Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbohydrate nanocarriers in biomedical applications: functionalization and construction
Biao
Kang
a,
Till
Opatz
b,
Katharina
Landfester
a and
Frederik R.
Wurm
*a
aMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: wurm@mpip-mainz.mpg.de
bInstitute of Organic Chemistry, University of Mainz, Duesbergweg 10-14, 55128 Mainz, Germany
First published on 17th August 2015
Abstract
The specific targeting of either tumor cells or immune cells in vivo by carefully designed and appropriately surface-functionalized nanocarriers may become an effective therapeutic treatment for a variety of diseases. Carbohydrates, which are prominent biomolecules, have shown their outstanding ability in balancing the biocompatibility, stability, biodegradability, and functionality of nanocarriers. The recent applications of sugar (mono/oligosaccharides and/or polysaccharides) for the development of nanomedicines are summarized in this review, including the application of carbohydrates for the surface-functionalization of various nanocarriers and for the construction of the nanocarrier itself. Current problems and challenges are also addressed.
1. Introduction
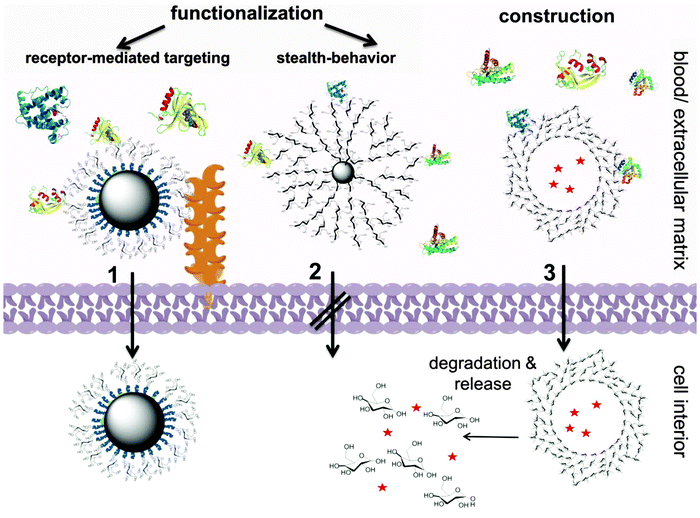
Since Paul Ehrlich has coined the term the “magic bullet” for modern medicine in the beginning of the 20th century, the development of targeted drug delivery has received immense interdisciplinary attention, ranging from chemistry over biology to medicine.1 In the last few decades, the idea has gradually evolved into the application of nanometer-sized vehicles for the delivery of drugs, due to their advantages including (i) protection of the payload from degradation in vivo, (ii) allowing specific targeting to the diseased tissue and thus (iii) reducing the risk of systemic toxicity, and, finally, (iv) the release of the drug, while the carrier is eliminated from the body without trace. All these properties have been realized partly in today's nanomedicine, however, have still not been accomplished completely. The innovative design and chemical functionalization of suitable nanocarriers is still challenging to finally generate “magic bullets”, selective drug delivery systems, of the 21st century.The early-stage nanocarriers were mostly prepared from artificial polymeric2–5 or inorganic materials.4,6–9 To increase their blood circulation times poly(ethylene glycol) (PEG) is often attached to their surface as the so called “stealth layer” decreasing protein adsorption.4–6,10 These nanocarriers suffered from several intrinsic drawbacks, especially regarding their biocompatibility and biodegradability. More recently, the research focus shifted to the use of natural materials for the fabrication of nanocarriers, which are inherently compatible with the metabolic system and have high potential for biological and biomimetic effects.
Together with lipids, proteins, and nucleic acids, carbohydrates (or saccharides) are one of the four major classes of biomolecules. The combination of several advantages of carbohydrates makes them unique candidates for application in nano-medicine:
(i) chemically well-defined structure
(ii) biocompatible/biodegradable
(ii) available on a large scale
(iv) protein-repellent
(v) highly water soluble
(vi) no aggregation
(vii) natural targeting agents
In contrast to proteins and nucleic acids, when oligo/poly saccharides are formed through chain elongation and branching, the linkage points between sugar units are not restricted to constant positions. Regioisomers can be formed by elongating the sugar chain at different hydroxy groups, resulting in a significantly enhanced code capacity. While 20 amino acids yield 6.4 × 107 hexapeptide isomers, the same amount of hexose repeating units in an oligosaccharide will result in 1.44 × 1015 different isomers.11 In addition, most of the carbohydrates are located on the outer surface of the cell, in the extracellular fluid and blood,12 which is the biological environment for the intravenously injected nano-medicines. The molecular understanding of the peculiarities of carbohydrates will help to pave the road for the translation of “sweet” nano-medicines to the clinic. Besides their role in biological signaling, carbohydrates also have other biological functions, including energy storage, protection of cell organelles,13 modification of the properties of peptides or proteins,14etc., which might grant the nano-medicine additional advanced properties. In addition, carbohydrates are responsible for cell/cell, and cell/matrix communications and interactions in cellular organelles or multicellular organs.15–17 Studying and utilizing the information from this natural “glyco-code” and exploiting the differences between healthy and malignant cells is a promising strategy for the diagnosis and treatment of cancer.18
Besides the biological origin of oligo- and polysaccharides and their important role in biological communication, their inherent hydrophilicity makes them even more attractive for biomedical polymer science. They are currently discussed as potential biodegradable substitutes for PEG, to reduce unspecific protein adsorption.19 It has been reported that hydroxyethyl starch (HES),20–24 a synthetically modified starch derivative, dextrin25–27 or other saccharides28,29 can reduce the protein adsorption on nanocarriers and prolong their circulation time in the blood stream similarly to PEG. This protein-repellent property, together with their active biological function to interact with certain proteins/cell surfaces, makes carbohydrates very promising elements for the construction of future therapeutics.
Another feature making carbohydrates interesting for drug delivery is their biodegradability. This not only ensures the eventual body clearance of the materials, but is an additional handle to trigger drug release or activation by certain enzymes.23–27 For HES, for example, degradation kinetics can be precisely adjusted by varying the degree of hydroxyethylation.23,24 In summary, the (i) biological activity, combined with (ii) the potential stealth properties, and (iii) the enzymatic stimulus makes carbohydrates interesting materials for the design of nanocarriers for biomedical applications. Both, surface-modification of preformed nanoparticles with carbohydrates or the direct construction of the nanocarriers from mono-, oligo-, or polysaccharides have attracted considerable attention during the last decade over the borders of single disciplines.
There are some reviews concerning the use of polysaccharides in nanomedicine, such as an – at that time comprehensive – article covering sugar-decorated nanoparticles from 2004.21 More recent reviews cover peptide- and saccharide-conjugated dendrimers (from 2012),30 and nanoparticles based on polysaccharides (200831 and 201432), which mainly focus on the synthetic methods. In 2013, a review disclosed comprehensively the application of carbohydrate functionalized nanoparticles as sensitive detection agents, inhibitors of bacterial adhesion, cancer vaccines in therapeutic systems, drug delivery agents, with focus on their imaging and detecting properties.33 Two interesting reviews about glyco-nanoparticles have also been published in 2013; both focusing on inorganic nanoparticles, like carbon nanomaterials, metal nanoparticles, quantum dots (QDs), magnetic (MNPs) and silica nanoparticles (SNPs). Marradi et al. elaborately discussed the density and orientation of sugars and their influence on the multivalency of binding,34 while Reichardt et al. summarized applications of glyco-nanoparticles in molecular imaging, biosensors for lectin/glycan, new concepts for the affinity separation and analysis, and vaccine development,35 all of which will not be the focus of the present review. A more recent review on glyco-nanoparticles was published in 2014,36 which also focused on inorganic nanoparticles and their application for imaging and diagnostics.
Herein, recent design strategies for carbohydrate-based nanocarriers will be reviewed: our article covers the surface-functionalization of nanoparticles as well as the full construction of nanocarriers from saccharides. In addition, both monomeric and oligo/polymeric carbohydrate-motifs are reviewed, as depicted in Scheme 1. The main focus of this review is to comprehensively present the advantages of carbohydrates as major components in drug delivery systems.
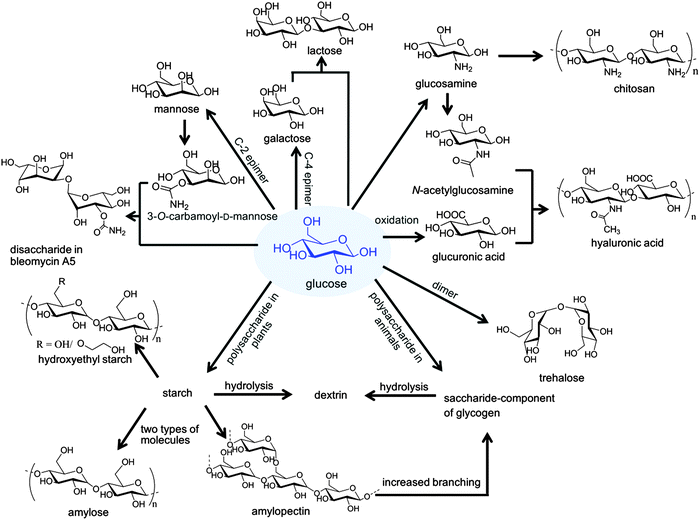
The review is structured as follows: the fundamental part will discuss the properties of carbohydrates for drug delivery, the chemical relations between different sugars, and how nature functionalizes proteins with saccharides to produce glycoproteins. The subsequent part will address the applications of carbohydrates for (i) the surface-functionalization of nanocarriers and (ii) the construction of nanocarriers. In the final part, the overall benefits gained from the application of carbohydrates are summarized and we give an outlook on potential future applications (Fig. 1 gives an overview on the structural relations of carbohydrates, which are discussed herein).
2. Fundamentals
2.1 Recognition of saccharides by cell surface receptors and their use for targeting of specific cells
Cells of higher organisms are in constant communication and interaction with their environment. In order to survive and maintain the appropriate functions, external signals must be received by the cell-surface, and subsequently delivered into the cell's interior.37 While much of this biological information is encoded and delivered by protein–protein interactions, carbohydrates also play a significant role.18,38,39 Carbohydrates act as recognition markers in different pathological and physiological processes, most of them occurring on the surfaces of cells. Three classes of proteins serve as receptors for the carbohydrate ligands: enzymes (for the synthesis, remodeling and degradation of carbohydrate), immunoglobulins and, most importantly, lectins40 which are membrane-bound receptors and assist during the process of endocytosis.41Through the binding with these receptors, many types of carbohydrates, including mono-, oligo-, and polysaccharides have been found to specifically bind to certain cell types. Mono/oligosaccharides like mannose derivatives exhibit strong binding to the C-type lectin DC-SIGN on the surface of dendritic cells,42 C-type lectin receptors on alveolar macrophages,43 and the plant lectin concanavalin A.44 Galactose can also bind selectively to C-type lectin receptors on alveolar macrophages43 and carbohydrate receptors on E. coli cells.45 Lactobionic acid can bind to asialoglycoprotein receptors (ASGP-R) of hepatic tumor cells.46 For rhamnose, a specific targeting effect to human skin cells was demonstrated.47 Polysaccharides like hyaluronic acid or chitosan have been found to specifically bind to ocular mucosa.48–50 Functionalized dextran has proven to specifically target vascular smooth muscle cells51 and human endothelial cells.52 Many cellular events are regulated by these sugar codes, including cell adhesion, proliferation, and cell death.53–56
Cancer still is one of the most prevalent deadly diseases worldwide and constitutes one of the two major causes of death in industrialized countries. While the complete eradication of malignant tumor is severely complicated by a tendency to form metastases, all malignant cells have special biological signatures which distinguish them from their healthy counterparts.
Carbohydrates, in particular glycoconjugates, play an essential role in cancer metastasis and communication, through the interaction with endogenous lectins present on the cancer cells.57–59 Presumably due to the fast metabolism of the tumor tissue, some of these lectins, e.g. galectins, are found to be expressed at an elevated level on malignant cells while they are not expressed detectably by their healthy counterparts.12,60 Defined by their role as β-galactose receptors,61 galectins have been reported as indicators for malignancies in stomach,62 liver,63 and the corresponding colon cancer.64–66 A high galectin-1 level was reported in papillary carcinomas, but not in the healthy tissues.67,68 A significant increase in the galecin-1 expression in adenocarcinoma cells was also reported, in contrast to the adjacent normal endometrium.69 In addition, other carbohydrates, like hyaluronic acid also shows specific binding to CD44 receptors70,71 which are expressed at low levels on hematopoietic, epithelial, and neuronal cells but at much higher levels in various tumor cells like lymphomas, melanomas, colorectal, and lung tumor cells.72,73 Thus, many carbohydrate-related biomarkers have been developed which individually exhibit specific binding to different cancer cells,74 and may open up possibilities to specifically target cancer cells by an appropriate carbohydrate functionalization of nanocarriers.
2.2 Protein repellent properties of carbohydrates
When a nanocarrier enters a biology fluid, e.g. by intravenous injection into the bloodstream, it will adsorb proteins on its surface, due to hydrophobic interactions and the high surface energy of most types of nanocarriers.75,76 This process, known as opsonization, can lead to phagocytosis of the nanocarrier by the Mononuclear phagocyte system (MPS). The adsorbed proteins will determine the fate of the nanocarrier in vivo (this process is often called the formation of a “biological identity”),75 typically resulting in the fast clearance of the nanocarriers from the blood. This makes any in vivo specific targeting a challenging task.75 In order to prolong the in vivo plasma half-life times of the nanocarriers, the opsonization needs to be reduced, either by the material of the nanocarrier itself, or by surface modification (dressing of the nanocarriers). Currently, PEGylation is the “gold standard” to achieve long blood circulation times and reduced unspecific cellular uptake due to the hydrophilicity and the steric repulsion by PEG-modified surfaces and proteins.19 PEGylation has achieved numerous successes in the past decades, and many PEG-related products both in consumer care and biomedical applications have improved the quality of life.19,77 In spite of these achievements, recent studies reported several drawbacks of PEG. The occurrence of renal tubular vacuolization in animal models has raised concerns that a prolonged therapy with PEGylated drugs may lead to an accumulation of PEG in the cytoplasm of kidney cells as the polymer is not biodegradable.78,79 In addition, PEG potentially forms toxic degradation products upon storage which could provoke adverse effects.19 These setbacks of PEG could be circumvented by using polysaccharides as substitutes which often show low hypersensitivity even after chemical functionalization.80 The structural similarity of many polysaccharides, for example HES or dextran, to the sugar component of glycogen, which is the form for the storage of sugar in animals, is a probable explanation why they lack immunogenicity. Moreover, the biodegradability of polysaccharides is advantageous over many other synthetic polymers that are currently discussed as alternatives for PEG.81 Not only the post-injection clearance of the nanocarriers is enhanced by its biodegradability, but also enzymatic induced masking–unmasking or encapsulation-release cascades of the payload are possible.23–27,44 However, care has to be taken depending on the chemical modification, e.g. anchoring or polymerizable groups that may alter both the degradation process and the cytotoxicity of the carbohydrates.Numerous studies have already proven that polysaccharide or their derivatives like HES20–22,82 or dextran exhibit a low protein affinity.28,29 Furthermore, the microbial polysaccharide pullulan, glycolipids, and dextran have shown their ability to decrease the uptake of nanocarriers into the MPS,83,84 while HES has been proven to suppress the unspecific uptake of the nanocarriers in vitro,85 and prolong the plasma halftime in vivo, the process of its attachment being called HESylation.23,24 While several mono- or oligosaccharides are responsible for the communication of biological information in the organism, some of them are capable of impeding the phagocytosis of native cells by the MPS. Sialic acid is one example of these saccharides and red blood cells without surface sialic acid are immediately removed from the blood by the MPS.86 It has been proven that when sialic acid is coupled to the surface of quantum dots, the in vivo plasma half-life time of the latter is prolonged.87
2.3 Glycoproteins: how nature uses carbohydrates
In nature, glycoproteins, i.e. glycosylated polypeptides, are of high importance and function as hormones,88 antibodies,89 antifreeze proteins,90 and proteins in the cell membrane.14 After glycosylation, the attached (oligo)saccharides provide additional properties for the protein, such as facilitating the protein folding and stabilizing the conformation of the peptidic backbone,91 protection,92 elongation of the in vivo plasma half-life,93 communication with the immune system,94 and adhesion to cognate receptors on other cell surfaces.95,96Inspired by these natural strategies, various researchers have prepared neoglycoproteins for diverse applications. Pharmacologically active peptides have been used for the treatment of various diseases.77,97 A major drawback, however, is their usually rapid degradation in vivo. To optimize the pharmacokinetic properties of such drugs, artificial polymers are frequently coupled to their surface. Typically PEG is used for this purpose but in modern literature, an increasing percentage of biodegradable biopolymers are coupled to proteins to optimize their therapeutic performance. For example, hyaluronan-functionalized insulin showed a prolonged and enhanced hypoglycemic effect, demonstrating the potential of hyaluronan in increasing the plasma half-life of peptides.98
Anakinra, a synthetically generated interleukin-1 antagonist, is used for the treatment of rheumatic arthritis, but has a plasma halftime of only 108 min; after conjugation with HES its blood circulation time was increased by a factor of 6.5.99
Dextrin, a glucose polymer with a molecular weight of 7700 and 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 and a degree of succinoylation of 9–32 mol% was used to functionalize trypsin (a serine protease) and thus masking its activity. The activity of the enzyme can be restored after the degradation of the polysaccharide by α-amylase.25–27 Also hyaluronic acid was used for the functionalization of trypsin, resulting in an increase of its activity to 145% over the native protein, while exhibiting a 52% higher stability in the presence of elastase (a protease).100 Although these studies are beyond the scope of this review, the idea of mimicking nature to utilize the advantageous properties of different sugars is identical.
200 g mol−1 and a degree of succinoylation of 9–32 mol% was used to functionalize trypsin (a serine protease) and thus masking its activity. The activity of the enzyme can be restored after the degradation of the polysaccharide by α-amylase.25–27 Also hyaluronic acid was used for the functionalization of trypsin, resulting in an increase of its activity to 145% over the native protein, while exhibiting a 52% higher stability in the presence of elastase (a protease).100 Although these studies are beyond the scope of this review, the idea of mimicking nature to utilize the advantageous properties of different sugars is identical.
3. Carbohydrate-functionalized nanocarriers
Being a C-2-epimer of glucose, mannose is an important monosaccharide for the glycosylation of proteins. Mannose-containing glycoproteins are produced in the liver and secreted into the blood, hence mannose is distributed throughout the body.101 Many mannose-binding proteins, like the C-type lectins, are crucial for cell-surface recognition and other communication events.102 Recently, mannose has been applied to functionalize mesoporous silica nanoparticles,44 magnetic nanoparticles,18 gold nanoparticles,42 and polyanhydride nanoparticles43 (Table 1, entry 1) to specifically target cells; distinct biological functionalities have been achieved in each case.| # | Type of sugar | Chemical structure | Nanocarrier | Properties | Chemistry | Note | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 1 | Mannose | a |
|
Mesoporous silica nano-particles | Pore closure by coupling of mannose with concanavalin A | Thiol–ene reaction | Re-opening of the pore controlled by pH or glucose level | 44 |
| b |
|
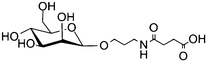
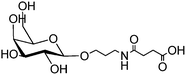
Magnetic nanoparticles | Increased binding affinity with different cell surface | Amidation | Selective binding to cancer cells | 18 | ||
| c | Different (oligo)mannosides functionalized with thiol groups | Gold nanoparticles | Inhibition of DC-SIGN/gp120 binding | Reaction between the thiol group and gold surface | A potential anti-HIV system | 42 | ||
| d |
|
Poly-anhydride nanoparticles | Targeting C-type lectin receptors on alveolar macrophages | EDC coupling | Enhanced expression of mannose receptor | 43 | ||
| 2 | Galactose | a |
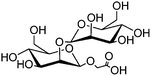
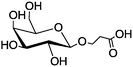
|
Magnetic nanoparticles | Increased binding affinity with different cell surface | Amide coupling reaction | Differentiates normal cells from cancer cells | 18 |
| b |
|
Polyanhydride nanoparticles | Targets C-type lectin receptors on alveolar macrophages | EDC coupling | Enhanced expression of galactose lectin | 43 | ||
| c |
|
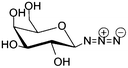
Self-assembled nanorods | High binding affinity to carbohydrate receptors on E. coli | Huisgen azide alkyne cycloaddition | Decreasing the toxicity of the nanorods | 45 | ||
| 3 | Fucose |
|
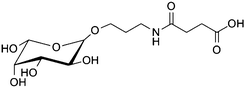
Magnetic nanoparticles | Increased binding affinity with different cell surfaces | Amidation | Distinction between isogenic sublines of cancer cells | 18 | |
| 4 | Sialic acid | a |
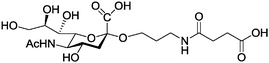
|
|||||
| b |
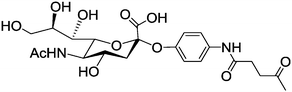
|
Quantum dots | Prolonged in vivo lifetime | Huisgen azide alkyne cycloaddition | 87 | |||
| c |
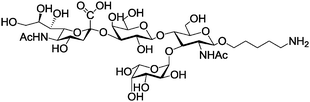 Sialyl-LewisX Sialyl-LewisX |
Core shell silica magnetic nanoparticle | Bind specifically to the endothelial transmembrane inflammatory proteins E and P selectin | Coupling between amine and NHS ester | Nanoparticles accumulated in the brain vasculature | 121 | ||
| 5 | Glucose |
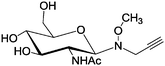
|
Magnetic nanoparticles | Increased binding affinity with different cell surfaces | Huisgen azide alkyne cycloaddition | 18 | ||
| 6 | Lactobionic acid |
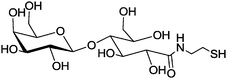
|
Micelles | Targeting liver cancer cells through asialoglycoprotein receptors (ASGP-R) | Thiol–disulfide exchange reaction | Un-coating in a reductive environment (mimicking the cell interior) | 46 | |
| 7 | Rhamnose |
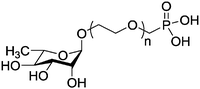
|
Fe3O4 nanoparticles | Targeting of human skin cells | Binding of phosphonate to Fe oxide | 47 | ||
| 8 | Disaccharide Moiety of Bleomycin A5 |
|
Microbubbles | Selective binding to different cancer cell types | Reacted with a NHS-ester coupled Cy5 dye | 127 | ||
| 9 | Trehalose |
|
Block copolymer self-assembly and siRNA complex | Colloidal stability of polyplexes at high salt concentrations and specific internalization into glioblastoma cells | RAFT block copolymerization, with aminoethylmethacrylamide | The amount of siRNA delivered can be controlled | 130 | |
| 10 | Starch |
|
Copper nanoparticles | Lower toxicity | Reduction of copper nitrate solution by ascorbic acid, starch as stabilizer for the nano-particle | Excellent bactericidal action | 131 | |
| 11 | Hydroxyethyl starch (HES) |
 Depends on the molar substitution Depends on the molar substitution |
DNA-polyplexes micelles | Reduced unspecific cell uptake | Schiff base formation and reductive amination | Deshielding of the nanocarrier possible | 23 and 24 | |
| 12 | Chitosan | a |
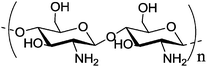 M
w ∼ 47 kDa
M
w ∼ 47 kDa |
Poly lactic-co-glycolic acid nanoparticle | Significantly increased (>5-fold) uptake by MCF-7 cells | Electrostatic interactions | 132 | |
| b |
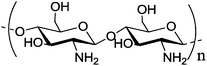 Molecular weight of 100 Molecular weight of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 000 g mol−1 |
Hyaluronic-paclitaxel nanoparticle | Protection of the payload | Electrostatic interactions | pH responsive release of paclitaxel | 133 | ||
| c |
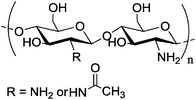 Depends on the deacetylation degree > 75% deacetylated Depends on the deacetylation degree > 75% deacetylated |
Silver nanoparticle | Lower toxicity | Chitosan as a stabilizer during preparation | Higher rate of killing cancer cell compared to PEGylated gold nanorod | 134 | ||
| d |
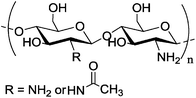 Depends on the deacetylation degree of deacetylation = 82.7%; Mw = 250 Depends on the deacetylation degree of deacetylation = 82.7%; Mw = 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Gold nanoparticles | Low unspecific cell uptake, enhanced stability and tumor targeting ability | Glycol-modified chitosan is used as reducing agent for gold(III) chloride in situ | Tomography of liver tissues with metastatic cancer | 135 | ||
| 13 | Hyaluronic acid | a |
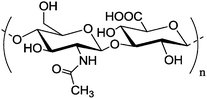
|
Micelles prepared from branched poly(ethylene imine) | Increased transfection efficiency and decreased cytotoxicity | Reductive amination | 136 | |
| b |
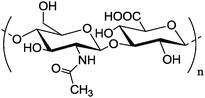
|
Re-constituted high density lipoprotein loaded with lovastatin | Lower accumulation in liver and higher atherosclerotic lesions targeting efficiency | Electrostatic interactions | Efficiently suppressed the advancement of atherosclerosis | 137 | ||
| c |
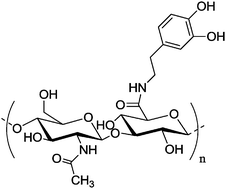
|
Gold nanocage | Specific binding to cancer cells via interaction with CD44, release in lysosome | Au–catechol bonds | Near-infrared irradiation accelerates the release | 138 | ||
When thiol-functionalized mannose is reacted with alkenyl-terminated silanes in a radical thiol–ene addition (Table 1, entry 1a), surface functionalization of mesoporous silica nanoparticles can be achieved, whose pores can be sealed by adding concanavalin A, a carbohydrate-binding protein, to the dispersion. The pores can be re-opened under acidic conditions (pH < 5.5) or in a glucose-rich environment. The release of the payload in the tumor tissue, where the pH value is typically lower than that in healthy tissue, or under high blood sugar level is thus possible.44 In another work, mannose-functionalized silica nanoparticles have been prepared, which showed specific binding to MCF-7 human breast cancer cells.103
Carboxylated derivatives of mannose (Table 1, entry 1b), galactose (Table 1, entry 2a), fucose (Table 1, entry 3), and sialic acid (Table 1, entry 4a) have been coupled to amino-functionalized magnetic nanoparticles via an amide linkage. When these nanoparticles were incubated with different malignant and non-malignant cells and investigated via magnetic resonance imaging, it was shown that the malignant cells can be differentiated by the changes in T2 relaxation time (% ΔT2),18 as shown in Fig. 2. Similar work was conducted using sialic acid-functionalized magnetic nanoparticles to detect the levels of β-amyloid, which is a pathological hallmark of Alzheimer's disease, both in vitro and ex vivo.104
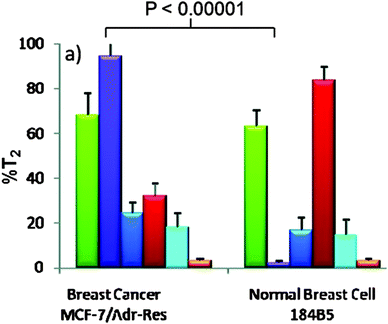 | ||
| Fig. 2 Discrimination of the breast cancer cells from their healthy counterparts by the changes in T2 relaxation time (% ΔT2) in magnetic resonance imaging, by magnetic particles functionalized with: mannose (green), galactose (violet), fucose (dark blue), sialic acid (red), glucose (light blue), compared to unmodified magnetic particles (orange), (adapted with permission from ref. 18. Copyright 2010 American Chemical Society). | ||
Different (oligo)mannosides have also been functionalized with thiols and coupled to gold nanoparticles.42 The obtained glycosylated gold nanoparticles show stronger binding to DC-SIGN (a C-type lectin) on the surface of dendritic cells compared to gp120, which is a protein essential for the entry of HI virus into cells, and thus could serve as a potential carbohydrate-based drug against HIV (Table 1, entry 1c). Similar glycosylated gold nanoparticles have also been prepared in another work and were observed to cross the blood–brain barrier (BBB) nearly 3-fold faster/more efficiently than unmodified gold nanoparticles.105
Both, α-1,2-linked dimannose (Table 1, entry 1d) and galactose have been coupled to polyanhydride nanoparticles through an amidation reaction via 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) as the coupling agent. The obtained mannose surface-functionalized particles, which were termed “pathogen-like” nanocarriers, exhibited specific binding to alveolar macrophages through the surface C-type lectin and enhanced the expression of the macrophage mannose receptor.43
It is the concern of some recent publications that the protein adsorption after contact with blood will hamper all specific targeting of nanocarriers due to the shielding of targeting groups, which might reduce the efficiency of “targeted” drug delivery systems remarkably.106–113 A current challenge is to understand the interaction of blood proteins with nanocarriers which carry additional targeting groups. The adsorption of plasma proteins onto the targeting agent could hinder the recognition of the targeting agent by the respective cells and hence could make any in vivo targeting impossible.75 The interactions of mannose-functionalized nanocarriers with plasma proteins have been studied to address this problem. It turned out that, in comparison with a PEGylated nano-carrier, additional functionalization of the PEGylated nanocarrier with mannose did not significantly change its protein corona formation. Furthermore, these mannose functionalized nanocarriers showed the same binding affinity to dendritic cells (DCs) both in the presence and absence of the plasma protein corona.114
Galactose is the C-4 epimer of glucose and is for example essential for the antigen structure of red blood cells which is the determinant of the blood type. For O and A antigens, two galactose units are contained in the saccharide portion while for the B antigen, three galactose units are contained.115 Galactose functionalized with an azide group at the C1-position, was coupled to pillar[5]arene by a Huisgen-type cycloaddition, while the latter is self-assembled into nanorods (Table 1, entry 2c), which have proven to show a high affinity for the carbohydrate receptors on E. coli as well as low toxicity, and can be utilized as excellent cell glues to agglutinate these bacteria.45 In another work, different statistical glycol-dithiocarbamate copolymers were prepared and used to functionalize gold nanoparticles on the surface, which were further coupled with gold(I) triphenylphosphine as an anticancer agent. Among these glyconanoparticles, the galactose-functionalized ones were found to be 4-fold more cytotoxic to HepG2 cells, in comparison with glucose and lactose functionalized particles.116
Sialic acid is a monosaccharide, which is widely distributed in animal tissues and mostly bound in the form of glycoproteins.117 It plays an important role in recognition and communication with the immune system,118–120 which is also proven by the fact that red blood cells without sialic acid on the surface are immediately removed from blood by the MPS.86 Ketone-functionalized sialic acid is reacted with aminooxy-functionalized quantum dots, namely phosphorylcholine self-assembled monolayer-coated quantum dots (PC-QDs), and their in vivo half-life times are extended compared to quantum dots functionalized by other monosaccharides (Table 1, entry 4b; and Fig. 3).87
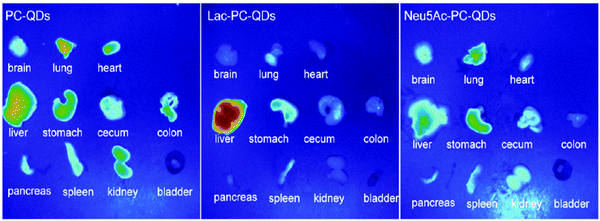 | ||
| Fig. 3 Images of major organs isolated from three tested mice, 2 h after the administration of phosphorylcholine quantum dots (PC-QDs), lactose-functionalized quantum dots (Lac–PC-QDs), and sialic acid-functionalized quantum dots (Nue5Ac-PC-QDs). (Reprinted with permission from ref. 87. Copyright 2011 American Chemical Society.) | ||
Sialyl-Lewisx is very important antigen for blood groups; it is displayed on the terminus of glycolipids that are present on the cell surface, has been used to functionalize superparamagnetic silica nanoparticles (Table 1, entry 4c), with the functionalization strategy shown in Fig. 4. These nanoparticles have diameters of around 18 nm and carry NH2-groups.121 Subsequent functionalization of these particles with an NHS-ester allows coupling to amino-functionalized Sialyl-LewisX. The obtained glycosylated nanoparticles bind specifically to the inflammation-associated endothelial transmembrane proteins E and P selectin, both cell adhesion molecules. In vivo studies have shown an accumulation in the brain vasculature by measuring the relaxation time of the nanoparticles via MRI.121
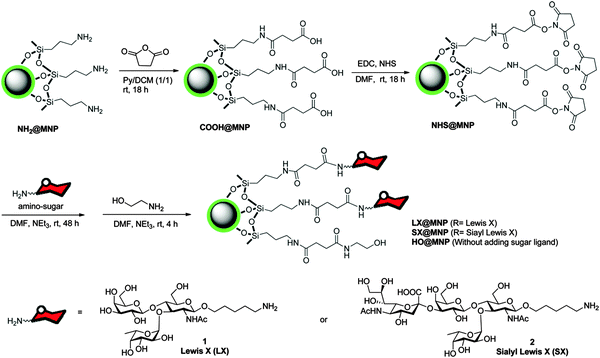 | ||
| Fig. 4 Functionalization of superparamagnetic nanoparticles with a silica core by Sialyl-LewisX. (Reprinted with permission from ref. 121. Copyright 2014 American Chemical Society.) | ||
Lactobionic acid (4-O-β-D-galactopyranosyl-D-gluconic acid, (Table 1, entry 6)) specifically bind to hepatocytes.122 Thiolated lactobionic acid was used to functionalize block copolymers, which were prepared by the ring-opening copolymerization of ε-caprolactone and a pyridyl disulfide containing cyclic carbonate, followed by post polymerization modification with thiolated lactobionic acid via the thiol–disulfide exchange reaction. The post-modified block copolymers then self-assembled into micelles with lactobionic acid on the surface. These micelles were shown to target liver cancer through asialoglycoprotein receptors (ASGP-R). Furthermore, the saccharide shells are cleavable under a reductive environment mimicking the interior of a cell.46
Rhamnose is a mannose-related 6-deoxy hexose which naturally occurs in the L-form. It is found mainly in bacteria and plants and is often present in the cell walls and is essential for the survival of bacteria.123 Phosphonated rhamnose has been prepared (Table 1, entry 7) and anchored to magnetic nanoparticles through the strong binding of phosphonate groups to metals. The rhamnose-functionalized magnetic nanoparticles exhibited the targeting effect on human skin cells. Since the iron oxide nanoparticles are superparamagnetic, they can be used as MRI contrast agents with specific cell targeting.47
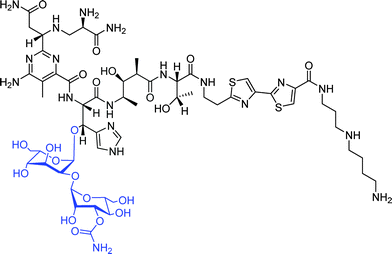
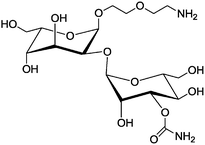
Bleomycin (BLM, Fig. 5), a glycopeptide-type antibiotic, has strong antitumor abilities and is used for the treatment of malignant lymphomas and squamous cell carcinomas.124,125 Additionally, BLM also has shown a specific tumor cell targeting effect, and hence has been used for tumor imaging.126 In order to understand the origin of the tumor targeting effect, it has been split into the BLM-analogue deglycobleomycin (devoid of the disaccharide moiety) and the disaccharide moiety itself. The difference in the ability for specific tumor targeting of these two derivatives has been investigated, and the disaccharide motif was found to be responsible for selective binding to MCF-7 human breast carcinoma cells and BxPC-3 pancreatic cancer cells, while having their healthy counterparts not being targeted.127 In contrast, deglycobleomycin (Bleomycin without the disaccharide moiety) did not show any specific targeting. Furthermore, after coupling of the disaccharide moiety to the surface of microbubbles (Table 1, entry 8), which consist of an empty core and a lipid shell, and originally used as contrast agents for ultrasonography,128 specific targeting of MCF-7 human breast carcinoma cells has been observed. Furthermore, the subsequent study verified that it is a single sugar unit from this disaccharide, namely the carbamoylmannose moiety, which is responsible for the tumor cell specific targeting effect.129
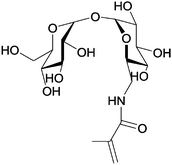
Trehalose (α-D-glucopyranosyl-(1 → 1)-α-D-glucopyranoside) is composed of two α-glucose units which are linked by an α,α′-1,1-glucosidic bond. It is widely found in animals, plants, and microorganisms. It is the blood-sugar of many insects, including locust, butterflies and bees. It is believed to transfer into a gel phase under dehydrating condition, protecting the cell internal organelles and hence the whole cell against desiccation.13 A monomer, namely methacrylamido trehalose (Table 1, entry 9) was polymerized followed by chain extension with aminoethyl methacrylamide (AEMA). The obtained polymer was used to complex siRNA with polyplexes which carry trehalose on the surface. These polyplexes show high stability in the presence of high salt concentrations and serum proteins and are specifically internalized into a brain tumor cell line (U-87 cells) as can be seen in Fig. 6.130
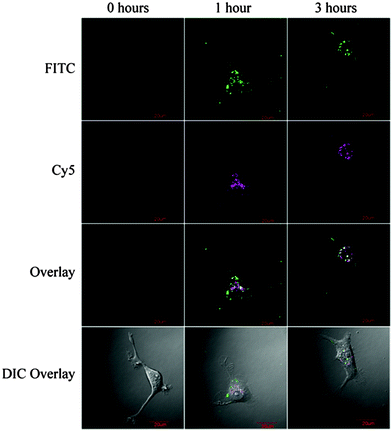 | ||
| Fig. 6 Confocal microscopy of U-87 cells transfected with siRNA containing polyplexes, both fluorescent intensity from Cy5-labeled siRNA (magenta) and FITC-labeled poly(methacrylamidotrehalose) is detected. (Reprinted with permission from ref. 130. Copyright 2013 American Chemical Society.) | ||
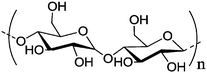
Starch is a polysaccharide with glucose as the monomer, which is coupled via glycosidic linkages. Two forms of starch are found in nature: amylose, a linear and helical polysaccharide with α-1,4-glycosidic bonds, and amylopectin, a branched poly(glucose) with 1,4- and 1,6-glycosidic bonds. It is the energy storage medium of green plants and the most common carbohydrate in human diets.139 The sugar part of glycogen, as another glucose polymer, is used to store glucose in animals with a similar structure to amylopectin, but with a higher degree of branching. Starch is used as the stabilizer during preparation of copper nanoparticles, while ascorbic acid is used as the reducing agent, and copper nitrate as the source of copper (Table 1, entry 10), which will result in starch-functionalized copper nanoparticles with a reduced toxicity, while retaining high antibacterial potential against both gram negative and gram positive strains.131
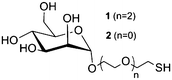
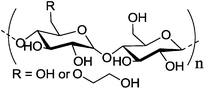
Hydroxyethyl starch. For some applications, the degradation kinetics of starch is too fast; starch is rapidly hydrolyzed by plasma amylases. In order to balance the biodegradability and stability, hydroxyethyl starch (HES) was introduced. It is prepared by ethoxylation of the hydroxyl groups with ethylene oxide, resulting in decreased biodegradation kinetics. The degree of hydroxyethyl-substitution is expressed by the molar substitution, which is the mean number of hydroxyethyl groups per glucose unit, and ranges between 0 and 3. The higher the molar substitution, or the higher the C2/C6 ratio of hydroxyethylation, the lower the rate of metabolization.80 Moreover, HES exhibits low hypersensitivity80 and depressed protein adsorption20–22,82 rendering it an interesting substitute for PEG for the preparation of stealth nanocarriers. HESylation of proteins and nanocarriers is of high potential as future drug delivery vehicles as it combines adjustable degradation with stealth properties.140 HES with different molecular weights and degrees of substitution were coupled to poly(ethylene imine) via Schiff base formation and reductive amination (Table 1, entry 11). Subsequent complexation of the polymer with DNA generated so called DNA-polyplexes, which presented HES on their surface. These polyplexes proved to exhibit stealth properties, as the nanocarrier is protected against α-amylase. The effect of deshielding is also affected by the degree of substitution of HES, as can be seen in Fig. 7.23,24
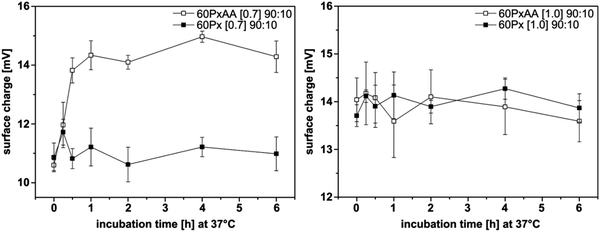 | ||
| Fig. 7 The effect of alpha amylase (AA) on biophysical characteristics of HESylated polyplexes. Two different HES species are coupled with poly(ethylene imine) (Px). Both of them have the molecular weight of 60 kDa, and the molar substitution of 0.7 and 1.0 respectively. The surface charge of HES60[0.7]-shielded (left) and HES60[1.0]-shielded (right) DNA-polyplexes under the effect of AA as a function of time at 37 °C. (Reprinted with permission from ref. 19.) | ||
Chitosan is a linear cationic polysaccharide, mainly prepared from the shells of shrimps or other crustaceans, composed of randomly distributed N-acetyl-D-glucosamine (acetylated unit) and glucosamine (deacetylated unit), with the ratio being referred to as the degree of deacetylation.141 Due to the cationic charges of chitosan, it can be electrostatically anchored onto the surface of anionically charged polymers or particles, such as poly(lactic-co-glycolic acid) nanoparticles (Table 1, entry 12a). Compared to the unmodified nanoparticles, the chitosan-coated particles showed a significant increased (>5-fold) uptake by MCF-7 cells, and the proapoptotic effect of chitosan providing synergistic cytotoxic activity to docetaxel, an anti-mitotic chemotherapeutic.132 In another work, chitosan was adsorbed to the surface of nanoparticles, which were formed by the self-assembly of hyaluronic-paclitaxel conjugates, by electrostatic interaction (Table 1, entry 12b). This enables the protection of the ester bond between hyaluronic acid and paclitaxel at acidic pH, and allows a controlled in vitro release of paclitaxel from the nanocarrier, which makes it suitable for oral administration.133
Nanoparticles based on effective Au and Ag photothermal transducers can be used to trigger localized hyperthermia of tumors. Chitosan has been used for the surface functionalization of silver nanoparticles (Table 1, entry 12c), and gold nanoparticles (Table 1, entry 12d). To a mixture of aqueous solutions of trisodium citrate, ascorbic acid, chitosan, and preformed Ag nanoparticles, a solution of AgNO3 was added dropwise, and chitosan surface functionalized Ag nanoparticles are obtained. These Ag nanoparticles show a lower toxicity compared to PEGylated gold nanorods, which are common hyperthermia agents.134 Ethylene glycol-modified chitosan is used as the reducing agent to produce gold nanoparticles along the polymer chain by reducing gold(III) chloride trihydrate in situ, as can be seen in Fig. 8. The obtained gold nanoparticles exhibited stealth properties, enhanced stability and tumor targeting ability.135
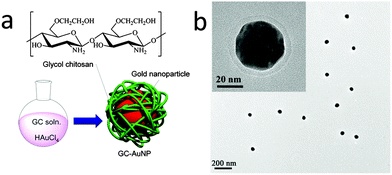 | ||
| Fig. 8 (a) Chemical structure of glycol chitosan (GC) and surface-modified AuNPs (GC-AuNP). (b) TEM images of GC-AuNPs (inset: magnified images). (Adapted from ref. 135 with permission.) | ||
Gum Arabic is the dried gum of acacia tree branches and stems. As a mixture of polysaccharides and proteins, it is mainly composed of galactose (44%), rhamnose (13%), arabinose (27%), glucuronic acid (16%) and peptides (2–3%),142 and possesses excellent emulsifying properties.143 Gum Arabic capped gold nanoparticles (GNP) have been prepared by using the leaf extract of Vitex negundo as a reducing agent and gum Arabic as a capping agent.144 Epirubicin was encapsulated in the GNP, while the surface of the GNP was functionalized by folic acid. These GNP showed increased stability at pH 7.4, together with enhanced cytotoxicity against A549 cells in comparison to free epirubicin. In another work, in vivo studies of gum Arabic functionalized GNP resulted in significant alterations in lung tumors in mice upon laser irradiation, including cyto-toxicity, apoptosis, decreased inflammation and angiogenesis, and enhanced lipid peroxidation.145
Hyaluronic acid is a polysaccharide distributed widely in all tissues and body fluids of vertebrates and is most abundantly found in the connective tissues, serves many physiological functions, including lubrication, filtering, water homeostasis, and regulation of plasma protein distribution. It is metabolized by receptor-mediated endocytosis, and subsequent lysosomal degradation.146 Hyaluronic acid was conjugated with branched poly(ethylene imine) via reductive amination (Table 1, entry 13a). Then, the polymer was self-assembled into micelles which were surface-modified by hyaluronic acid and showed increased transfection efficiency and decreased cytotoxicity.136 A reconstituted high density lipoprotein loaded with lovastatin (a statin which blocks the de novo-synthesis of cholesterol) was functionalized by hyaluronic acid (Table 1, entry 13b), through electrostatic adsorption of hyaluronic acid to a cationic lipid core of the nanoparticle. After surface-modification, the nanocarrier has lower accumulation in liver and a better atherosclerotic lesion targeting efficiency, and efficiently suppressed the advancement of atherosclerosis.137 Dopamine coupled hyaluronic acid has also been used to surface functionalize a gold nano-cage (Table 1, entry 13c), as can be seen in Fig. 9.
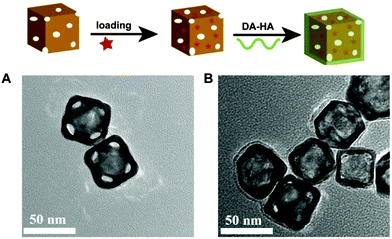 | ||
| Fig. 9 Schematic representation of the coating of the doxorubicin loaded gold nano-cage with hyaluronic acid, and the TEM image of the gold nano-cage before (A), and after (B) hyaluronic acid coating. (Adapted from ref. 138 with permission.) | ||
The hyaluronic acid layer can seal the nanoporous in the gold nanocage to protect the encapsulated dopamine, while the interaction between hyaluronic acid and the excess CD44 acceptors on the cancer cells can lead to specific cellular internalization of the nanocage.138 After the functionalized gold nanoparticles enter the lysosomes, the degradation of the hyaluronic acid layer in situ will result in the release of the payload, furthermore, the release can be accelerated upon near-infrared (NIR) irradiation.
4. Carbohydrate-constructed nanocarriers
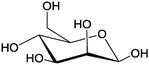
Due to their outstanding biocompatibility, biodegradability, high diversity of chemical functionalities, and versatile biological functions, carbohydrates are also useful for the construction of nanocarriers in biomedical applications.Amphiphilic dendrimers carrying both long alkyl chains and mono- or disaccharides as the hydrophilic part (Table 2, entry 1a, 2 and 3 with mannose, galactose, and lactose, respectively) can be formulated into vesicles by the addition of their THF or ethanolic solution into water. So called “glycodendrimersomes” (Fig. 10) are generated via the self-assembly of the amphiphile. They exhibited multivalent binding with lectins from both plants and humans.147
| # | Type of sugar | Chemical structure | Nanocarrier | Properties | Chemistry | Note | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 1 | Mannose | a |
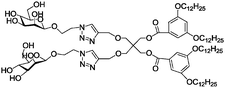
|
Glyco-dendrimersome | Multivalent binding with human and plant lectins | Self-assembly in water into glycodendrimersomes | Controlled over the size by adjusting the concentration | 147 |
| b |
|
Nanocapsule | Encapsulation of hydrophilic guest in the core of the capsule | Polycondensation with diisocyanate in a miniemulsion system | A preferential deposition in the lung | 148 | ||
| 2 | Galactose |
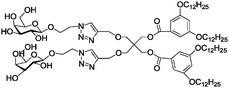
|
Glyco-dendrimersome | Multivalent binding with human and plant lectins | Self-assembly in water into glycodendrimersomes | Controlled over the size by adjusting the concentration | 147 | |
| 3 | Lactose |
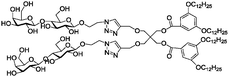
|
Glyco-dendrimersome | Multivalent binding with human and plant lectins | Self-assembly in water into glycodendrimersomes | Controlled over the size by the concentration | 147 | |
| 4 | Trehalose |
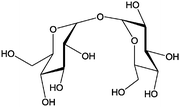
|
Nanoparticle | Protect the protein from chemical and physical degradation during storage | Self-assembly into inverse micelles | 155 | ||
| 5 | Sucrose |
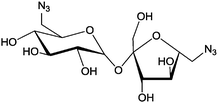
|
Nanocapsule | Encapsulation of hydrophobic guest in the core of the capsule | Huisgen azide alkyne cycloaddition | Prepared by bio-orthogonal reactions | 152 | |
| 6 | Dextran |
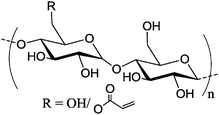 Depends on the degree of substitution Depends on the degree of substitution |
Nanocapsule | Encapsulation of hydrophilic guest in the core of the capsule | Olefin cross metathesis on the interface of miniemulsion | Prepared by bio-orthogonal reactions | 154 | |
| 7 | Starch | a |
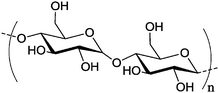 Water-soluble potato starch Mw 15 Water-soluble potato starch Mw 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 000 g mol−1 |
Nanocapsule | Encapsulation of water soluble guests in the core and functionalization of the shell possible | Polycondensation with diisocyanate in a miniemulsion system | Silver nano particles in the aqueous core, as antibacterial agent | 150 |
| b |
|
Multilayered polysaccharide vesicle | The amylase treatment of the nanoparticles allows the presence of a void/hollow inner core (resulting from the degradation starch molecules) within the fabricated particles | Rehydration of a thin film of hyaluronate-starch to form vesicles | 156 | |||
| c |
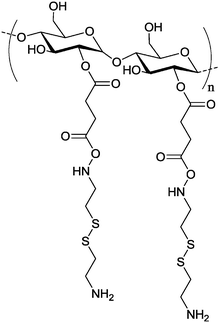
|
siRNA complex | Protect siRNA from enzymatic degradation | Self-assembled with siRNA to form nanocarriers | Efficiently induced P-glycoprotein gene silencing in the human ovarian adenocarcinoma cell line | 157 | ||
| 8 | Hyaluronic acid | a |
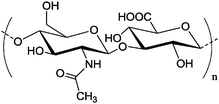 M
w = 140
M
w = 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 000 g mol−1 |
Nanocapsule | Encapsulation of polyhexanide in the core of the capsule | Polycondensation with diisocyanate in a miniemulsion system | Release of polyhexanide upon the contact with bacterial | 149 |
| b |
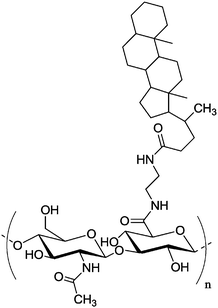
|
Nanoparticle | Specific uptake by SCC7 cancer cells, encapsulation of doxorubicin and camptothecin | Amphiphilic HA–CA is self-assembled to nanoparticles in PBS | Size can be tuned between 237–424 nm, rapid drug release in the presence of enzyme Hyal-1 | 158–161 | ||
| c |
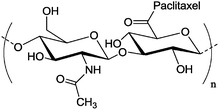
|
Nanoparticle | Higher cellular uptake than free paclitaxel in HepG2 cells | Hyaluronic – paclitaxel self-assemble in water to form the nano-particles | Paclitaxel could accumulate remarkably into tumor sites after oral administration | 133 | ||
| d |
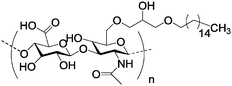
|
Nanoparticle | Binding of the particle with CD44 over-expressed cancer cells | Self-assembled in water into nanoparticles | Higher therapeutic potential in the presence of a green tea polyphenol, epigallocatechin-3-gallate | 162 | ||
| e |
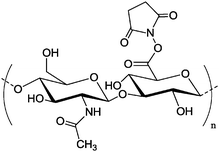
|
Multilayered polysaccharide vesicle | incubation with hyaluronidase contributed to accelerate the release | Rehydration of a thin film of hyaluronate-starch to form vesicles | Drug release in the presence of hyaluronidase | 156 | ||
| 9 | HES | a |
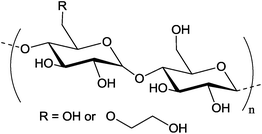 Depends on the molar substitution Mw = 200 000 g mol−1 Depends on the molar substitution Mw = 200 000 g mol−1 |
Nanocapsule | Encapsulation of hydrophilic guest in the core of the capsule | Polycondensation with diisocyanate in a miniemulsion system | Suppressed unspecific uptake into HeLa cells, preferential deposition in the liver | 85 and 148 |
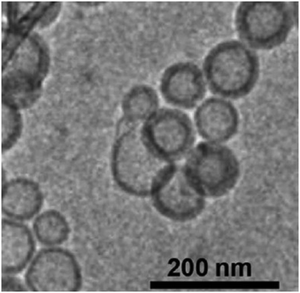 | ||
| Fig. 10 Cryo-TEM images of glycodendrimersomes assembled from amphiphilic glycodendrimer composed of mannose (adapted with permission from ref. 147. Copyright 2013 American Chemical Society.) | ||
Apart from self-assembly, emulsion techniques are interesting and versatile methods for the in situ formation of carbohydrate-based nanocarriers: nanocapsules can be prepared in an inverse miniemulsion (i.e., a stable dispersion of water droplets in an organic solvent, compare Fig. 11) by the polyaddition of the sugar-hydroxyls (dissolved inside the aqueous droplets) at the interface to strong electrophiles. Mannose-nanocapsules were prepared by the polyaddition of mannose to toluene diisocyanate (TDI) which occurs exclusively at the interface of a water-in-oil miniemulsion (Table 2, entry 1a). By the inverse miniemulsion technique, hydrophilic guests can be encapsulated with high efficiencies in the aqueous core of the capsule if they do not take part in the polyaddition reaction. After intravenous injection mannose nanocapsules are preferentially deposited in the lungs.148
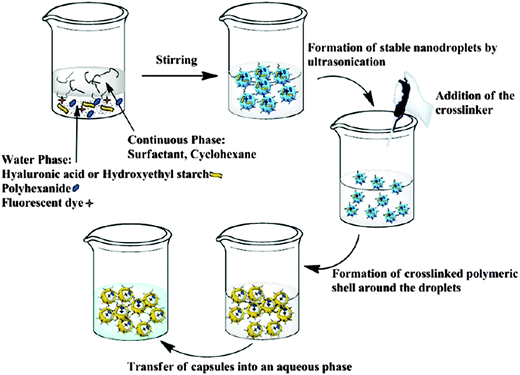 | ||
| Fig. 11 Schematic illustration of the nanocapsule formation through interfacial polyaddition in the inverse miniemulsion system with hyaluronic acid as the polyol component and toluene diisocyanate (TDI) served as the bifunctional electrophilic crosslinker. (Reprinted with permission from ref. 149. Copyright 2013 American Chemical Society.) | ||
Water-soluble potato starch has been used to prepare nanocapsules (Table 2, entry 7a) by the above mentioned inverse miniemulsion technique, while silver nanoparticles were generated in situ in the core to serve as an antibacterial agent.150
HES with a molecular weight of Mw = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a degree of substitution of 0.5 has also been used to prepare nanocapsules through the inverse miniemulsion technique. The obtained HES nanocapsules showed a suppressed uptake into HeLa cells85 and a preferential deposition in the liver (Table 2, entry 9).148
000 g mol−1 and a degree of substitution of 0.5 has also been used to prepare nanocapsules through the inverse miniemulsion technique. The obtained HES nanocapsules showed a suppressed uptake into HeLa cells85 and a preferential deposition in the liver (Table 2, entry 9).148
The in vivo plasma half-life times of the HES nanocapsules obtained by this strategy can be further tailored by different surface functionalization methods. PEGylation of the capsule surface by isocyanate-terminated PEG results in increased plasma half-life times with 20% and 5% of the nanocapsules remaining in the blood plasma after 24 h and 72 h, respectively.151
Despite the straightforward reaction setup, this strategy has limited feasibility, when used to encapsulate and protect pharmaceutical agents, which often contain nucleophiles like amines, thiols, or alcohols, and consequently will participate in the polycondensation reaction with the diisocyanate electrophile. Recent work presents strategies to use bioorthogonal reactions to generate the nanocarriers allowing the encapsulation of more complex molecules.
Two different strategies have been developed to meet this demand so far. In the first strategy, the copper-catalyzed azide-alkyne cycloaddition (CuAAC) is utilized in an oil-in-water miniemulsion: an aqueous solution of azide-functionalized sucrose and a miglyol solution of a dialkyne (bis-(propargyloxy)butane) as the oil phase were allowed to react at the interface of surfactant-stabilized hydrophobic droplets.152 Sucrose was functionalized with azide groups under Mitsunobu conditions (Table 2, entry 5). The obtained nanocapsules have a diameter below 200 nm, and a core filled with miglyol, as can be seen in Fig. 12, allowing loading of the nanocarriers with hydrophobic molecules. However, the removal of the copper catalyst may be problematic with this protocol and copper-free click chemistry could be used in future studies to prevent this, which has been demonstrated for non-carbohydrate systems in miniemulsion recently.153
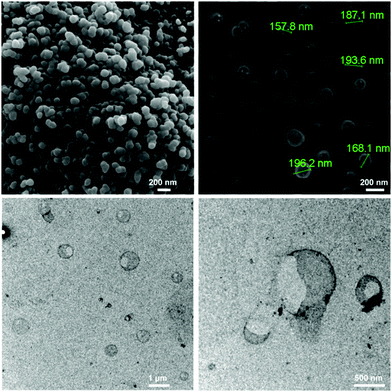 | ||
| Fig. 12 SEM (top) and TEM (bottom) images of the sucrose nanocapsules generated by interfacial CuAAC polyaddition. (Reprinted with permission from ref. 152. Copyright 2012 American Chemical Society.) | ||
In the second strategy, olefin cross metathesis was carried out in a water-in-oil miniemulsion by the reaction of acrylated dextran dissolved in water droplets and dispersed in a cyclohexane solution of phenyldi(undec-10-en-1-yl)-phosphate as the oil phase (Table 2, entry 6).154 The TEM and SEM images of the obtained capsules can be seen in Fig. 13. The ruthenium catalyst for the olefin metathesis can be easily removed by centrifugation as it is only soluble in the continuous (outer) phase. These nanocapsules offer the possibility to be degraded by enzymes that cleave dextran or the phosphate crosslinkers and in addition by pH changes due to ester cleavage.
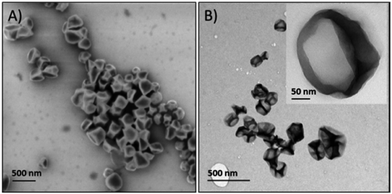 | ||
| Fig. 13 (A) Scanning electron microscopy image and (B) transmission electron microscopy image of the nanocapsules prepared in a miniemulsion process by olefin cross metathesis. (Reprinted with permission from ref. 154. Copyright 2014 American Chemical Society.) | ||
Another emulsion technique has been used to encapsulate hydrophilic guests (e.g. proteins) into inverse micelles of trehalose (Table 2, entry 4 and Fig. 14): dioctyl sodium sulfosuccinate (AOT) is dissolved in isooctane as the oil phase, while different proteins are dissolved together with trehalose in the water phase. After mixing of the two phases, stable water-in-oil micelle dispersions will be obtained, which can be subsequently freeze-dried by flash-freezing, and result in AOT-coated sugar-glass nanoparticles. The surfactant coating on the nanoparticle surface provides colloidal stability in organic solvent–polymer solutions. The trehalose, which transfers into the gel phase under dehydrating conditions, protects the cell internal organelles and hence protect the cells in desiccation and serves to protect the protein from chemical and physical degradation during storage.155
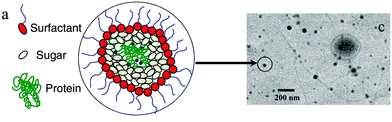 | ||
| Fig. 14 Schematic representation and TEM image of sugar–glass nanoparticles. (Reprinted with permission from ref. 155.) | ||
Multilayered polysaccharide vesicles are generated from starch as the core and hyaluronic acid (HA) as the shell (Table 2, entry 7b). The hydroxyl groups of starch were activated by succinic anhydride and then reacted with an excess of cysteamine by Steglich esterification (i.e. N,N-dimethylamino pyridine (DMAP), dicyclohexyl carbodiimide (DCC), and N-hydroxy succinimide (NHS) in dimethyl sulfoxide (DMSO)) to produce amino-functionalized starch with additional disulfide bonds. The amines were then reacted with the activated ester groups of HA. Rehydration of a thin film of this core–shell HA–starch conjugate in PBS will result in self-assembled nanoparticles with a starch core and a hyaluronic acid shell, which are subsequently treated by amylase, and result in vesicles with a hollow inner core in the end. Proteins/peptides can be encapsulated in these vesicles, when they are dissolved in the PBS buffer used. In addition, the enzymatic degradation of the HA shell by hyaluronidase (HYAL) enzyme contributed to accelerate the release of the payload.156 In another work, starch modified with ammonium groups is complexed with siRNA by electrostatic interaction to self-assemble into nanocarriers (Table 2, entry 7c), the starch can protect the siRNA from enzymatic degradation on its delivery route. It has high cellular uptake into a human ovarian adenocarcinoma cell line and efficiently induced P-glycoprotein (P-gp) gene silencing.157
Antibacterial nanodevices are interesting for coatings and wound dressings if the release of antibacterial agents can be triggered by the presence of bacteria. HA-nanocapsules (Table 2, entry 8a) containing the antimicrobial agent Polyhexanide were prepared by the interfacial polycondensation with TDI in an inverse miniemulsion. They can be specifically cleaved in the presence of the enzyme hyaluronidase, a factor of pathogenicity and invasion for bacteria like Staphylococcus aureus and Escherichia coli.149
Hyaluronan–cholanic acid conjugates (HA–CA conjugates) were synthesized by the chemical conjugation of the hydrophobic bile acid (a steroic acid) to the hydrophilic HA backbone through amide formation (Table 2, entry 8b, Fig. 15). The amphiphilic HA–CA can self-assemble into nanoparticles and can be loaded with doxorubicin and camptothecin, both of which are strongly cytotoxic compounds, and exhibited an efficient intracellular uptake into SCC7 cancer cells. The size of the nanoparticles was varied between 200 and 400 nm by varying the degree of substitution. Enzyme-triggered drug release was induced by the enzyme Hyal-1, as can be seen in Fig. 16.158–161
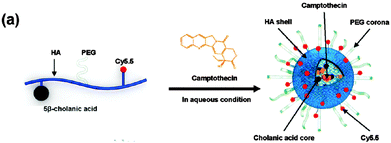 | ||
| Fig. 15 Schematic illustration of the formation of drug-loaded HA-NPs. (Reprinted with permission from ref. 158. Copyright 2011 American Chemical Society.) | ||
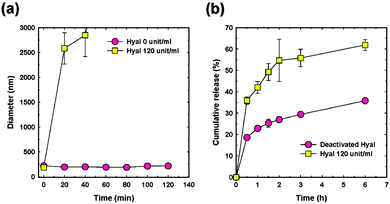 | ||
| Fig. 16 (a) Particle size changes of P-HA-NPs and (b) release patterns of CPT from P-HA-NPs in the presence and the absence of Hyal-1. Error bars represent the standard deviation (n = 5). (Reprinted with permission from ref. 158. Copyright 2011 American Chemical Society.) | ||
Esterification between hyaluronic acid and paclitaxel, a mitotic inhibitor used in cancer chemotherapy, was conducted via dicyclohexyl carbodiimide (DCC) coupling in anhydrous DMSO (Table 2, entry 8c). These conjugates then self-assemble in water to nanoparticles, which demonstrated higher cellular uptake than free paclitaxel against HepG2 cells, a human liver carcinoma cell line. The oral administration of these nanoparticles can result in remarkable accumulation of paclitaxel into the tumor (Fig. 17).133
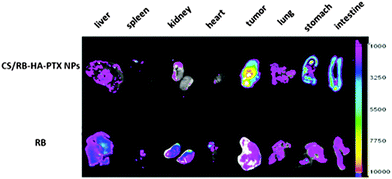 | ||
| Fig. 17 Ex vivo fluorescence intensity images of tumors and major organs after oral administration of rhodamine B labelled hyaluronic acid–paclitaxel nanoparticles (CS/RB-HA–PTX CNPs) and free rhodamine B (RB). (Taken from ref. 133 with permission.) | ||
Amphiphilic hexadecylated polysaccharides were synthesized (Table 2, entry 8d) and self-assembled in water into nanoparticles. Doxorubicin can be encapsulated in these nanoparticles, which showed specific binding with CD44 over-expresseing cancer cells. Higher therapeutic potential in the presence of a green tea polyphenol, epigallocatechin-3-gallate, was also observed for this nanoparticle system.162
Other polysaccharides like cellulose and chitin, which intrinsically are water insoluble, have also been used to construct nanocarriers. In some cases, their water solubility has been increased by chemical modifications, for example, carboxymethylation. Carboxymethyl cellulose has been conjugated with docetaxel and poly(ethylene glycol) through acetylation, self-assembled into nanoparticles, which was stable against dilution, and induced significantly higher toxic effects against EMT-6 murine mammary carcinoma cells and murine Pan02 tumors cells.163 Carboxymethyl chitin nanoparticles have also been prepared by crosslinking of the polysaccharide with CaCl2 and FeCl3.164 The obtained nanoparticle showed anti-bacterial activity by itself, and a sustained and controlled release of the payload. While different functions are enabled by cellulose and chitin, the intrinsic low water solubility and poor bio-degradability in animals rendered them to be used more as wound dressing, scaffolds for tissue engineering and medical implants, which is not the focus of this review and is comprehensively reviewed in detail elsewhere.165,166
5. Summary and outlook
This review summarizes current approaches on the use of (poly)saccharides in nanometer-sized drug delivery systems. Two major strategies have been discussed: (a) the surface-functionalization and (b) the construction of nanocarriers with/from carbohydrates.In summary, functionalization of nanocarriers with mono/oligosaccharides has proven to show the following advantageous properties:
(i) specific targeting to different cell types;43,45–47,127,130
(ii) diagnostics and differentiation of healthy and malignant population of the same cell type;18,127
(iii) protecting the payload130 and prolonged in vivo plasma half-life time (due to reduced protein adsorption);87
(iv) controlled release of a payload by specific protein interaction;44
(v) competing with and inhibiting the binding of other saccharide containing bacteria, virus, or pathogen with the corresponding cells.42
For nanocarriers that are functionalized with poly(saccharide)s are:
(i) protecting the payload133 and increasing the plasma half-life times;23,24,137
(ii) specific targeting;132,136,137,167
(iii) enzyme-induced release or activation of a therapeutic agent;23–27
(v) decreased toxicity of the payload;131,134,136,167
(vi) kinetics of metabolization are tunable by chemical functionalization to balance biocompatibility and stability.23,24
As carbohydrates are omnipresent as functional surface coatings in nature, their use in biomedical applications is obvious. They have been used to construct nanocarriers, which enable the encapsulation and protection155,157 of different (mainly) water-soluble guests,85,148–150,158–162 while maintaining the biological properties such as specific targeting,133,147,158–162,168 suppressed unspecific cell uptake,85 and enzyme-triggered release.149,156,158–161 In addition, the inherent high chemical functionality of different polysaccharides (mainly: hydroxyls, carboxylic acids, amines) allows straightforward crosslinking or on top functionalization of these molecules which are often major factors for their application compared to synthetic macromolecules. However, it always must be considered that the molecular weight distributions of these biopolymers are typically rather broad, making fractionation necessary in some cases. Nevertheless, due to the biodegradability of polysaccharides, their non-uniform molecular weight might not be too problematic for nanometer-sized drug delivery devices, however, the toxicity after any chemical modification and also that of the degradation products after chemical modification need to be considered.
A major feature of all nanocarriers, either modified or constructed of carbohydrates is their low protein interaction: the high level of hydrophilicity induces for many of them a “stealth” behavior, and the unspecific cell uptake due to opsonization is low. Furthermore, targeting is achieved by carbohydrates in multiple cases. This behavior plus the inherent biodegradability makes carbohydrate-based nanocarriers a high potential platform for developing the “magic bullet” that was coined by Paul Ehrlich more than 100 years ago and makes research in this direction promising for many scientists. It is certain that several new developments in treating diseases or enabling sophisticated diagnostics will rely on carbohydrates in the future.
Acknowledgements
F.R.W. thanks the Max Planck Graduate Center for support. Financial support of the Deutsche Forschungsgemeinschaft (DFG, SFB1066) is highly appreciated.References
- K. Strebhardt and A. Ullrich, Nat. Rev. Cancer, 2008, 8, 473–480 CrossRef CAS PubMed.
- Z. Liu, W. Cai, L. He, N. Nakayama, K. Chen, X. Sun, X. Chen and H. Dai, Nat. Nanotechnol., 2007, 2, 47–52 CrossRef CAS PubMed.
- N. Nasongkla, E. Bey, J. Ren, H. Ai, C. Khemtong, J. S. Guthi, S.-F. Chin, A. D. Sherry, D. A. Boothman and J. Gao, Nano Lett., 2006, 6, 2427–2430 CrossRef CAS PubMed.
- J. Cheng, B. A. Teply, I. Sherifi, J. Sung, G. Luther, F. X. Gu, E. Levy-Nissenbaum, A. F. Radovic-Moreno, R. Langer and O. C. Farokhzad, Biomaterials, 2007, 28, 869–876 CrossRef CAS PubMed.
- W. Wu, S. Wieckowski, G. Pastorin, M. Benincasa, C. Klumpp, J. P. Briand, R. Gennaro, M. Prato and A. Bianco, Angew. Chem., Int. Ed., 2005, 44, 6358–6362 CrossRef CAS PubMed.
- X. Qian, X. H. Peng, D. O. Ansari, Q. Yin-Goen, G. Z. Chen, D. M. Shin, L. Yang, A. N. Young, M. D. Wang and S. Nie, Nat. Biotechnol., 2008, 26, 83–90 CrossRef CAS PubMed.
- X. Gao, Y. Cui, R. M. Levenson, L. W. Chung and S. Nie, Nat. Biotechnol., 2004, 22, 969–976 CrossRef CAS PubMed.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889–896 CrossRef CAS PubMed.
- A. G. Tkachenko, H. Xie, D. Coleman, W. Glomm, J. Ryan, M. F. Anderson, S. Franzen and D. L. Feldheim, J. Am. Chem. Soc., 2003, 125, 4700–4701 CrossRef CAS PubMed.
- Q. Dai, C. Walkey and W. C. Chan, Angew. Chem., Int. Ed., 2014, 53, 5093–5096 CrossRef CAS PubMed.
- R. A. Laine, Glycosciences, Wiley-VCH Verlag GmbH, 2008, ch. 1, pp. 1–14, DOI:10.1002/9783527614738.
- A. Danguy, I. Camby and R. Kiss, Biochim. Biophys. Acta, 2002, 1572, 285–293 CrossRef CAS.
- J. H. Crowe, L. M. Crowe and D. Chapman, Science, 1984, 223, 701–703 CAS.
- P. D. Kwong, R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski and W. A. Hendrickson, Nature, 1998, 393, 648–659 CrossRef CAS PubMed.
- Y.-L. Xie, M.-J. Wang and S.-J. Yao, Langmuir, 2009, 25, 8999–9005 CrossRef CAS PubMed.
- N. Sharon and H. Lis, Sci. Am., 1993, 268, 82–89 CrossRef CAS PubMed.
- S. Q. Ye, C. Y. Wang, X. X. Liu and Z. Tong, J. Biomater. Sci., Polym. Ed., 2005, 16, 909–923 CrossRef CAS PubMed.
- K. El-Boubbou, D. C. Zhu, C. Vasileiou, B. Borhan, D. Prosperi, W. Li and X. Huang, J. Am. Chem. Soc., 2010, 132, 4490–4499 CrossRef CAS PubMed.
- K. Knop, R. Hoogenboom, D. Fischer and U. S. Schubert, Angew. Chem., Int. Ed., 2010, 49, 6288–6308 CrossRef CAS PubMed.
- A. Besheer, J. Vogel, D. Glanz, J. Kressler, T. Groth and K. Mader, Mol. Pharmaceutics, 2009, 6, 407–415 CrossRef CAS PubMed.
- C. Lemarchand, R. Gref and P. Couvreur, Eur. J. Pharm. Biopharm., 2004, 58, 327–341 CrossRef CAS PubMed.
- M. Orlando, PhD thesis, University of Giessen, 2003.
- M. Noga, D. Edinger, R. Klaeger, S. V. Wegner, J. P. Spatz, E. Wagner, G. Winter and A. Besheer, Biomaterials, 2013, 34, 2530–2538 CrossRef CAS PubMed.
- M. Noga, D. Edinger, W. Rodl, E. Wagner, G. Winter and A. Besheer, J. Controlled Release, 2012, 159, 92–103 CrossRef CAS PubMed.
- E. L. Ferguson and R. Duncan, Biomacromolecules, 2009, 10, 1358–1364 CrossRef CAS PubMed.
- R. Duncan, H. R. P. Gilbert, R. J. Carbajo and M. J. Vicent, Biomacromolecules, 2008, 9, 1146–1154 CrossRef CAS PubMed.
- J. Hardwicke, E. L. Ferguson, R. Moseley, P. Stephens, D. W. Thomas and R. Duncan, J. Controlled Release, 2008, 130, 275–283 CrossRef CAS PubMed.
- R. E. Marchant, S. Yuan and G. Szakalas-Gratzl, J. Biomater. Sci., Polym. Ed., 1994, 6, 549–564 CrossRef CAS PubMed.
- E. Osterberg, K. Bergstrom, K. Holmberg, T. P. Schuman, J. A. Riggs, N. L. Burns, J. M. Van Alstine and J. M. Harris, J. Biomed. Mater. Res., 1995, 29, 741–747 CrossRef CAS PubMed.
- J. Liu, W. D. Gray, M. E. Davis and Y. Luo, Interface Focus, 2012, 2, 307–324 CrossRef PubMed.
- Z. Liu, Y. Jiao, Y. Wang, C. Zhou and Z. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1650–1662 CrossRef CAS PubMed.
- J. K. Oh and Y. Wen, Macromol. Rapid Commun., 2014, 35, 1815–1818 CrossRef PubMed.
- N. Kottari, Y. M. Chabre, R. Sharma and R. Roy, Adv. Polym. Sci., 2013, 254, 297–342 CrossRef CAS.
- M. Marradi, F. Chiodo, I. Garcia and S. Penades, Chem. Soc. Rev., 2013, 42, 4728–4745 RSC.
- N. C. Reichardt, M. Martin-Lomas and S. Penades, Chem. Soc. Rev., 2013, 42, 4358–4376 RSC.
- X. Chen, O. Ramstrom and M. Yan, Nano Res., 2014, 7, 1381–1403 CrossRef CAS.
- C. F. Brewer, M. C. Miceli and L. G. Baum, Curr. Opin. Struct. Biol., 2002, 12, 616–623 CrossRef CAS.
- H. J. Gabius, Biochem. Soc. Trans., 2008, 36, 1491–1496 CrossRef CAS PubMed.
- H. J. Gabius, S. Andre, H. Kaltner and H. C. Siebert, Biochim. Biophys. Acta, 2002, 1572, 165–177 CrossRef CAS.
- N. Yamazaki, S. Kojima, N. V. Bovin, S. Andre, S. Gabius and H. J. Gabius, Adv. Drug Delivery Rev., 2000, 43, 225–244 CrossRef CAS.
- T. B. H. Geijtenbeek, R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, G. J. Adema, Y. van Kooyk and C. G. Figdor, Cell, 2000, 100, 575–585 CrossRef CAS.
- O. Martinez-Avila, K. Hijazi, M. Marradi, C. Clavel, C. Campion, C. Kelly and S. Penades, Chem. – Eur. J., 2009, 15, 9874–9888 CrossRef CAS PubMed.
- A. V. Chavez-Santoscoy, R. Roychoudhury, N. L. B. Pohl, M. J. Wannemuehler, B. Narasimhan and A. E. Ramer-Tait, Biomaterials, 2012, 33, 4762–4772 CrossRef CAS PubMed.
- S. Wu, X. Huang and X. Du, Angew. Chem., Int. Ed., 2013, 52, 5580–5584 CrossRef CAS PubMed.
- G. Yu, Y. Ma, C. Han, Y. Yao, G. Tang, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2013, 135, 10310–10313 CrossRef CAS PubMed.
- W. Chen, Y. Zou, F. Meng, R. Cheng, C. Deng, J. Feijen and Z. Zhong, Biomacromolecules, 2014, 15, 900–907 CrossRef CAS PubMed.
- L. Lartigue, K. Oumzil, Y. Guari, J. Larionova, C. Guerin, J. L. Montero, V. Barragan-Montero, C. Sangregorio, A. Caneschi, C. Innocenti, T. Kalaivani, P. Arosio and A. Lascialfari, Org. Lett., 2009, 11, 2992–2995 CrossRef CAS PubMed.
- A. K. Zimmer, P. Chetoni, M. F. Saettone, H. Zerbe and J. Kreuter, J. Controlled Release, 1995, 33, 31–46 CrossRef CAS.
- A. M. Durrani, S. J. Farr and I. W. Kellaway, Int. J. Pharm., 1995, 118, 243–250 CrossRef CAS.
- C. M. Lehr, J. A. Bouwstra, E. H. Schacht and H. E. Junginger, Int. J. Pharm., 1992, 78, 43–48 CrossRef CAS.
- D. Letourneur, C. Parisel, S. Prigent-Richard and M. Cansell, J. Controlled Release, 2000, 65, 83–91 CrossRef CAS.
- M. Cansell, C. Parisel, J. Jozefonvicz and D. Letourneur, J. Biomed. Mater. Res., 1999, 44, 140–148 CrossRef CAS.
- M. Demetriou, M. Granovsky, S. Quaggin and J. W. Dennis, Nature, 2001, 409, 733–739 CrossRef CAS PubMed.
- J. C. Sacchettini, L. G. Baum and C. F. Brewer, Biochemistry, 2001, 40, 3009–3015 CrossRef CAS PubMed.
- J. B. Lowe, Cell, 2001, 104, 809–812 CrossRef CAS.
- G. A. Rabinovich, L. G. Baum, N. Tinari, R. Paganelli, C. Natoli, F. T. Liu and S. Iacobelli, Trends Immunol., 2002, 23, 313–320 CrossRef CAS.
- S. Hakomori, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 10231–10233 CrossRef CAS PubMed.
- S. J. Danishefsky and J. R. Allen, Angew. Chem., Int. Ed., 2000, 39, 836–863 CrossRef CAS.
- H. Lis and N. Sharon, Chem. Rev., 1998, 98, 637–674 CrossRef CAS PubMed.
- F. T. Liu and G. A. Rabinovich, Nat. Rev. Cancer, 2005, 5, 29–41 CrossRef CAS PubMed.
- S. H. Barondes, V. Castronovo, D. N. Cooper, R. D. Cummings, K. Drickamer, T. Feizi, M. A. Gitt, J. Hirabayashi, C. Hughes and K. Kasai, et al. , Cell, 1994, 76, 597–598 CrossRef CAS.
- R. Lotan, H. Ito, W. Yasui, H. Yokozaki, D. Lotan and E. Tahara, Int. J. Cancer, 1994, 56, 474–480 CrossRef CAS PubMed.
- D. K. Hsu, C. A. Dowling, K. C. G. Jeng, J. T. Chen, R. Y. Yang and F. T. Liu, Int. J. Cancer, 1999, 81, 519–526 CrossRef CAS.
- T. Irimura, Y. Matsushita, R. C. Sutton, D. Carralero, D. W. Ohannesian, K. R. Cleary, D. M. Ota, G. L. Nicolson and R. Lotan, Cancer Res., 1991, 51, 387–393 CAS.
- H. L. Schoeppner, A. Raz, S. B. Ho and R. S. Bresalier, Cancer, 1995, 75, 2818–2826 CrossRef CAS.
- X. Sanjuan, P. L. Fernandez, A. Castells, V. Castronovo, F. van den Brule, F. T. Liu, A. Cardesa and E. Campo, Gastroenterology, 1997, 113, 1906–1915 CrossRef CAS.
- X. C. Xu, A. K. el-Naggar and R. Lotan, Am. J. Pathol., 1995, 147, 815–822 CAS.
- P. L. Fernandez, M. J. Merino, M. Gomez, E. Campo, T. Medina, V. Castronovo, X. Sanjuan, A. Cardesa, F. T. Liu and M. E. Sobel, J. Pathol., 1997, 181, 80–86 CrossRef CAS.
- F. A. VandenBrule, C. Buicu, A. Berchuck, R. C. Bast, M. Deprez, F. T. Liu, D. N. W. Cooper, C. Pieters, M. E. Sobel and V. Castronovo, Hum. Pathol., 1996, 27, 1185–1191 CrossRef CAS.
- E. Auzenne, S. C. Ghosh, M. Khodadadian, B. Rivera, D. Farquhar, R. E. Price, M. Ravoori, V. Kundra, R. S. Freedman and J. Klostergaard, Neoplasia, 2007, 9, 479–486 CrossRef CAS.
- D. Peer and R. Margalit, Neoplasia, 2004, 6, 343–353 CrossRef CAS PubMed.
- M. Zoller, J. Mol. Med., 1995, 73, 425–438 CrossRef CAS.
- F. Smadja-Joffe, S. Legras, N. Girard, Y. Li, B. Delpech, F. Bloget, K. Morimoto, C. Le Bousse-Kerdiles, D. Clay, C. Jasmin and J. P. Levesque, Leuk. Lymphoma, 1996, 21, 407–420 CAS , color plates following 528.
- S. Jin, Y. Cheng, S. Reid, M. Li and B. Wang, Med. Res. Rev., 2010, 30, 171–257 CAS.
- A. Salvati, A. S. Pitek, M. P. Monopoli, K. Prapainop, F. B. Bombelli, D. R. Hristov, P. M. Kelly, C. Aberg, E. Mahon and K. A. Dawson, Nat. Nanotechnol., 2013, 8, 137–143 CrossRef CAS PubMed.
- B. P. Pablo del Pino, Q. Zhang, P. Maffre, G. Ulrich Nienhaus and W. J. Parak, Mater. Horiz., 2014, 1, 301–313 RSC.
- S. N. S. Alconcel, A. S. Baas and H. D. Maynard, Polym. Chem., 2011, 2, 1442–1448 RSC.
- A. Bendele, J. Seely, C. Richey, G. Sennello and G. Shopp, Toxicol. Sci., 1998, 42, 152–157 CrossRef CAS PubMed.
- C. D. Conover, C. W. Gilbert, K. L. Shum and R. G. Shorr, Artif. Organs, 1997, 21, 907–915 CrossRef CAS PubMed.
- J. Treib, J. F. Baron, M. T. Grauer and R. G. Strauss, Intensive Care Med., 1999, 25, 258–268 CrossRef CAS.
- E. M. Pelegri-O'Day, E. W. Lin and H. D. Maynard, J. Am. Chem. Soc., 2014, 136, 14323–14332 CrossRef PubMed.
- A. Besheer, G. Hause, J. Kressler and K. Maeder, Biomacromolecules, 2007, 8, 359–367 CrossRef CAS PubMed.
- M. C. Woodle and D. D. Lasic, Biochim. Biophys. Acta, 1992, 1113, 171–199 CrossRef CAS.
- T. M. Allen, Adv. Drug Delivery Rev., 1994, 13, 285–309 CrossRef CAS.
- G. Baier, D. Baumann, J. M. Siebert, A. Musyanovych, V. Mailaender and K. Landfester, Biomacromolecules, 2012, 13, 2704–2715 CrossRef CAS PubMed.
- J. M. Jancik, R. Schauer, K. H. Andres and M. Vonduring, Cell Tissue Res., 1978, 186, 209–226 CrossRef.
- T. Ohyanagi, N. Nagahori, K. Shimawaki, H. Hinou, T. Yamashita, A. Sasaki, T. Jin, T. Iwanaga, M. Kinjo and S. Nishimura, J. Am. Chem. Soc., 2011, 133, 12507–12517 CrossRef CAS PubMed.
- J. G. Pierce and T. F. Parsons, Annu. Rev. Biochem., 1981, 50, 465–495 CrossRef CAS PubMed.
- J. Kruse, R. Mailhammer, H. Wernecke, A. Faissner, I. Sommer, C. Goridis and M. Schachner, Nature, 1984, 311, 153–155 CrossRef CAS PubMed.
- Y. Yeh and R. E. Feeney, Chem. Rev., 1996, 96, 601–618 CrossRef CAS PubMed.
- J. C. Paulson, Trends Biochem. Sci., 1989, 14, 272–276 CrossRef CAS.
- S. Ebbinghaus, K. Meister, B. Born, A. L. DeVries, M. Gruebele and M. Havenith, J. Am. Chem. Soc., 2010, 132, 12210–12211 CrossRef CAS PubMed.
- A. G. Morell, G. Gregoriadis, I. H. Scheinberg, J. Hickman and G. Ashwell, J. Biol. Chem., 1971, 246, 1461–1467 CAS.
- K. Hase, K. Kawano, T. Nochi, G. S. Pontes, S. Fukuda, M. Ebisawa, K. Kadokura, T. Tobe, Y. Fujimura, S. Kawano, A. Yabashi, S. Waguri, G. Nakato, S. Kimura, T. Murakami, M. Iimura, K. Hamura, S.-I. Fukuoka, A. W. Lowe, K. Itoh, H. Kiyono and H. Ohno, Nature, 2009, 462, 226-U101 CrossRef PubMed.
- S. F. Lichtenthaler, D. I. Dominguez, G. G. Westmeyer, K. Reiss, C. Haass, P. Saftig, B. De Strooper and B. Seed, J. Biol. Chem., 2003, 278, 48713–48719 CrossRef CAS PubMed.
- W. S. Nesbitt, S. Kulkarni, S. Giuliano, I. Goncalves, S. M. Dopheide, C. L. Yap, I. S. Harper, H. H. Salem and S. P. Jackson, J. Biol. Chem., 2002, 277, 2965–2972 CrossRef CAS PubMed.
- F. M. Veronese and G. Pasut, Drug Discovery Today, 2005, 10, 1451–1458 CrossRef CAS.
- A. Mero, M. Pasqualin, M. Campisi, D. Renier and G. Pasut, Carbohydr. Polym., 2013, 92, 2163–2170 CrossRef CAS PubMed.
- R. Liebner, R. Mathaes, M. Meyer, T. Hey, G. Winter and A. Besheer, Eur. J. Pharm. Biopharm., 2014, 87, 378–385 CrossRef CAS PubMed.
- E. L. Ferguson, A. M. Alshame and D. W. Thomas, Int. J. Pharm., 2010, 402, 95–102 CrossRef CAS PubMed.
- J. A. Davis and H. H. Freeze, Biochim. Biophys. Acta, 2001, 1528, 116–126 CrossRef CAS.
- W. I. Weis, K. Drickamer and W. A. Hendrickson, Nature, 1992, 360, 127–134 CrossRef CAS PubMed.
- J. H. Ahire, I. Chambrier, A. Mueller, Y. Bao and Y. Chao, ACS Appl. Mater. Interfaces, 2013, 5, 7384–7391 CAS.
- H. Kouyoumdjian, D. C. Zhu, M. H. El-Dakdouki, K. Lorenz, J. Chen, W. Li and X. Huang, ACS Chem. Neurosci., 2013, 4, 575–584 CrossRef CAS PubMed.
- J. Frigell, I. Garcia, V. Gomez-Vallejo, J. Llop and S. Penades, J. Am. Chem. Soc., 2014, 136, 449–457 CrossRef CAS PubMed.
- T. Cedervall, I. Lynch, S. Lindman, T. Berggard, E. Thulin, H. Nilsson, K. A. Dawson and S. Linse, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 2050–2055 CrossRef CAS PubMed.
- P. Aggarwal, J. B. Hall, C. B. McLeland, M. A. Dobrovolskaia and S. E. McNeil, Adv. Drug Delivery Rev., 2009, 61, 428–437 CrossRef CAS PubMed.
- D. Walczyk, F. B. Bombelli, M. P. Monopoli, I. Lynch and K. A. Dawson, J. Am. Chem. Soc., 2010, 132, 5761–5768 CrossRef CAS PubMed.
- C. Rocker, M. Potzl, F. Zhang, W. J. Parak and G. U. Nienhaus, Nat. Nanotechnol., 2009, 4, 577–580 CrossRef PubMed.
- Z. J. Deng, M. Liang, M. Monteiro, I. Toth and R. F. Minchin, Nat. Nanotechnol., 2011, 6, 39–44 CrossRef CAS PubMed.
- C. D. Walkey and W. C. Chan, Chem. Soc. Rev., 2012, 41, 2780–2799 RSC.
- M. P. Monopoli, D. Walczyk, A. Campbell, G. Elia, I. Lynch, F. B. Bombelli and K. A. Dawson, J. Am. Chem. Soc., 2011, 133, 2525–2534 CrossRef CAS PubMed.
- A. Albanese, C. D. Walkey, J. B. Olsen, H. Guo, A. Emili and W. C. Chan, ACS Nano, 2014, 8, 5515–5526 CrossRef CAS PubMed.
- B. Kang, P. Okwieka, S. Schottler, S. Winzen, J. Langhanki, K. Mohr, T. Opatz, V. Mailander, K. Landfester and F. R. Wurm, Angew. Chem., Int. Ed., 2015, 54, 7436–7440 CrossRef CAS PubMed.
- W. M. Watkins, Science, 1966, 152, 172–181 CAS.
- C. K. Adokoh, S. Quan, M. Hitt, J. Darkwa, P. Kumar and R. Narain, Biomacromolecules, 2014, 15, 3802–3810 CrossRef CAS PubMed.
- T. Angata and A. Varki, Chem. Rev., 2002, 102, 439–469 CrossRef CAS PubMed.
- R. Schauer, Trends Biochem. Sci., 1985, 10, 357–360 CrossRef CAS.
- S. Kelm, J. Gerlach, R. Brossmer, C. P. Danzer and L. Nitschke, J. Exp. Med., 2002, 195, 1207–1213 CrossRef CAS.
- L. Jin, P. A. McLean, B. G. Neel and H. H. Wortis, J. Exp. Med., 2002, 195, 1199–1205 CrossRef CAS.
- T. D. Farr, C. H. Lai, D. Grunstein, G. Orts-Gil, C. C. Wang, P. Boehm-Sturm, P. H. Seeberger and C. Harms, Nano Lett., 2014, 14, 2130–2134 CrossRef CAS PubMed.
- K. M. K. Selim, Y.-S. Ha, S.-J. Kim, Y. Chang, T.-J. Kim, G. H. Lee and I.-K. Kang, Biomaterials, 2007, 28, 710–716 CrossRef PubMed.
- M. F. Giraud and J. H. Naismith, Curr. Opin. Struct. Biol., 2000, 10, 687–696 CrossRef CAS.
- J. A. Levi, D. Raghavan, V. Harvey, D. Thompson, T. Sandeman, G. Gill, R. Stuart-Harris, R. Snyder, M. Byrne and Z. Kerestes, et al. , J. Clin. Oncol., 1993, 11, 1300–1305 CAS.
- R. deWit, G. Stoter, S. B. Kaye, D. T. Sleijfer, W. G. Jones, W. W. T. Huinink, L. A. Rea, L. Collette and R. Sylvester, J. Clin. Oncol., 1997, 15, 1837–1843 CAS.
- D. A. Goodwin, C. F. Meares, L. H. DeRiemer, C. I. Diamanti, R. L. Goode, J. E. Baumert Jr., D. J. Sartoris, R. L. Lantieri and H. D. Fawcett, J. Nucl. Med., 1981, 22, 787–792 CAS.
- Z. Yu, R. M. Schmaltz, T. C. Bozeman, R. Paul, M. J. Rishel, K. S. Tsosie and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 2883–2886 CrossRef CAS PubMed.
- J. K. Willmann, R. Paulmurugan, K. Chen, O. Gheysens, M. Rodriguez-Porcel, A. M. Lutz, I. Y. Chen, X. Chen and S. S. Gambhir, Radiology, 2008, 246, 508–518 CrossRef PubMed.
- C. Bhattacharya, Z. Yu, M. J. Rishel and S. M. Hecht, Biochemistry, 2014, 53, 3264–3266 CrossRef CAS PubMed.
- A. Sizovs, L. Xue, Z. P. Tolstyka, N. P. Ingle, Y. Wu, M. Cortez and T. M. Reineke, J. Am. Chem. Soc., 2013, 135, 15417–15424 CrossRef CAS PubMed.
- M. Valodkar, P. S. Rathore, R. N. Jadeja, M. Thounaojam, R. V. Devkar and S. Thakore, J. Hazard. Mater., 2012, 201–202, 244–249 CrossRef CAS PubMed.
- S. Asthana, P. K. Gupta, R. Konwar and M. K. Chourasia, J. Nanopart. Res., 2013, 15 Search PubMed.
- J. Li, P. S. Huang, L. L. Chang, X. W. Long, A. J. Dong, J. J. Liu, L. P. Chu, F. Q. Hu, J. F. Liu and L. D. Deng, Macromol. Res., 2013, 21, 1331–1337 CrossRef CAS.
- S. C. Boca, M. Potara, A. M. Gabudean, A. Juhem, P. L. Baldeck and S. Astilean, Cancer Lett., 2011, 311, 131–140 CrossRef CAS PubMed.
- I. C. Sun, J. H. Na, S. Y. Jeong, D. E. Kim, I. C. Kwon, K. Choi, C. H. Ahn and K. Kim, Pharm. Res., 2014, 31, 1418–1425 CrossRef CAS PubMed.
- C. J. Needham, A. K. Williams, S. A. Chew, F. K. Kasper and A. G. Mikos, Biomacromolecules, 2012, 13, 1429–1437 CrossRef CAS PubMed.
- L. Liu, H. He, M. Zhang, S. Zhang, W. Zhang and J. Liu, Biomaterials, 2014, 35, 8002–8014 CrossRef CAS PubMed.
- Z. Wang, Z. Chen, Z. Liu, P. Shi, K. Dong, E. Ju, J. Ren and X. Qu, Biomaterials, 2014, 35, 9678–9688 CrossRef CAS PubMed.
- D. M. Stark, K. P. Timmerman, G. F. Barry, J. Preiss and G. M. Kishore, Science, 1992, 258, 287–292 CAS.
- T. Hey, H. Knoller and P. Vorstheim, Therapeutic Proteins, Wiley-VCH Verlag GmbH & Co. KGaA, 2012, ch. 7, pp. 117–140 DOI:10.1002/9783527644827.
- M. N. Kumar, R. A. Muzzarelli, C. Muzzarelli, H. Sashiwa and A. J. Domb, Chem. Rev., 2004, 104, 6017–6084 CrossRef PubMed.
- S. Al-Assaf, M. Sakata, C. McKenna, H. Aoki and G. O. Phillips, Struct. Chem., 2009, 20, 325–336 CrossRef CAS.
- A. M. Dias, A. Hussain, A. S. Marcos and A. C. Roque, Biotechnol. Adv., 2011, 29, 142–155 CrossRef CAS PubMed.
- P. R. Devi, C. S. Kumar, P. Selvamani, N. Subramanian and K. Ruckmani, Mater. Lett., 2015, 139, 241–244 CrossRef PubMed.
- A. M. Gamal-Eldeen, D. Moustafa, S. M. El-Daly and K. V. Katti, Eur. J. Cancer, 2014, 50, e46 CrossRef PubMed.
- J. R. Fraser, T. C. Laurent and U. B. Laurent, J. Intern. Med., 1997, 242, 27–33 CrossRef CAS.
- V. Percec, P. Leowanawat, H. J. Sun, O. Kulikov, C. D. Nusbaum, T. M. Tran, A. Bertin, D. A. Wilson, M. Peterca, S. Zhang, N. P. Kamat, K. Vargo, D. Moock, E. D. Johnston, D. A. Hammer, D. J. Pochan, Y. Chen, Y. M. Chabre, T. C. Shiao, M. Bergeron-Brlek, S. Andre, R. Roy, H. J. Gabius and P. A. Heiney, J. Am. Chem. Soc., 2013, 135, 9055–9077 CrossRef CAS PubMed.
- P.-N. Anette, M. Fichter, M. Dedters, L. Pretsch, S. H. Gregory, C. Meyer, A. Doganci, M. Diken, K. Landfester, G. Baier and S. Gehring, Biomacromolecules, 2014, 15, 2378–2388 CrossRef PubMed.
- G. Baier, A. Cavallaro, K. Vasilev, V. Mailaender, A. Musyanovych and K. Landfester, Biomacromolecules, 2013, 14, 1103–1112 CrossRef CAS PubMed.
- S. Taheri, G. Baier, P. Majewski, M. Barton, R. Forch, K. Landfester and K. Vasilev, J. Mater. Chem. B, 2014, 2, 1838–1845 RSC.
- B. Kang, P. Okwieka, S. Schottler, O. Seifert, R. E. Kontermann, K. Pfizenmaier, A. Musyanovych, R. Meyer, M. Diken, U. Sahin, V. Mailander, F. R. Wurm and K. Landfester, Biomaterials, 2015, 49, 125–134 CrossRef CAS PubMed.
- R. Roux, L. Sallet, P. Alcouffe, S. Chambert, N. Sintes-Zydowicz, E. Fleury and J. Bernard, ACS Macro Lett., 2012, 1, 1074–1078 CrossRef CAS.
- E. M. Alexandrino, P. Buchold, M. Wagner, A. Fuchs, A. Kreyes, C. K. Weiss, K. Landfester and F. R. Wurm, Chem. Commun., 2014, 50, 10495–10498 RSC.
- K. Malzahn, F. Marsico, K. Koynov, K. Landfester, C. K. Weiss and F. R. Wurm, ACS Macro Lett., 2014, 3, 40–43 CrossRef CAS.
- J. Giri, W. J. Li, R. S. Tuan and M. T. Cicerone, Adv. Mater., 2011, 23, 4861–4867 CrossRef CAS PubMed.
- D. S. Kwag, K. T. Oh and E. S. Lee, J. Controlled Release, 2014, 187, 83–90 CrossRef CAS PubMed.
- E. Amar-Lewis, A. Azagury, R. Chintakunta, R. Goldbart, T. Traitel, J. Prestwood, D. Landesman-Milo, D. Peer and J. Kost, J. Controlled Release, 2014, 185, 109–120 CrossRef CAS PubMed.
- K. Y. Choi, H. Y. Yoon, J.-H. Kim, S. M. Bae, R.-W. Park, Y. M. Kang, I.-S. Kim, I. C. Kwon, K. Choi, S. Y. Jeong, K. Kim and J. H. Park, ACS Nano, 2011, 5, 8591–8599 CrossRef CAS PubMed.
- K. Y. Choi, H. Chung, K. H. Min, H. Y. Yoon, K. Kim, J. H. Park, I. C. Kwon and S. Y. Jeong, Biomaterials, 2010, 31, 106–114 CrossRef CAS PubMed.
- K. Y. Choi, K. H. Min, J. H. Na, K. Choi, K. Kim, J. H. Park, I. C. Kwon and S. Y. Jeong, J. Mater. Chem., 2009, 19, 4102–4107 RSC.
- K. Y. Choi, K. H. Min, H. Y. Yoon, K. Kim, J. H. Park, I. C. Kwon, K. Choi and S. Y. Jeong, Biomaterials, 2011, 32, 1880–1889 CrossRef CAS PubMed.
- L. Ray, P. Kumar and K. C. Gupta, Biomaterials, 2013, 34, 3064–3076 CrossRef CAS PubMed.
- M. J. Ernsting, W.-L. Tang, N. MacCallum and S.-D. Li, Bioconjugate Chem., 2011, 22, 2474–2486 CrossRef CAS PubMed.
- A. Dev, J. C. Mohan, V. Sreeja, H. Tamura, G. R. Patzke, F. Hussain, S. Weyeneth, S. V. Nair and R. Jayakumar, Carbohydr. Polym., 2010, 79, 1073–1079 CrossRef CAS PubMed.
- M. Jorfi and E. J. Foster, J. Appl. Polym. Sci., 2015, 132 DOI:10.1002/app.41719.
- R. Jayakumar, D. Menon, K. Manzoor, S. V. Nair and H. Tamura, Carbohydr. Polym., 2010, 82, 227–232 CrossRef CAS PubMed.
- X. Zhang, Q. Zhang, Q. Peng, J. Zhou, L. Liao, X. Sun, L. Zhang and T. Gong, Biomaterials, 2014, 35, 6130–6141 CrossRef CAS PubMed.
- M. Fichter, G. Baier, M. Dedters, L. Pretsch, A. Pietrzak-Nguyen, K. Landfester and S. Gehring, Nanomedicine, 2013, 9, 1223–1234 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |