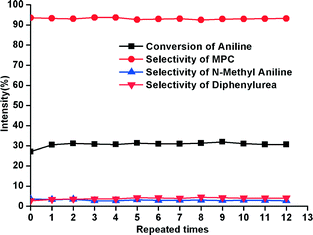
The selectivity of MPC on the best catalyst is very close to that of the best homogeneous catalyst, i.e. zinc acetate (Table 1), on which TOF could not be determined because the concentration of Zn2+ is not constant in the reaction mixture in the beginning of the reaction when the solid zinc acetate is being dissolved.13a–d Integrated reaction results show that the yield of MPC can reach 91.6% (conversion of aniline = 94.6%, selectivity = 96.8%) after 2 hours reaction at 170 °C on the catalyst 1.01P/0.72Zn2+, 330 °C, which is comparable to that on zinc acetate being 94.2% (conversion of aniline = 96.6%, selectivity = 97.5%), under identical conditions and amount of Zn2+ (Zn2+/aniline = 1/50, molar ratio). The result on zinc acetate is consistent with that reported by Li et al. that the yield of MPC could reach 92.7% in the presence of zinc acetate as the catalyst, under reaction conditions very similar to ours.16bFig. 3 shows the results of repeated evaluation of a typical catalyst for 12 repeats under a moderate conversion of aniline. The almost unchanged activity proves the excellent recyclability of the catalyst, which highlights the potential for industrialization.
For comparison, activated carbon supported zinc acetate catalyst (0.75 mmol Zn2+ gcat−1) prepared by the impregnation method was evaluated under conditions identical to those of the integrated reaction described above. The yield of MPC on the fresh catalyst is 85.8%, while it decreases to 9.7% on the reused catalyst. The serious deactivation of the catalyst may be due to dissolution of the zinc acetate into the reaction mixture, since it is only physisorbed on the support. Although the silica supported zinc acetate catalyst reported by Wang et al. exhibits a better recyclability, the yield of MPC decreases from 93.8% to 38.1% after repeated use for 5 times.16b
Undoubtedly zinc alkyl carboxylate is the active site and it is stable until the calcination temperature exceeds 400 °C, as is reflected by the performance of the catalyst calcined at 420 °C (Table 1) and thermogravimetric analysis (TGA) results (Fig. S5†). Serious weight loss starts from about 400 °C on the TGA curve, indicating the decomposition of the grafted alkyl carboxylate, which is confirmed by the FT-IR results in that almost no characteristic bands exist on the spectrum of the catalyst calcined at 420 °C (Fig. S4†).
Table 1 shows that catalysts prepared under different conditions exhibit rather different performances, especially in terms of the TOF of MPC. The difference seems not to be derived from the physical property of the catalysts (surface area, pore size and pore volume listed in Table S1†) and exchanging degree of Zn2+ (Table S2†). The difference in surface chemical state and active site may be responsible, as discussed below.
The zinc alkyl carboxylate groups confirmed by solid NMR and FT-IR results may exist on the catalyst surface in three forms, as shown in Fig. 4. Site a was investigated by ion chromatography analysis and the results shown in Table S3† indicate that only a very small proportion of acetate can be detected on the catalysts which have undergone ion-exchange with the zinc acetate solution, and it becomes less following the loading of alkyl carboxylate. On the catalysts which have undergone ion-exchange with zinc nitrate, formate and propionate solutions, respectively, negligible amounts of nitrate, formate and propionate anions could be found. This implies that the anions in the zinc salts for ion-exchange contribute little in the construction of the surface active sites. Accordingly, we suggest that the main active sites may comprise site b and site c. If site c dominates, then the ratio of alkyl carboxylate to Zn2+ should be close to 1. However, the loading of alkyl carboxylate is obviously higher than that of Zn2+ on most catalysts. So we conclude that cyclic active site b must exist on the catalyst surface and is the main and most efficient active center for producing MPC, which is as active as homogeneous zinc acetate, and the stable cyclic structure may be responsible for the high stability of the catalyst. Site c may exist on the surface, but the proportion should be small due to its rigid structure, and the catalytic activity should be lower because of steric hindrance to reactants. Since site b is a single site, isolated from each other and easily accessible to reactants, a highly dispersed site b can provide the highest atomic efficiency of Zn2+ on the catalyst. The flexible alkyl chain affords high feasibility to form the cyclic active site b through pairing Zn2+ with two alkyl carboxylate groups in a relatively wide range of distance. Higher loading of alkyl carboxylate groups results in the formation of more cyclic active sites on the catalyst surface as well as a higher activity of the catalyst, while excessive high loading may result in crowded cyclic active sites not well accessible to reactants, so the catalytic activity is lowered (Table 1). Catalysts which have undergone ion-exchange with zinc nitrate, formate and propionate solution, respectively, possesses an obviously lower content of Zn2+, compared with those that have undergone ion-exchange with zinc acetate, although the loadings of alkyl carboxylate are identical (Table 1). This is because the acetate anion is more compatible with alkyl carboxylate groups on the catalyst surface than the nitrate and formate, so the diffusion as well as the ion-exchange takes place more readily. In addition, a smaller size benefits acetate in ion-exchange compared to propionate. So the catalyst exchanged with zinc acetate contains more Zn2+ and exhibits a better performance. On the other hand, difficulty in ion-exchange and the weak acidic character of the ion-exchange solutions lead to excessive hydrolysis of the alkyl carboxylate groups into carboxyl groups on the catalysts which have undergone ion-exchange with zinc nitrate and zinc formate, as evidenced by the higher intensity ratios of the band at 1715 cm−1 to the band at 1556 cm−1 (Fig. S4†). On the catalysts calcined at lower temperatures (<330 °C), zinc alkyl carboxylate groups may not be well distributed on the surface because the condensation reaction does not occur sufficiently and surface hydroxyls are more prominent, so both the selectivities and TOFs are lower.
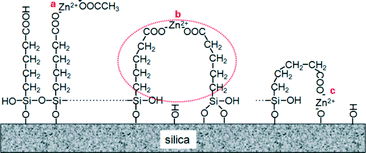 | ||
| Fig. 4 Proposed chemical state and active sites on a catalyst which has undergone ion-exchange with zinc acetate solution. | ||
In conclusion, we have designed and prepared a new type of heterogeneous salt catalyst with zinc alkyl carboxylate covalently bonded on silica by a special grafting method in aqueous media. An elaborately designed precursor agent and pH defined modifying agent enable the active site to form precisely as its homogeneous counterpart. The catalyst is highly active and recyclable for MPC synthesis from aniline and dimethylcarbonate. The high atomic efficiency and robust repeated usability highlight the potential for commercialization of the catalyst, and the preparation strategy provides new opportunities to develop other heterogeneous catalysts or surface functionalized materials with variable active metal ions, organic anion groups, supporting substrates and additional ligands.
Acknowledgements
We thank HarBin Science and Technology Bureau for financial support (Project No. 2007RFLXG014).Notes and references
- (a) J. A. Gladysz, Chem. Rev., 2002, 102, 3215 CrossRef CAS PubMed; (b) A. E. C. Collis and I. T. Horváth, Catal. Sci. Technol., 2011, 1, 912 RSC; (c) C. Sarmah, D. Sahu and P. Das, Catal. Commun., 2013, 41, 75 CrossRef CAS PubMed; (d) J. K. Dombrovskis, H. Y. Jeong, K. Fossum, O. Terasaki and A. E. C. Palmqvist, Chem. Mater., 2013, 25, 856 CrossRef CAS; (e) J. Sun, Q. B. Kan, Z. F. Li, G. L. Yu, H. Liu, X. Y. Yang, Q. S. Huo and J. Q. Guan, RSC Adv., 2014, 4, 2310 RSC.
- (a) M. Shimokawabe, H. Asakawa and N. Takezawa, Appl. Catal., 1990, 59, 45 CrossRef CAS; (b) Z. L. Wang, Q. S. Liu, J. F. Yu, T. H. Wu and G. J. Wang, Appl. Catal., A, 2003, 239, 87 CrossRef CAS; (c) M. Thammachart, V. Meeyoo, T. Risksomboon and S. Osuwan, Catal. Today, 2001, 68, 53 CrossRef CAS.
- A. Corma and H. García, Chem. Rev., 2002, 102, 3837 CrossRef CAS PubMed.
- (a) C. A. McNamara, M. J. Dixon and M. Bradley, Chem. Rev., 2002, 102, 3275 CrossRef CAS PubMed; (b) P. Barbaro and F. Liguori, Chem. Rev., 2009, 109, 515 CrossRef CAS PubMed.
- (a) Z. Wang, G. Chen and K. L. Ding, Chem. Rev., 2009, 109, 322 CrossRef CAS PubMed; (b) T. R. Cook, Y. R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734 CrossRef CAS PubMed.
- (a) C. E. Song and S. G. Lee, Chem. Rev., 2002, 102, 3495 CrossRef CAS PubMed; (b) D. E. D. Vos, M. Dams, B. F. Sels and P. A. Jacobs, Chem. Rev., 2002, 102, 3615 CrossRef PubMed; (c) J. M. Fraile, J. I. García and J. A. Mayoral, Chem. Rev., 2009, 109, 360 CrossRef CAS PubMed; (d) A. F. Trindade, P. M. P. Gois and C. A. M. Afonso, Chem. Rev., 2009, 109, 418 CrossRef CAS PubMed.
- O. N. Temkin, H. I. Abanto-Chavez and K. B. Hoang, Kinet. Catal., 2000, 41, 638 CrossRef CAS.
- A. Talebian-Kiakalaieh, N. A. S. Amin and H. Mazaheri, Appl. Energy, 2013, 104, 683 CrossRef CAS PubMed.
- M. Ree, Y. Hwang, J. S. Kim, H. Kim, G. Kim and H. Kim, Catal. Today, 2006, 115, 134 CrossRef CAS PubMed.
- (a) Y. Meng, B. Liang and S. W. Tang, Appl. Catal., A, 2012, 439, 1 CrossRef PubMed; (b) J. Zhu, A. Robertson and S. C. Tsang, Chem. Commun., 2002, 2044 RSC.
- M. Wang, J. J. Tian, L. J. Liu, H. Jiang, H. Gong and R. Wang, Petrochem. Technol., 2003, 32, 234 Search PubMed.
- Y. Ono, Appl. Catal., A, 1997, 155, 133 CrossRef CAS.
- (a) The Dow Chemical Company, US Pat., 4 268 683, 1981 Search PubMed; (b) T. Baba, A. Kobayashi, T. Yamauchi, H. Tanaka, S. Aso, M. Inomata and Y. Kawanami, Catal. Lett., 2002, 82, 193 CrossRef CAS; (c) E. Reixach, N. Bonet, F. X. Rius-Ruiz, S. Wershofen and A. Vidal-Ferran, Ind. Eng. Chem. Res., 2010, 49, 6362 CrossRef CAS; (d) X. Q. Zhao, L. J. Kang, N. Wang, H. L. An, F. Li and Y. J. Wang, Ind. Eng. Chem. Res., 2012, 51, 11335 CrossRef CAS; (e) E. Reixach, R. M. Haak, S. Wershofen and A. Vidal-Ferran, Ind. Eng. Chem. Res., 2012, 51, 16165 CrossRef CAS; (f) Z. H. Fu and Y. Ono, J. Mol. Catal., 1994, 91, 399 CrossRef CAS; (g) T. Baba, M. Fujiwara, A. Oosaku, A. Kobayashi, R. G. Deleon and Y. Ono, Appl. Catal., A., 2002, 227, 1 CrossRef CAS; (h) S. P. Wang, G. L. Zhang, X. B. Ma and J. L. Gong, Ind. Eng. Chem. Res., 2007, 46, 6858 CrossRef CAS; (i) C. Han and J. A. Porco, Org. Lett., 2007, 9, 1517 CrossRef CAS PubMed; (j) M. Curini, F. Epifano, F. Maltese and O. Rosati, Tetrahedron Lett., 2002, 43, 4895 CrossRef CAS; (k) M. Distaso and E. Quaranta, J. Catal., 2008, 253, 278 CrossRef CAS PubMed; (l) S. Kumar and S. L. Jain, New J. Chem., 2013, 37, 2935 RSC; (m) R. Juárez, A. Padilla, A. Corma and H. García, Ind. Eng. Chem. Res., 2008, 47, 8043 CrossRef.
- (a) F. Li, Y. J. Wang, W. Xue and X. Q. Zhao, J. Chem. Technol. Biotechnol., 2009, 84, 48 CrossRef CAS; (b) A. Orejón, A. M. Masdeu-Bultó, P. Salagre, S. Castillón, C. Claver, A. Padilla, B. Almena and F. L. Serrano, Ind. Eng. Chem. Res., 2008, 47, 8032 CrossRef.
- (a) S. Grego, F. Aricò and P. Tundo, Pure Appl. Chem., 2012, 84, 695 CAS; (b) S. Grego, F. Aricò and P. Tundo, Org. Process Res. Dev., 2013, 17, 679 CrossRef CAS.
- (a) X. Q. Zhao, Y. J. Wang, S. F. Wang, H. J. Yang and J. Y. Zhang, Ind. Eng. Chem. Res., 2002, 41, 5139 CrossRef CAS; (b) F. Li, W. B. Li, J. Li, W. Xue, Y. J. Wang and X. Q. Zhao, Appl. Catal., A, 2014, 475, 355 CrossRef CAS PubMed.
- X. C. Guo, Z. F. Qin, W. B. Fan, G. F. Wang, R. H. Zhao, S. Y. Peng and J. G. Wang, Catal. Lett., 2009, 128, 405 CrossRef CAS.
- (a) J. L. Speier, J. A. Webster and G. H. Barnes, J. Am. Chem. Soc., 1957, 79, 974 CrossRef CAS; (b) D. Troegel and J. Stohrer, Coord. Chem. Rev., 2011, 255, 1440 CrossRef CAS PubMed.
- (a) A. Cauvel, G. Renard and D. Brunel, J. Org. Chem., 1997, 62, 749 CrossRef CAS; (b) Y. V. Subba Rao, D. E. De Vos and P. A. Jacobs, Angew. Chem., Int. Ed. Engl., 1997, 36, 2661 CrossRef; (c) K. Moller and T. Bein, Chem. Mater., 1998, 10, 2950 CrossRef CAS; (d) A. P. Wight and M. E. Davis, Chem. Rev., 2002, 102, 3589 CrossRef CAS PubMed.
- G. E. Maciel and D. W. Sindorf, J. Am. Chem. Soc., 1980, 102, 7606 CrossRef CAS.
- M. Geppi, S. Borsacchi, G. Mollica and C. A. Veracini, Appl. Spectrosc. Rev., 2009, 44, 1 CrossRef CAS.
- T. W. G. Solomons and C. B. Fryhle, in Organic Chemistry, Wiley, New York, 8th edn, 2004, ch. 2, pp. 79–97 Search PubMed.
- K. Ito and H. J. Bernstein, Can. J. Chem., 1956, 34, 170 CrossRef CAS.
- T. Baba, A. Kobayashi, Y. Kawanami, K. Inazu, A. Ishikawa, T. Echizenn, K. Murai, S. Aso and M. Inomata, Green Chem., 2005, 7, 159 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supporting data. See DOI: 10.1039/c4cy01130a |
| This journal is © The Royal Society of Chemistry 2015 |