Proline-induced enantioselective heterogeneous catalytic hydrogenation of isophorone on basic polymer-supported Pd catalysts
Abstract
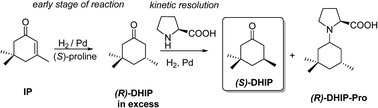
The mode of enantioselection in the proline-modified asymmetric hydrogenation of isophorone (3,5,5-trimethyl-2-cyclohexenone) on polymer-supported Pd catalysts has been studied. Based on earlier results, polymers of basic nature, such as poly(vinyl-pyridine) (PVP), aminomethylated polystyrene (AMPS) and Amberlyst-OH (AOH), have been applied. The study has been focused on the early events in the reaction. The effect of different parameters such as hydrogen pressure and proline configuration and concentration has been studied. The pristine and proline pretreated catalysts have also been investigated with FT-IR spectroscopy. In the case of the AMPS-supported catalyst, the spectra indicated the formation of an amido group-anchored proline, which was potentially formed by the reaction of the surface amino groups with the carboxylic acid unit of proline. Our results provide convincing support for the existence of heterogeneous enantioselection in this system. These studies indicate that the basic nature of the support is clearly able to contribute to the observed enantioselectivities through the strong, potentially covalent, adsorption of the modifier.
- This article is part of the themed collection: Catalysis on Chiral Surfaces: From Fundamental Aspects to Application

 Please wait while we load your content...
Please wait while we load your content...