Diketopyrrolopyrrole: brilliant red pigment dye-based fluorescent probes and their applications
Matinder
Kaur
and
Dong Hoon
Choi
*
Department of Chemistry, Research Institute for Natural Sciences, Korea University, 5 Anam-dong, Sungbuk-Gu, 136-701 Seoul, Korea. E-mail: dhchoi8803@korea.ac.kr; Fax: +82-2-925-4284; Tel: +82-2-3290-3140
First published on 4th September 2014
Abstract
The development of fluorescent probes for the detection of biologically relevant species is a burgeoning topic in the field of supramolecular chemistry. A number of available dyes such as rhodamine, coumarin, fluorescein, and cyanine have been employed in the design and synthesis of new fluorescent probes. However, diketopyrrolopyrrole (DPP) and its derivatives have a distinguished role in supramolecular chemistry for the design of fluorescent dyes. DPP dyes offer distinctive advantages relative to other organic dyes, including high fluorescence quantum yields and good light and thermal stability. Significant advancements have been made in the development of new fluorescent probes based on DPP in recent years as a result of tireless research efforts by the chemistry scientific community. In this tutorial review, we highlight the recent progress in the development of DPP-based fluorescent probes for the period spanning 2009 to the present time and the applications of these probes to recognition of biologically relevant species including anions, cations, reactive oxygen species, thiols, gases and other miscellaneous applications. This review is targeted toward providing the readers with deeper understanding for the future design of DPP-based fluorogenic probes for chemical and biological applications.
Key learning points(1) Importance of diketopyrrolopyrrole (DPP) in supramolecular chemistry(2) Biological roles of anions, cations, reactive oxygen species, and thiols (3) Design strategies for DPP-based fluorescent probes (4) Recognition mechanisms of DPP-based fluorescent probes (5) Diverse applications of DPP-based small molecules and polymers |
1. Introduction
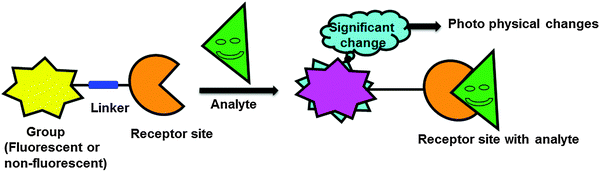
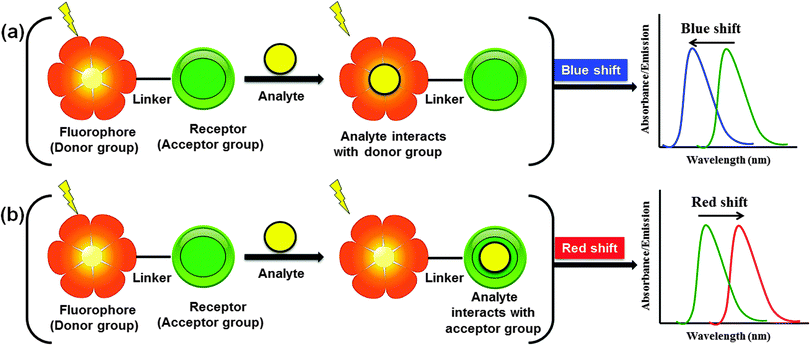
Biologically important species such as anions, cations, reactive oxygen species (ROS), and thiols are essential for human health and environment.1 An appropriate amount of these species is crucial for the effective operation of biological systems as well as environment and for maintaining the mandatory balance between them. However, these species are highly hazardous once their concentration exceeds a maximum limit, and therefore detection, quantitative determination, and removal of these species from environmental and biological systems are the main objectives of many studies. One of the most facile ways to recognize these species is to use fluorescent probes that are capable of detecting these species in solution, in the solid state, and in living cells with high sensitivity. Fluorogenic probes basically comprise an ion recognition unit (e.g., ionophore) attached to a signaling unit (e.g., fluorophore) that converts molecular recognition into highly discriminative and easily detected/measured optical signals that are associated with changes in the photophysical properties of the probes (Fig. 1). These changes are associated with various signaling mechanisms such as fluorescence enhancement/quenching and ratiometric changes due to interaction of the probes with particular analytes (e.g., Lewis acid–base interaction, deprotonation, photoinduced processes such as charge transfer, energy transfer, intermolecular excited state proton transfer).The binding of a specific analyte to a fluoroionophore (i.e., fluorophore linked with an ionophore) can cause either enhancement (turn-on) or quenching (turn-off) of the fluorescence intensity, coupled with a red or blue shift of the emission band or absorption band. Upon excitation, intramolecular charge transfer from a donor to an acceptor unit occurs within the fluoroionophore. However, complexation of a metal ion with the donor or acceptor group results in significantly different photophysical responses. When the metal ion binds with the donor group of the receptor, there is a consequent reduction of the conjugation length that decreases the electron-donating ability of the donor group, which results in a blue-shift in the absorption spectrum (Fig. 2a). On the other hand, when the metal ion interacts with the acceptor unit, there is an enhanced conjugative effect that increases the electron-withdrawing nature of the acceptor group and hence, generally induces a red-shift of the absorption or emission spectrum (Fig. 2b).
2. Diketopyrrolopyrrole
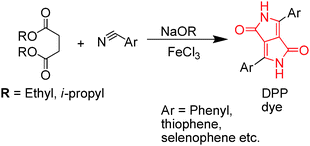
Diketopyrrolopyrrole (DPP) is synthesized by the reaction of aromatic nitrile with dialkyl succinate (Scheme 1).2 DPP has attracted considerable research interest over the last few years. DPP has a planar structure and can induce strong intermolecular H-bonding and π–π stacking with neighboring molecules. The molecular frame of DPP has many reactive centers such as (i) the aryl rings that undergo electrophilic and nucleophilic reactions and (ii) the bicyclic lactam chromophoric unit with three different functional groups: (1) –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– double bonds, (2) carbonyl, and (3) secondary amine (NH) groups that may potentially undergo structural modification for further derivatization (Scheme 1). The significant advantages of DPP derivatives are as follows: (a) they can undergo several synthetic modifications, (b) act as strong acceptor units, (c) exhibit high fluorescence quantum yields, and (d) possess exceptional thermal and photostability, making them excellent building blocks for many applications. Owing to the above features, DPP and its derivatives have been widely used as high-performance pigments (e.g., paints, plastics, and inks)3 and exhibit outstanding semiconducting properties that have been primarily utilized in field-effect transistors and photovoltaic cells. These DPP-based materials and their various applications have been reviewed recently.4–7
C– double bonds, (2) carbonyl, and (3) secondary amine (NH) groups that may potentially undergo structural modification for further derivatization (Scheme 1). The significant advantages of DPP derivatives are as follows: (a) they can undergo several synthetic modifications, (b) act as strong acceptor units, (c) exhibit high fluorescence quantum yields, and (d) possess exceptional thermal and photostability, making them excellent building blocks for many applications. Owing to the above features, DPP and its derivatives have been widely used as high-performance pigments (e.g., paints, plastics, and inks)3 and exhibit outstanding semiconducting properties that have been primarily utilized in field-effect transistors and photovoltaic cells. These DPP-based materials and their various applications have been reviewed recently.4–7
Over the past decade, the design and synthesis of novel fluorescent probes using various fluorogenic units such as coumarin, rhodamine, fluorescein, cyanine, boron-dipyrromethene (BODIPY), and DPP for the recognition of various biologically important species have been extensively reported.8 Rhodamine and fluorescein dyes exhibit high fluorescence quantum yields and good photostability (Table 1). However, their biological applications are limited because their absorption and emission maxima appear below 600 nm. Similarly, although cyanine dyes are fluorophores of biological interest, the poor photostability of these dyes has hampered their applications. BODIPY derivatives have attracted much interest because of their excellent absorption and emission spectra and high molar absorption coefficient (Table 1). On the other hand, DPP has earned distinction in the chemistry community because of its wide application as a fluorescent probe over other organic dyes because of excellent photostability and good fluorescence quantum yields. To date, DPP-based high-performance materials have been well developed,4–7 while the ability of DPP to recognize biologically important species still remains relatively underdeveloped. However, because of the high fluorescence quantum yields, exceptional photostability, and moderately high molar absorption coefficients of DPP-containing fluorescent probes in both solution and membrane-bound forms, considerable effort has recently been dedicated to the development of such probes and investigation of their applications in supramolecular chemistry. Despite many emerging applications, a comprehensive review systematically addressing DPP-based fluorescent probes has not yet been presented. In this tutorial review, we summarize the development of various DPP-based fluorescent probes and their applications for tracing biologically and environmentally important species. The DPP-based fluorescent probes are broadly categorized as (a) the probes for various analytes such as anions, cations, reactive oxygen species (ROS), thiols, pH, CO2, and H2; (b) the probes for molecular imaging, (c) structurally modified DPP-based near-infrared (NIR) dyes, and (d) DPP-based polymers as fluorescent probes. Specific focus is placed on the research progress and scholarly contributions made in recent years. Overall, this review provides an outline of the design strategy for each class of DPP-based fluorescent probes and their respective applications in the format of a tutorial review.
| Organic dyes | Absorption range (nm) | Fluorescence emission range (nm) | Photo stability | Molar extinction coefficient (M−1 cm−1) | Fluorescence quantum yield |
|---|---|---|---|---|---|
| DPP | 400–550 | 500–650 | Very high | ∼25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.4–0.9 |
| Coumarin | 320–520 | 450–590 | Good | ∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
∼0.8 |
| Rhodamine | 470–528 | 510–610 | High | ∼116![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
∼0.95 |
| Fluorescein | 400–550 | 460–700 | High | ∼80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
∼0.92 |
| Cyanine | 400–800 | 500–800 | Moderate | ∼100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.12–0.67 |
| BODIPY | 500–645 | 506–760 | Very high | ∼80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
∼0.92 |
3. DPP-based fluorescent probes for various analytes
3.1 Probes for anions
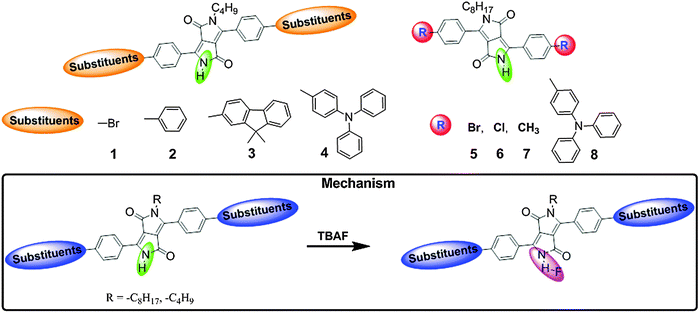
Anions play significant roles in environmental, medicinal, chemical, and biological applications. Among the various important anions (e.g., fluoride, chloride, cyanide, and phosphate), the fluoride (F−) and cyanide (CN−) ions are of considerable interest because of their well-known roles in biological systems. Despite the significant benefits of F−, excessively high or low concentrations have been associated with gastric and kidney disorder, dental and skeletal fluorosis, urolithiasis in humans, and even death. Similarly, a small amount of CN− can cause death within a few minutes because of its strong preference for the active site of cytochrome c oxidase and its ability to inhibit cellular respiration. Owing to the serious toxicity of F− and CN− ions, development of fluorogenic probes for detection of these ions has been widely pursued. Thus, in this section, we focus on the recent developments of DPP-based fluorescent probes for F− and CN− ion detection.Hua et al. utilized the DPP moiety to synthesize fluorescent probes bearing bromo 1, phenyl 2, and fluorene 3 moieties for the colorimetric and ratiometric detection of F− (Fig. 3).9 The hydrogen atoms on the nitrogen of the amide units of these probes 1–3 were used as a receptor for F− sensing due to the strong H–F interactions. The absorption maxima of probes 1–3 were observed at 477 nm, 485 nm, and 497 nm, respectively. The absorbance decreased, accompanied by large bathochromic shifts to 571 nm, 585 nm, and 594 nm, respectively, upon addition of tetrabutylammonium fluoride (TBAF), leading to significant color changes. Furthermore, the emission spectra of probes 1–3 underwent a F− induced red-shift from 532 to 613 nm for probe 1, from 545 to 630 nm for probe 2, and from 563 to 635 nm for probe 3.
The colorimetric and ratiometric changes were mainly ascribed to the intermolecular proton transfer (IPT) between the hydrogen atom of the amide unit and F−. Probe 3 can detect F− at concentrations in the range of 0–10 μM in the visible part of the electromagnetic spectrum. Furthermore, to improve the detection limit of F− and enhance the intramolecular charge transfer (ICT) from the amide unit of the DPP moiety to the conjugated donor system, the new fluorescent probe 4 was synthesized by introducing two electron-donating substituents (triphenylamine) at the DPP unit (Fig. 3).10 Recognition of F− by probe 4 was studied in acetone and acetonitrile solvents, giving rise to similar absorption changes but different fluorescence behavior. Probe 4 showed ratiometric behavior in absorption spectra upon titration with TBAF in acetone and acetonitrile with a decrease in absorbance at ∼520 nm and formation of a new red-shifted band at ∼594 nm. In acetone, probe 4 exhibited fluorogenic ratiometric behavior upon addition of F− with a decrease in emission intensity at 590 nm and formation of a new emission band at 641 nm (Fig. 4a and b). However, in acetonitrile, probe 4 exhibited a very weak fluorescence band at ca. 530 nm due to aggregation-induced fluorescence quenching. With addition of F− ions, the fluorescence intensity was enhanced by ca. 40-fold at 631 nm, which is attributed to deprotonation of the amide unit by F−, resulting in charge transfer (CT) from the deprotonated DPP unit to the triphenylamine donor units (Fig. 4c and d). The F− detection limit of probe 4 was 4.20 μM in acetone and 2.46 μM in acetonitrile. Furthermore, the ratiometric absorption behavior and turn-on fluorescence emission of probe 4 with F− in acetonitrile has been employed to mimic a parallel double-INH logic gate in the presence of HSO4−. Probe 4 demonstrated two-input–two-output INH gate (parallel double-INH logic gate) with F− (In1) and HSO4− (In2) as two chemical inputs and ratiometric absorption and turn-on fluorescence emission as two outputs (Fig. 4e–g).
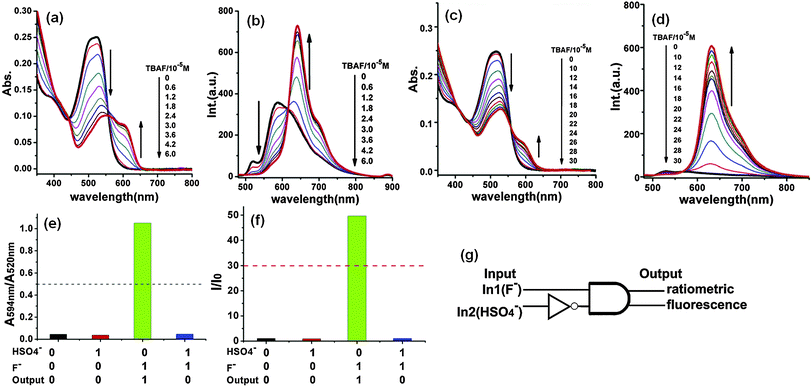 | ||
| Fig. 4 (a) UV-Vis absorption and (b) fluorescence spectral changes of probe 4 upon the addition of F− in acetone; (c) UV-Vis absorption and (d) fluorescence spectral changes of probe 4 upon the addition of F− in acetonitrile. (e) Ratiometric responses of absorption spectra (A594nm/A520nm) of 4 (10 μM) in the absence and presence of HSO4− and/or F− in acetonitrile; (f) turn on responses of emission spectra (λem = 631 nm) of 4 (10 μM) in the absence and presence of HSO4− and/or F− in acetonitrile; and (g) schematic representation of a parallel double INH logic gate. Reproduced from ref. 10 with permission from Elsevier. | ||
A similar research was conducted by Yang et al. in the synthesis of DPP-based one-photon and two-photon probes 5–7 for detection of F− ions (Fig. 3).11 These probes exhibited ratiometric absorption and fluorescence behavior with F− ions due to the IPT between the amide moiety and F−. In the presence of F−, ICT from the amide to the conjugated region is increased, resulting in a bathochromic shift of the spectra. Probes 5–7 exhibited practical two-photon absorption (TPA) cross-sections (δ = 58–87 GM) that depend on the substituents in the benzene rings of DPP.
In order to improve the TPA cross-section, they further synthesized the donor–π–acceptor–π–donor type DPP-based compound 8 and evaluated the F− sensing properties using one- and two-photon absorption and emission spectroscopy (Fig. 3).12 The red-shift of the absorption (from 538 to 608 nm) and emission bands (from 594 to 645 nm) of probe 8 in the presence of F− was attributed to deprotonation of the NH group in DPP by the F− ion. On the other hand, the TPA cross-section (δ) of probe 8 increased (up to 1030 GM) because of the incorporation of the triphenylamine groups.
These results revealed that structural modification of DPP with different donor units can change the sensitivity of probes towards detection of F−, and the modified-DPP probes can be used for designing efficient optical probes for different analytes.
Based on fluoride–boron interactions, Wang et al. synthesized a DPP-based colorimetric and ratiometric probe 9, bearing a pinacol boronate group via the Suzuki–Miyaura reaction for the efficient detection of F− ions (Fig. 5).13 A peak was observed at 535 nm in the emission spectrum of probe 9, and this peak showed a five-fold enhancement of the emission intensity along with a blue-shift from 535 nm to 527 nm after the addition of F− ions, accompanied by a color change from yellow green to green. The binding behavior of probe 9 was proved by 19F and 1H NMR spectroscopy. The combined results of these experiments showed the existence of two species 9a and 9b; finally, 9b was found to be more stable than 9a. The interaction of probe 9 with F− ions was based on the strong interactions of F− ions with the sp2-hybridized boron center (Fig. 5). Probe 9 exhibits CT from the fluorophore to the empty p-orbital of the boron atom; however, after the addition of F− ions, the CT was inhibited because of the occupied p-orbital at the boron atom.
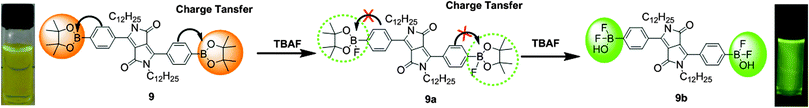 | ||
| Fig. 5 Structure of fluorescent probe 9 and mechanism of F− ion detection. Reproduced from ref. 13 with permission from Elsevier. | ||
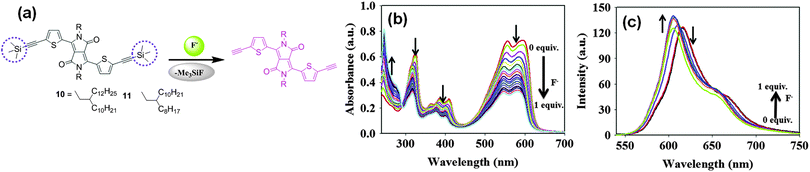
Choi et al. utilized the chemodosimetric approach for recognizing F− using DPP-based probes 10 and 11 with different alkyl chains bearing two trimethylsilyl groups (Fig. 6a).14 The design strategy exploited the high selectivity of F− towards Si. The respective absorption and emission maxima of probe 10 at 597 nm and at 617 nm were blue-shifted to 589 and 604 nm upon addition of F− (Fig. 6b and c). Probe 10 could be used for F− detection not only in solution but also in the solid state (e.g., test strips, glass films). The detection limit of probe 10 for F− analysis was found to be 2 × 10−7 M.
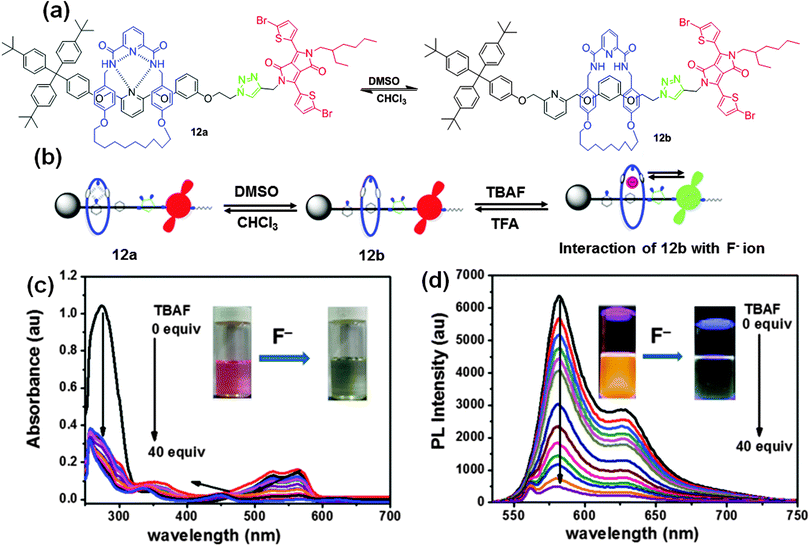 | ||
| Fig. 7 (a) Chemical structure of fluorescent probe 12, (b) representation of two translational isomers, 12a and 12b, in CDCl3 and DMSO and interaction with F− ions. (c) UV-visible absorption, and (d) fluorescence spectral changes of probe 12b upon the addition of F− ions in DMSO. Reproduced from ref. 15 with permission from American Chemical Society. | ||
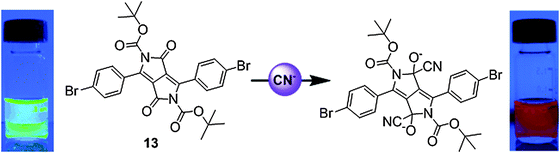 | ||
| Fig. 8 Structure of fluorescent probe 13 and the mechanism of CN− detection. Reproduced from ref. 16 with permission from John Wiley and Sons. | ||
Thus, recent efforts in the development of DPP-based anion probes have provided new ideas for modification of the DPP core in the design and synthesis of new colorimetric and fluorescent anion probes. The hydrogen atoms on the nitrogen of the amide units of DPP were utilized for the sensing of F− and the electron-deficient lactam ring of DPP was used for selective detection of CN− ions. Further introduction of electron-donating substituents onto the DPP moiety improves the ICT effect for better sensitivity towards F−. Therefore, the present reports should prove useful for further progress and modification of the sensing properties of DPP-based fluorescent probes.
3.2 Probes for metal ions
All living organisms require metal ions for their growth and development given the significant role of metal ions in biological processes. Metal ions such as sodium, potassium, magnesium, zinc, copper, manganese, iron, in particular, are vital for various physiological functions such as transmission of nerve impulses, osmoregulation, metabolism, biomineralization, and signaling. Optimal concentrations of these ions are essential for proper growth and development, and any change in their concentrations can be dangerous and may lead to various disorders in the body. Therefore, the development of optical probes for specific detection of metal ions is a very important objective for protecting living systems and the environment from the toxic effects of metals.Wang et al. synthesized fluorescence turn-on DPP-based probe 1417 (Fig. 9a) bearing the N,N-di(pyridin-2-ylmethyl)amine (DPA) group, and probe 1518 bearing the N,N-di(pyridin-2-ylmethyl)ethane-1,2-diamine group (Fig. 10a) for Zn2+. Probe 14 exhibited very weak red fluorescence at λem = 630 nm; however, after complexation with Zn2+, a spectral blue-shift (Δλ = 70 nm) from 630 nm to 560 nm was observed with an enhancement of the yellow fluorescence by 25-fold. Similar fluorescence behavior was observed for probe 15 upon interaction with Zn2+. The large blue-shift was attributed to photo-induced electron-transfer (PET) and ICT from the nitrogen atom to the DPP core through the conjugation pathway. On the other hand, probe 16 containing the acylated linker (Fig. 10b) exhibited no fluorescence enhancement with Zn2+ under similar conditions because of the decrease in the electron-donating capability of the conjugated N atom, which short-circuited the PET process. Probe 14 was the first DPP-based fluorescent probe used for the successful detection of Zn2+ in HeLa cells using Leica TCSSP2 confocal fluorescence microscopy using both green and red channels (Fig. 9b).
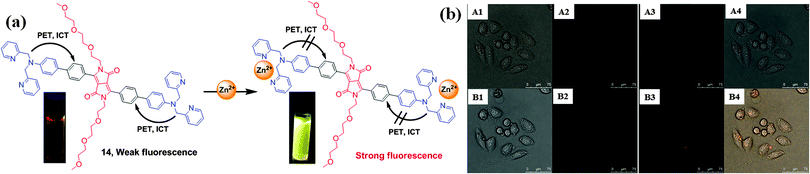 | ||
| Fig. 9 (a) Structure of fluorescent probe 14 and the mechanism of binding with Zn2+ and (b) confocal fluorescence images of Zn2+ in HeLa cells: (top (A1–A4)) incubated with a 10 μM solution of 14 for 30 min at 25 °C; (bottom (B1–B4)) incubated with a 10 μM of Zn2+ for 15 min. Bright-field transmission images (A1 and B1) and double channel fluorescence images (A2 and B2: channels centered: 570 ± 15 nm; A3 and B3: channels centered: 650 ± 15 nm), and overlap field (A4 and B4). Scale bar of HeLa cells 0 to 75 μM. Reproduced from ref. 17 with permission from Royal Society of Chemistry. | ||
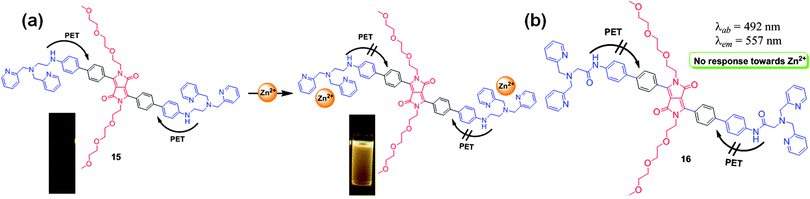 | ||
| Fig. 10 (a) Structure of probe 15 and mechanism of binding with Zn2+ and (b) structure of derivative 16. Reproduced from ref. 18 with permission from Elsevier. | ||
Choi et al. reported the DPP-based probe 17 with bis-alkyne groups as a fluorescent chemodosimeter for the recognition of Hg2+ based on the Kucherov reaction (Fig. 11a).20 Probe 17 exhibited absorption bands at 316 nm, 377 nm, 399 nm, 542 nm, and 582 nm, and addition of Hg2+ resulted in a decrease in the intensity of the absorptions at 542 nm and 582 nm, with a small increase at 341 nm and 422 nm. The band at 582 nm underwent a bathochromic shift to 623 nm after addition of Hg2+, which was due to the conversion of the alkyne groups to carbonyl groups (Fig. 11b). On the other hand, addition of Cu2+ also resulted in a decline of the absorption intensity at 582 nm with an increase at 274 nm, 440 nm, and 690 nm (Fig. 11b). The maximum emission of probe 17 at 603 nm was quenched by addition of Hg2+ and Cu2+ (Fig. 11c).
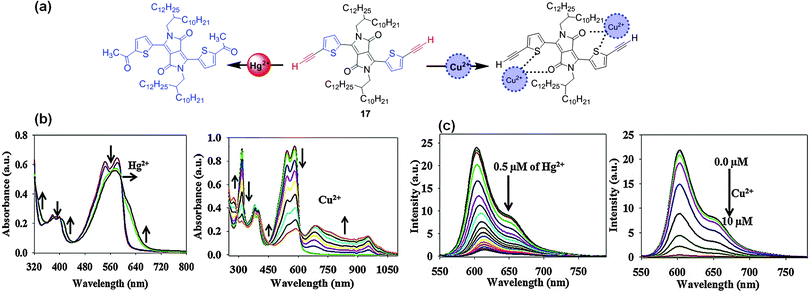 | ||
| Fig. 11 (a) Structure of fluorescent probe 17 and its interactions with Hg2+ and Cu2+; (b) UV-Vis absorption spectral changes; (c) fluorescence spectral changes of probe 17 with Hg2+ and Cu2+. | ||
Thus, DPP-based fluorescent probes have emerged as promising candidates not only for sensing anions, but also for the selective and sensitive detection of metal ions. Structural modification of the DPP core with specific metal binding units such as DPA, DEPA, and terminal alkynes generates DPP derivatives that lend themselves to fast, selective, and sensitive metal ion determination.
3.3 Probes for reactive oxygen species and thiols
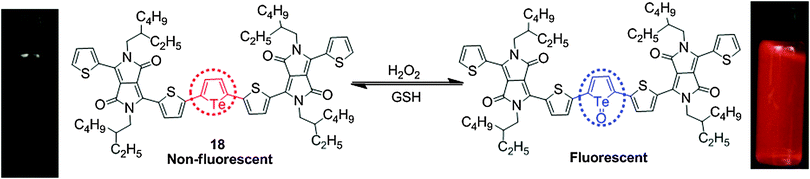
Choi et al. reported the DPP–tellurophene conjugate 18 based on the redox properties of the tellurium atom for the turn-on selective and sensitive fluorescence detection of H2O2 over other ROS (Fig. 12).21 Probe 18 exhibited an absorption band at 610 nm that completely disappeared upon addition of H2O2 accompanied by the appearance of a new small band at 982 nm. The fluorescence peak of probe 18 at ca. 565 nm was enhanced by titration with H2O2 with a spectral red-shift from 565 nm to 579 nm. Moreover, the oxidized form of 18, tellurophene-1-oxide, was reduced to regenerate the free probe 18 in the presence of glutathione (GSH). Thus, probe 18 works as a reversible H2O2 probe that is responsive to the oxidative environment and could be reduced in the presence of GSH. The detection limits were determined to be 6.0 μM for H2O2 and 8 μM for GSH.
Thus, combining the favorable features of DPP with tellurophene generates innovative optical probes that can detect H2O2 with high selectivity and sensitivity. This approach should be useful for the further development of fluorescent probes for detecting ROS species.
Zhao et al. utilized the Michael addition mechanism in constructing the DPP-based fluorescent probe 19 with a malonitrile moiety (Fig. 13a).22 Probe 19 exhibited a blue-shift of the absorption bands and enhancement of the fluorescence emission after addition of cysteine (Cys). This is attributed to the Michael addition reaction of Cys to the ethylenic double bonds of the probe, resulting in reduced π-conjugation. The recognition mechanism was deduced by means of mass analysis, which demonstrated that along with Michael addition, the –CN group of the malonitrile moieties reacted with thiols to form the 4,5-dihydrothiazole structure. It was found that probe 19 could be utilized as a ratiometric probe for bio-imaging of intracellular thiols (Fig. 13b).
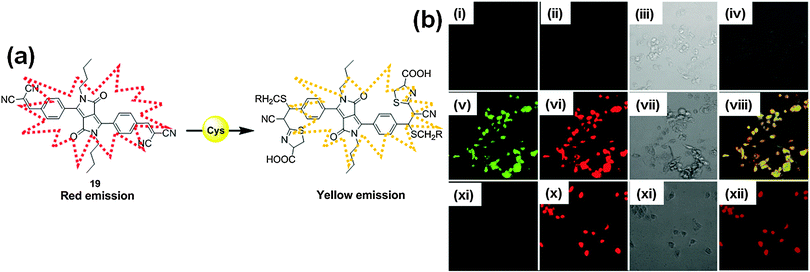 | ||
| Fig. 13 (a) Structure of probe 19 and proposed mechanism of Cys detection; (b) differential interference contrast and fluorescence images of living MDA-231 cells with probe 19; 1st row (i–iv): without probe 19, 2nd row (v–viii): MDA-231 cells incubated with 10 μM of probe 19, and 3rd row (xi–xii) MDA-231 cells pretreated with 1 mM N-methylmaleimide and then incubated with 10 μM probe 19. Reproduced from ref. 22 with permission from American Chemical Society. | ||
Thus, this research indicates that DPP-based fluorescent probes are not strictly limited to the detection of anions and cations, but are also functional for the determination of intracellular thiols, opening a new avenue for the design of DPP-based fluorescent probes for the recognition of intracellular thiols.
3.4 Probes for pH, carbon dioxide and H2 gas
Determination of pH is an extremely important issue because pH plays a governing role in various physiological processes. The intracellular or extracellular pH sensitively affects many cellular processes such as cell growth, various enzyme activities, and transport of ionic species through biological membranes.23 Alterations in the intracellular pH caused by metabolic processes such as metabolic acid production and membrane transport processes may lead to cellular dysfunctions. Therefore, determination of extracellular/intracellular pH is very important for monitoring the physiological and pathological status of organisms. Among the various available methods for pH measurement, the use of fluorescent probes is one of the most effective methods for pH determination in solutions as well as in living cells. The importance of pH for both the environment and living systems is the driving force for the design of new and efficient fluorescent probes for determination of the intracellular or extracellular pH. | ||
| Fig. 14 (a) Structure of fluorescent probe 20 and (b) photographs of a thin layer silica gel sheet showing the changes in the fluorescence of probe 20 in the presence of formic acid and TFA vapor under UV light (λ = 365 nm). Reproduced from ref. 24 with permission from Elsevier. | ||
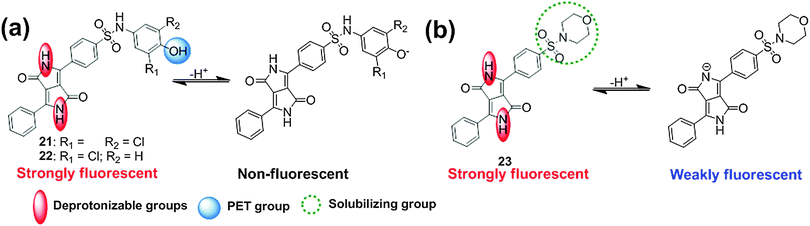
Borisov et al. demonstrated the design and synthesis of the new pH indicators 21–23 (Fig. 15)25 based on deprotonation of NH groups in the amide unit of DPP. These indicators could operate by means of two different mechanisms as shown in Fig. 15. Deprotonation of the phenolic groups in the indicators 21 and 22 resulted in the PET effect, which was associated with the fluorescence quenching in the pH range of 5.9–9.3 with no alteration of the absorption spectra. At higher pH (9.7–11.6), these indicators exhibit bathochromic shifts in the absorption spectra. On the other hand, the bathochromic shift in the absorption and emission spectra of the indicator 23 with a morpholino group was ascribed to the deprotonation of the NH groups in the amide unit of the DPP. By utilizing appropriate immobilizing polymer matrices, Hydromed® D4 (a commercially available polyurethane-based hydrogel), poly(2-hydroxyethylmethacrylate) in the form of planar sensors, and Eudragit® RL100 (a positively charged acrylate polymer) in the form of sensor nanobeads, these indicators worked as efficient pH probes (e.g., planar or sensor beads) over a wide pH range from 5–12. The use of sensor beads was more effective than the use of planar sensors because of the larger surface area of the beads and higher flexibility which can reveal instantaneous response whereas the planar probe was intact. The probe beads in Eudragit® RL100 polymer can be practically applied in microfluidic systems and also in fluorescence imaging and microscopy.
Mizuguchi et al. reported a H2 gas sensor using DPP derivative 27 containing pyridyl units (Fig. 16).27 The high proton affinity of the N atoms of the pyridyl units significantly affected the shade and electrical conductivities of compound 27. The H2 gas sensing behavior was evaluated in the vapor and solution phases; the vapor phase showed a high tendency for protonation, thus leading to substantial changes in H2 gas sensing. Furthermore, the effect of a high electric field on the dissociation of H2 into protons in the presence of Pd or Pt was evaluated.28 It was observed that H2 dissociated exponentially with the electric field, which was further enhanced by the presence of a DPP moiety with a high affinity for protons.
Furthermore, Kratochvılova et al. reported the hydrogenation of derivative 28, modulating its electronic properties such as ionization potential, surface potential, and conductivity (Fig. 16).29 Upon the interaction of the pyridyl units with protons, the conductivity of derivative 28 was significantly increased; however, the surface potential decreased, indicating its potential use as a H2 sensor.
Thus, it is crucial to develop optical probes for environmental and biological pH detection. By utilizing the strong absorbance and fluorescence properties of the DPP core, efficient pH probes have been developed. These pH probes provided new approaches for the development of CO2 sensors. Further investigations revealed the usefulness of DPP derivatives containing a pyridyl unit as H2 sensors.
4. DPP-based fluorescent probes for molecular imaging
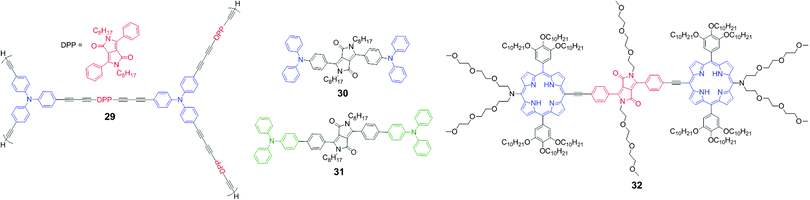
Two-photon excitation fluorescence (TPEF) microscopy emerged as the most promising technique for acquiring images of deep tissue (>500 μm) and is a non-invasive tool for living cell and tissue imaging. Recently, a number of reports have explored the two-photon excitation and two-photon absorption properties of DPP-based conjugated molecules. The conjugation of the DPP unit, having strong acceptor properties with different donor units, was exploited in the development of efficient two-photon probes. The DPP-based two-photon probes showed significant TPA cross-section values (∼500–3000 GM), making them prospectively useful for deep tissue imaging for disease detection.Tian et al. synthesized the DPP-based hyperbranched polyyne 29 with triphenylamine groups connected to the DPP unit and investigated its two-photon absorption properties (Fig. 17).30 Upon excitation of 140 femtosecond laser pulses (λ = 800 nm), polymer 29 emitted strong frequency up-converted fluorescence at 584 nm. Using the solution in tetrahydrofuran, the TPA cross-section of polymer 29 was measured to be 579 GM per repeating unit.
Yang et al. developed two donor–π–acceptor–π–donor type DPP derivatives 30 and 31 with electron-donating diphenylamine and triphenylamine groups with TPA properties (Fig. 17).31 These two derivatives had large TPA cross-sections (δmax = 900–1200 GM) and emitted strong red two-photon excitation fluorescence. These derivatives exhibited large bathochromic shifts because of enhancement of the ICT. Derivative 31 exhibited weaker ICT than derivative 30, which indicates that weak electron donating benzene is not an effective conjugation bridge. Consequently, derivative 30 exhibited red emission at 604 nm but with low quantum yield (ϕ = 0.37) as compared to derivative 31, which exhibited emission at 594 nm with a slightly higher quantum yield (ϕ = 0.46). These derivatives served as prototypes for the design of new DPP-based candidates as two-photon fluorescent probes.
Gryko et al. developed the new probe molecule 32 bearing a DPP bridge with two porphyrin units, which was synthesized by coupling the porphyrin moiety to the DPP-alkyne partner (Fig. 17).32 Probe 32 displayed maximum fluorescence emission at λmax = 710 nm and possesses large intrinsic TPA in the near-IR wavelength range, with maximum TPA cross-section values of 2500–3000 GM at 940 nm.
Bolze et al. reported the synthesis of DPP-based non-ionic water-soluble TPEF microscopy dyes 33–39 (Fig. 18).33 The one-photon photophysical properties of all of the synthesized dyes in dichloromethane (CH2Cl2) and water gave rise to an absorption in the 470 nm region and intense green fluorescence at around 530 nm. In the case of dye 37, the quantum yield decreased because of the remaining NH group, and the quantum yield of probe 37 dropped to 2% in water. The TPA cross-sections of probes 35 and 36 were very similar with a moderate effect of the DPP core substituent (150 GM at 830 nm). On the other hand, probes 38 and 39 with π-extended conjugated systems had significantly increased TPA cross-sections (950 GM at 835 nm for probe 38). These probes are highly photostable and were explored for their application in confocal and TPEF microscopic evaluation of HeLa cell cultures (Fig. 18).
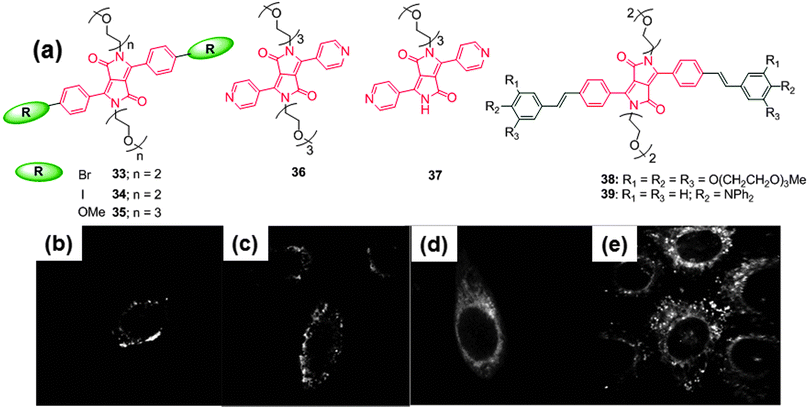 | ||
| Fig. 18 (a) Structures of water soluble non-ionic DPP-based probes 33–39, two-photon excitation microscopy (TPEM) image of HeLa cells stained with (10−6 M with 1% DMSO) (b) 35, (c) 36, (d) 38, and (e) 39. Reproduced from ref. 33 with permission from Elsevier. | ||
The structures of these fluorophores were further modified for bio-conjugation with the amino group of biomolecules. The same group reported the synthesis of DPP-based fluorescent probes, such as monomers 40 and 41, and dimers 42 and 43 (Fig. 19).34 The authors utilized two approaches for the bio-conjugation of these probes to the HIV Trans-Activator of Transcription (Tat) protein. These bio-conjugates were used as two-photon fluorescent probes for imaging HeLa cells using TPEF microscopy. In the first approach, the Tat (44-61) peptide was attached to the reactive end of the fluorophore 41 and in the second approach the Tat (44-61) peptide was attached to the central DPP core 43 (Fig. 19). The Tat peptides 41-βAla–Tat and 43-βAla–Tat tagged with the fluorophores were employed in TPEF microscopic analysis of HeLa cell cultures (λex = 800 nm, laser power <5 mW) (Fig. 19c and d). These fluorescently tagged Tat peptides are prospectively useful in microscopy for living cells with low dose excitation (e.g. <1 mW for 43) and also in multicolor labeling of biomolecules utilizing optimized two-photon bio-conjugated fluorophores using two-photon excited microscopy (TPEM).
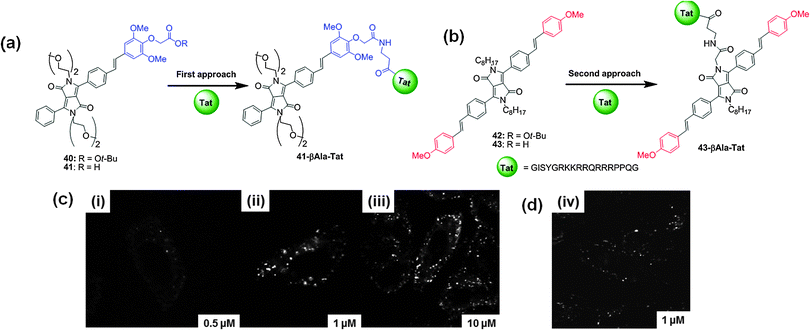 | ||
| Fig. 19 (a) and (b) Structures of DPP-based two photon fluorescent probes 40–43 and the structures of Tat (44-51) bio-conjugates 41-βAla–Tat and 43-βAla–Tat; (c) TPEM images of HeLa cells incubated in solutions of 41-βAla–Tat (i) 0.5 μM, (ii) 1 μM, (iii) 10 μM, and (d) TPEM image of HeLa cells incubated in solutions of 43-βAla–Tat (iv) 1 μM. Reproduced from ref. 34 with permission from American Chemical Society. | ||
Thus, the development of two-photon probes is very attractive in light of their advantages such as deeper tissue penetration, and reduced photobleaching and photodamage during tissue imaging. These reports are a source of valuable insights for the construction of DPP-based two-photon probes with versatile applications.
5. Structurally modified DPP-based near-infrared (NIR) dyes
Considerable efforts have been devoted to develop new near-infrared (NIR) dyes for applications in chemical biology35 and materials science. Dyes with strong NIR absorption and emission offer important advantages over the traditional dyes operating in the visible region. The NIR absorption of such dyes has been primarily utilized in materials science; however, NIR fluorescence attracted considerable interest in cell biology because of the following reasons: (1) deep tissue penetration for cell recognition, (2) minimal autofluorescent background from biological cells, (3) in vivo imaging, and (4) minimum photodamage to tissues. Various NIR dyes such as cyanines, squaraines, and BODIPYs have been developed; however, their applications are limited because of poor photostability or low fluorescence quantum yields. Therefore, the development of new NIR dyes with high fluorescence quantum yields and good photostability provides avenues for various applications.Thus, the structural modifications of the DPP unit have been carried out for the development of highly stable NIR dyes. The DPP core can be functionalized in many ways to provide suitable derivatives with desired properties. The carbonyl O atoms of DPP can be replaced with different arylacetonitriles, S atoms, and aromatic amines.36 The reaction of carbonyl O atoms with arylacetonitriles is interesting as it leads to π-expanded bathochromically shifted dyes exhibiting absorption in the NIR wavelength region.
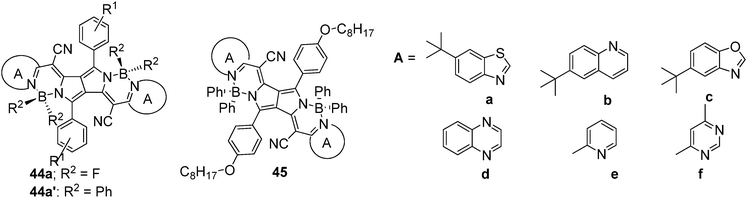
Daltrozzo et al. modified the DPP core to synthesize cyanine-type NIR absorbing dyes, pyrrolopyrrole cyanine (PPCy), containing a pyrrolopyrrole moiety as the central component (Fig. 20).37 The reaction of DPPs with heteroarylacetonitriles followed by the substitution of protons with BF2 or BPh2 group rigidifies the chromophore to afford the corresponding NIR dyes. These dyes exhibited fluorescence quantum yields of 0.59 (λem = 773 nm) and 0.53 (λem = 831 nm) for 44a and 44a′, respectively.
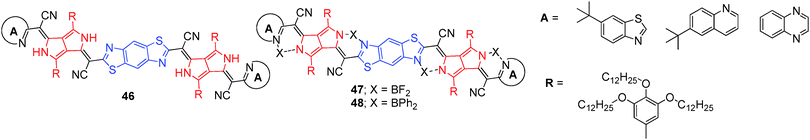
Using a similar strategy, a series of PPCys 45a–f (Fig. 20)38 and bis(pyrrolopyrrole) cyanines 46–48 containing different heterocyclic peripheral groups were synthesized (Fig. 21).39 Dyes 45a–f exhibited weak absorption in the visible spectral range, but strong and narrow absorptions in the NIR region between 684 and 864 nm, high quantum yields, and good photostability. The photostability of dye 45e was compared to that of indocyanine green (ICG), which is used as a standard for new NIR fluorophores. Dye 45e showed much better photostability than ICG, indicating promising features for its application in cell biology. On the other hand, bis(pyrrolopyrrole) cyanines 46–48 possess extended π-electron systems, thus providing a very strong and narrow NIR absorption (in the range 815–941 nm) and no significant absorption in the visible spectral wavelength range. Dyes 47 and 48 are selective NIR absorbers, highly photochemical stable, and exhibited strong fluorescence, which could be successfully applied to many applications.
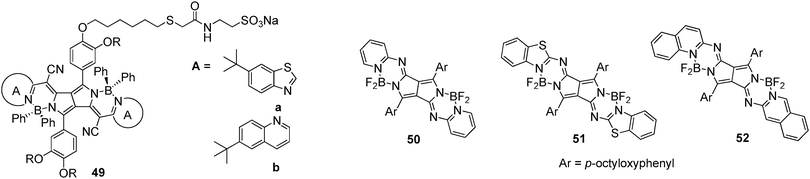
Furthermore, water-soluble PPCy dyes were synthesized as NIR fluorophores, 49a and 49b, and found to be more photostable than ICG (Fig. 22).40 Live cell imaging showed the easy internalization of 49a and 49b into mammalian cells through endocytosis processes, demonstrating their advantages for imaging applications at the cellular level.
Kobayashi et al. reported the synthesis of pyrrolopyrrole aza-BODIPY analogs by the reaction of DPP with heteroaromatic amines (pyridine; 50, benzothiazole; 51, and quinolone; 52) in the presence of titanium tetrachloride (Fig. 22).41 In CHCl3, these analogs exhibited red-shifted absorption bands at 638, 655, and 671 nm and emission bands at 661, 676, and 692 nm for 50, 51, and 52, respectively. These analogues exhibit high fluorescence quantum yields of >0.8. These analogs may be useful in the field of molecular electronics and optoelectronics and also in designing molecular probes for bioimaging.
Gryko et al. used a different strategy to expand the DPP core and synthesized a series of π-expanded S-shaped DPP-based fluorescent dyes 53–58 (Fig. 23).42 The six-membered rings were formed by placing two vinylene moieties between the nitrogen atoms and the aromatic substituents. The synthetic methodology involved the three step synthesis of DPP, followed by N-alkylation and electrophilic substitution to aromatic rings. The final products were S-shaped, stable, π-expanded DPP analogues that were obtained in good yields. This synthetic methodology could be applied to the synthesis of various DPP-based dyes with electron-rich or electron-neutral aromatic and heteroaromatic rings. The dyes displayed spectroscopic properties that differed from that of the DPP core. The dye 57 was further used for labeling mammalian cells indicating its potential for bio-applications.
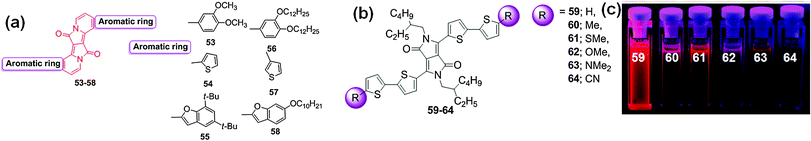 | ||
| Fig. 23 (a) Structures of DPP-based fluorescent dyes 53–58, (b) structures of bithiophene functionalized DPP-based dyes 59–64 and (c) photographs of the solutions of these dyes showing the decrease in fluorescence intensity based on the terminal groups. Reproduced from ref. 43 with permission from American Chemical Society. | ||
The effect of substitution on the optical properties of bithiophene-functionalized DPP-based dyes was explored by Wurthner et al. who reported the synthesis of a series of DPP-based dyes 59–64 with electron-donating groups (Me, SMe, OMe, NMe2) and an electron-withdrawing group (CN) (Fig. 23).43 The effect of substituents on the electronic properties of the respective DPP chromophores was evaluated using UV-Vis absorption and fluorescence spectroscopy and cyclic voltammetry. All of the synthesized dyes exhibit a broad absorption band that covers the visible and NIR region (i.e., 450–800 nm) in CH2Cl2. The fluorescence spectra of these dyes covered the range of 637–753 nm. Among these dyes, dye 63 displayed absorption at ca. 684 nm and emission at 753 nm because of the incorporation of the strong electron donating group (NMe2) into the dye, which resulted in planarization of the bithiophenes through π-extended conjugation to the DPP electron acceptor. The fluorescence intensity based on the substituents decreased in the order: H > Me > CN > SMe > OMe > NMe2 (Fig. 23c). The intriguing properties of these dyes should motivate the synthesis of new candidate materials for organic photovoltaics.
Similarly, the effect of peripheral substituents around the DPP core on the optical properties of dyes was evaluated by Borsato et al. who synthesized a new class of soluble DPP-based dyes with di-tert-butyl dicarbonate (t-Boc) groups 65a–g. After removal of the t-Boc groups by thermal treatment, the new insoluble pigments 66a–g were formed (Fig. 24a).44 The UV-Vis absorption and fluorescence spectra of dyes 65a–g were acquired in CH2Cl2; the absorptions and emissions were, respectively, bathochromically shifted in the range of 451–518 nm and 528–642 nm. These dyes display an average Stoke's shift of 70–80 nm, except for 65c for which the Stoke's shift was 124 nm. The optical properties of pigments 66a–g were also recorded in DMSO, where bathochromic shifts of the absorption spectra (in the range of 540–566 nm) and emission spectra (in the range of 550–629 nm) were observed. These dyes are prospective candidates for use as molecular probes for bio-imaging.
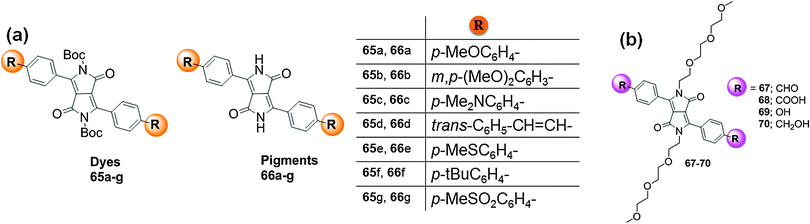 | ||
| Fig. 24 (a) Structure of DPP-based dyes 65a–g, pigments 66a–g and (b) structures of symmetric DPP derivatives 67–70. | ||
Wang et al. reported the synthesis of four symmetric DPP derivatives with substituents 67–70 (Fig. 24b).45 In THF, these derivatives ( 67, 68, 69, and 70) exhibited absorption bands at 496 nm, 485 nm, 482 nm, and 470 nm. The green emission bands of these derivatives in THF were located at 575 nm, 555 nm, 530 nm, and 527 nm. The effects of alkaline conditions on derivatives 68 and 69 were evaluated, illustrating remarkable changes in the photophysical properties. The UV-Vis absorption and emission spectra of derivative 68 were blue-shifted by 20 nm in the presence of sodium hydroxide. On the other hand, derivative 69 displayed a red-shift of the absorption with no change of the emission because of the increased electron-donating ability of 69. These derivatives may be explored for the design and synthesis of new optoelectronic materials and may also be used in bio-imaging because of their good solubility in water derived from the oligoethyleneglycol chains.
Thus, the present research shows that structural modification of DPP may be exploited to generate a series of functionalized DPP-based dyes with versatile optoelectronic properties that function as laser dyes, NIR dyes, and have prospective application in the synthesis of new DPP-based organic molecules for optoelectronic and biological application.
6. DPP-based polymers as fluorescent probes
Recently, Lin et al. reported the synthesis of mechanically interlocked polymeric architectures (MIPAs) possessing a 9-alkylidiene-9H-fluorene monomer, DPP linked with a dumbbell unit, metalated [2]rotaxane, demetalated [2]rotaxane and simple alkyl chain-tethering monomers 71–74 (Fig. 25a).46 In THF, polymer 73 exhibited absorption bands at 642 nm and 725 nm and an emission band at 766 nm. After addition of TFA, the intensity of the absorption and emission bands decreased simultaneously. The binding constant of polymer 73 with TFA was found to be 1.22 × 105 M−1. The complexation of TFA with the orthogonal H-bonded unit of polymer 73 was reversible in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and this on–off–on cycling process was reversible for up to four cycles. The reversible self-assembly process of polymer 73 was substantiated by SEM and TEM analysis. SEM images of polymer 73 illustrated spherical vesicular type particles (Fig. 25b) which converted to distinctive flake and nanofiber structures after protonation with TFA (Fig. 25c). The reversibility was achieved by treating polymer 73–TFA with DBU to regenerate the spherical vesicles observed in the initial SEM images of polymer 73 (Fig. 25d).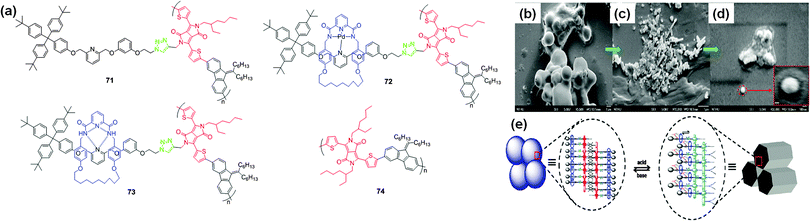 | ||
| Fig. 25 (a) Structures of mechanically interlocked DPP-based polymers 71–74 and SEM images of (b) polymer 73, (c) 73 with TFA, and (d) 73–TFA with DBU, (e) graphical representation of a possible hierarchical self-assembly process of polymer 73 on the nanoscale. Reproduced from ref. 46 with permission from American Chemical Society. | ||
Polymer 73 underwent a controllable self-assembly process on the nanoscale, achieved by a reversible acid–base stimulated molecular switch mechanism. The orthogonal H-bonded [2]rotaxane side chain unit in polymer 73 originally exists as metal-free vesicle-shaped particles and further morphs into hexagonal nano-objects (e.g. sheets and fibers) in the presence of acid (Fig. 25e).
Recently, Hua et al. reported the synthesis of the conjugated polymer 75 with DPP (electron acceptor) and fluorene monomers (electron donor) (Fig. 26a)47 for the sensitive and selective detection of F−. The UV-Vis absorption spectrum of polymer 75 (7.5 μg mL−1) in THF was characterized by a transition band at 511 nm, the intensity of which decreased upon addition of F−, accompanied by the formation of a new band at 625 nm that corresponds to the deprotonated species. The color of the solution changed from red to purple (Fig. 26b). Initial addition of F− resulted in fluorescence quenching at 570 nm, and further addition of F− resulted in the appearance of a new emission peak at 656 nm, corresponding to the deprotonated DPP species (Fig. 26c). The unalkylated imide groups in DPP function as emission traps for excited state transport, and F− disrupts the electronic energy transfer by acting as a quencher. Furthermore, polymer 75 was used for the fabrication of nanoparticles with diameters ranging from 100–200 nm that were applied to live cell imaging.
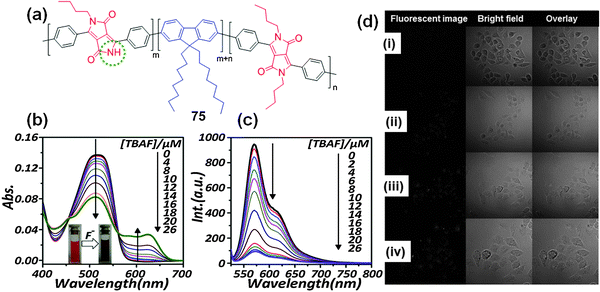 | ||
| Fig. 26 (a) Structure of DPP-based conjugated polymer 75; (b) UV-Vis absorption; (c) fluorescence titration spectra of probe 75 with addition of F− in THF; (d) real-time fluorescence images of polymer 75 nanoparticles incubated in KB cells for: (i) 30 min, (ii) 60 min, (iii) 90 min, and (iv) 120 min. Reproduced from ref. 47 with permission from Elsevier. | ||
Confocal laser scanning microscopy was used to study the applicability of polymer 75 nanoparticles for real-time imaging using KB tumor cells. Initially, no fluorescence signal was detected; however, with elapse of the incubation time, red fluorescence signals were observed (Fig. 26d). This scientific contribution is potentially useful for the design and synthesis of DPP-based fluorescent polymers for diverse applications in sensing and bio-imaging.
He et al. reported three water soluble conjugated polyfluorene polymers 76–78 in which the ratio of fluorene to DPP units varied (Fig. 27).48 The fluorene: DPP ratios of these polymers were 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 90
5, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 50
10, and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 for 76, 77, and 78, respectively. Intramolecular energy transfer from the fluorene units to the DPP units took place, and aqueous solution of the polymers displayed red-shifted emissions. Polymer 77 has strong electrostatic interaction with Cy5-labeled ssDNA and the fluorescence intensity of red Cy5 dye significantly increased through fluorescence resonance energy transfer.
50 for 76, 77, and 78, respectively. Intramolecular energy transfer from the fluorene units to the DPP units took place, and aqueous solution of the polymers displayed red-shifted emissions. Polymer 77 has strong electrostatic interaction with Cy5-labeled ssDNA and the fluorescence intensity of red Cy5 dye significantly increased through fluorescence resonance energy transfer.
Cao et al. demonstrated the synthesis of two cationic conjugated polyelectrolytes (CPEs) 79 and 80 having fluorene, DPP, and diacetylene moieties (Fig. 28a).49 The interaction of these polymers with DNA was studied by UV-Vis absorption and fluorescence spectroscopy. The emission maximum of polyelectrolyte 79 was present at 417 nm (originating from the fluorene ethynylene segment). After the addition of DNA, the emission intensity at 417 nm decreases, accompanied by the appearance of a new emission band at 431 nm (originating from the DPP unit). The interaction of positively charged polyelectrolytes with negatively charged DNA results in complex formation as a result of cooperative electrostatic attractions, which leads to aggregation of the DPP-containing conjugated polyelectrolyte. Fluorescence resonance energy transfer from the blue-emitting fluorene ethynylene segments to the DPP units resulted in the observed visible color change from blue to red allowing efficient detection of DNA (Fig. 28b).
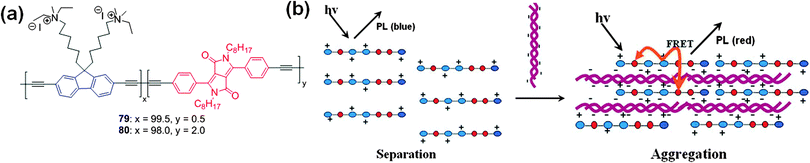 | ||
| Fig. 28 (a) Structures of water soluble cationic polyelectrolytes 79 and 80, (b) pictorial representation of the analysis of optical changes caused by complexation of 79 and 80 with CT DNA. Reproduced from ref. 49 with permission from John Wiley and Sons. | ||
To improve the sensitivity of CPEs towards DNA, the water soluble cationic polymers 81 and 82 with fluorene, DPP, triphenylamine, and diacetylene units in the main chain were synthesized for studying the detection capability of DNA (Fig. 29a).50 Similar UV-Vis absorption spectral changes were observed as in the case of polymers 79 and 80. Interaction of polymer 82 with CT DNA resulted in more efficient quenching of the blue emission (from the fluorine ethynylene-triphenylamine units) than the red emission (from the DPP segments), accompanied by a color change from light blue to light red. Hyperbranched polymer 82 showed better sensitivity for DNA detection because of the presence of a large number of branches in the polymer structure, which facilitated exciton migration and energy transfer than the linear polymer 81 (Fig. 29b).
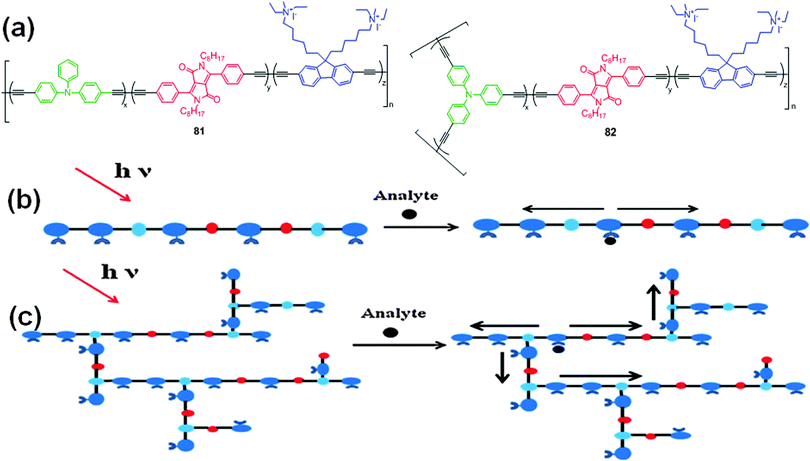 | ||
| Fig. 29 (a) Structures of water soluble cationic polymers 81 and 82; (b) pictorial representation of fluorescence amplification of (b) polymer 81 and (c) polymer 82. Reproduced from ref. 50 with permission from Elsevier. | ||
Liu et al. reported the synthesis of anionic conjugated polyelectrolytes 83 and 84 consisting of water soluble DPP derivatives linked to phenylenevinylene (PV)/phenylene ethynylene (PE) units (Fig. 30a).51 The absorption maxima of these polyelectrolytes were red-shifted in water (from 524 nm to 547 nm for 83 and from 507 nm to 522 nm for 84). Fluorescence quenching of polyelectrolyte 83, in contrast to 84, was observed in aqueous solutions. This change is attributed to J-aggregation in the polyelectrolytes. The hydrophobic main-chains in the polyelectrolytes interact strongly to form stacked layers and the hydrophilic sulfonium side chains are freely oriented to water molecules. This J-aggregation results in a higher conjugative effect, with facile transfer of excitons between the main chains, which might account for the fluorescence quenching in 83. The surfactochromicity of these polyelectrolytes was further evaluated in different solvents and in the presence of cationic surfactants or polymer non-ionic surfactants. In the presence of oppositely charged surfactants, the homogeneous aggregates were broken and heterogeneous aggregates between the polyelectrolytes and the surfactant were formed, resulting in destruction of the J-aggregation.
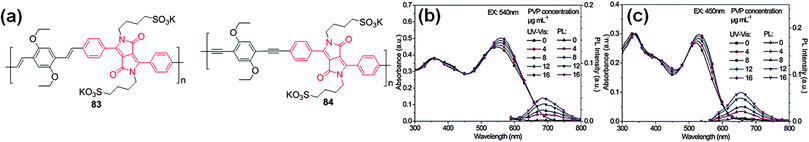 | ||
| Fig. 30 (a) Structures of water soluble anionic conjugated polyelectrolytes 83 and 84; UV-Vis absorption and emission spectra of polyelectrolytes in aqueous solutions with different concentrations of PVP: (b) polyelectrolyte 83, and (c) polyelectrolyte 84. Reproduced from ref. 51 with permission from John Wiley and Sons. | ||
This results in variation of the conjugation length of the polymers and therefore lowering of the energy dissipation between the main chains, followed by fluorescence enhancement. The fluorescence of these polyelectrolytes could be enhanced by adding the specific non-ionic polymer surfactant polyvinylpyrrolidone (PVP) (Fig. 30b and c).
7. Conclusions
DPP is an emerging dye component in supramolecular chemistry that has a vast array of possibilities for functionalization. Tremendous research effort has been expended in the development of DPP-related chemistry and for utilizing DPP derivatives in the design of highly efficient materials for various applications. This manuscript summarizes the progress achieved from 2009 to the present time in the design and development of DPP-based fluorescent probes. As described herein, considerable achievements have been accomplished by researchers in the exploration of DPP-based fluorescent probes and their applications in the detection of biologically important species (e.g., anions, cations, thiols, ROSs and gases).The structures of different DPP-based fluorescent probes, detection mechanisms, optical properties, and other miscellaneous applications were discussed, demonstrating that the application of the DPP acceptor is not strictly limited to the creation of new organic materials for materials chemistry, but can also be exploited in the design of new derivatives for a plethora of applications. The applications of DPP-based small molecules and polymers in the development of two-photon probes, NIR dyes, and DNA detection were discussed. It is anticipated that this research area will become more active and productive and that the studies highlighted in this review will emphasize the great potential of the DPP moiety to researchers. This is the first review focusing on DPP-based fluorescent probes, and it is hoped that the information provided herein will provide new directives for the chemistry scientific community in the exploration of novel ideas for structural modification of DPP for the design and synthesis of new fluorescent probes to extend the range of practical applications.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF2012R1A2A1A01008797) and by Key Research Institute Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF20100020209).References
- X. Li, X. Gao, W. Shi and H. Ma, Chem. Rev., 2014, 114, 590–659 CrossRef CAS PubMed
.
-
A. Iqbal and L. Cassar, US Pat., 4415685 A, 1983 Search PubMed
.
- Z. Hao and A. Iqbal, Chem. Soc. Rev., 1997, 26, 203–213 RSC
.
- Y. Li, P. Sonar, L. Murphy and W. Hong, Energy Environ. Sci., 2013, 6, 1684–1710 CAS
.
- C. B. Nielsen, M. Turbiez and I. McCulloch, Adv. Mater., 2013, 25, 1859–1880 CrossRef CAS PubMed
.
- D. Chandran and K.-S. Lee, Macromol. Res., 2013, 21, 272–283 CrossRef CAS PubMed
.
-
O. Wallquist, R. Lenz, in High Performance Pigments, ed. E. B. Faulkner and R. J. Schwartz, Wiley-VCH Verlag, 2009, pp. 165–194 Search PubMed
.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC
.
- Y. Qu, J. Hua and H. Tian, Org. Lett., 2010, 12, 3320–3323 CrossRef CAS PubMed
.
- Y. Qu, S. Qu, L. Yang, J. Hua and D. Qu, Sens. Actuators, B, 2012, 173, 225–233 CrossRef CAS PubMed
.
- C. Yang, M. Zheng, Y. Li, B. Zhang, J. Li, L. Bu, W. Liu, M. Sun, H. Zhang, Y. Tao, S. Xue and W. Yang, J. Mater. Chem. A, 2013, 1, 5172–5178 CAS
.
- Y. Li, M. Zheng, J. Wang, Y. Gao, B. Zhang and W. Yang, Dyes Pigm., 2014, 104, 97–101 CrossRef CAS PubMed
.
- G. Zhang, L. Wang, X. Cai, L. Zhang, J. Yu and A. Wang, Dyes Pigm., 2013, 98, 232–237 CrossRef CAS PubMed
.
- M. Kaur, M. J. Cho and D. H. Choi, Dyes Pigm., 2014, 103, 154–160 CrossRef CAS PubMed
.
- M. V. R. Raju and H.-C. Lin, Org. Lett., 2013, 15, 1274–1277 CrossRef CAS PubMed
.
- Y.-H. Jeong, C.-H. Lee and W.-D. Jang, Chem. – Asian J., 2012, 7, 1562–1566 CrossRef PubMed
.
- G. Zhang, H. Li, S. Bi, L. Song, Y. Lu, L. Zhang, J. Yu and L. Wang, Analyst, 2013, 138, 6163–6170 RSC
.
- G. Zhang, S. Bi, L. Song, F. Wang, J. Yu and L. Wang, Dyes Pigm., 2013, 99, 779–786 CrossRef CAS PubMed
.
- G. Aragay, J. Pons and A. Merkoci, Chem. Rev., 2011, 111, 3433–3458 CrossRef CAS PubMed
.
- M. Kaur and D. H. Choi, Sens. Actuators, B, 2014, 190, 542–548 CrossRef CAS PubMed
.
- M. Kaur, D. S. Yang, K. Choi, M. J. Cho and D. H. Choi, Dyes Pigm., 2014, 100, 118–126 CrossRef CAS PubMed
.
- L. Deng, W. Wu, H. Guo, J. Zhao, S. Ji, X. Zhang, X. Yuan and C. Zhang, J. Org. Chem., 2011, 76, 9294–9304 CrossRef CAS PubMed
.
- M. Schaferling, Angew. Chem., Int. Ed., 2012, 51, 3532–3554 CrossRef PubMed
.
- T. Yamagata, J. Kuwabara and T. Kanbara, Tetrahedron Lett., 2010, 51, 1596–1599 CrossRef CAS PubMed
.
- D. Aigner, B. Ungerbock, T. Mayr, R. Saf, I. Klimant and S. M. Borisov, J. Mater. Chem. C, 2013, 1, 5685–5693 RSC
.
- S. Schutting, S. M. Borisov and I. Klimant, Anal. Chem., 2013, 85, 3271–3279 CrossRef CAS PubMed
.
- J. Mizuguchi, T. Imoda, H. Takahashi and H. Yamakami, Dyes Pigm., 2006, 68, 47–52 CrossRef CAS PubMed
.
- H. Takahashi and J. Mizuguchia, J. Appl. Phys., 2006, 100, 034908 CrossRef PubMed
.
- O. Salyk, J. Vynuchal, I. Kratochvılova, T. Todorciuc, J. Pavluch and P. Toman, Phys. Status Solidi A, 2010, 207, 2327–2333 CrossRef CAS
.
- Y. Jiang, Y. Wang, J. Hua, S. Qu, S. Qian and H. Tian, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4400–4408 CrossRef CAS
.
- E. Q. Guo, P. H. Ren, Y. L. Zhang, H. C. Zhang and W. J. Yang, Chem. Commun., 2009, 5859–5861 RSC
.
- A. Nowak-Krol, M. Grzybowski, J. Romiszewski, M. Drobizhev, G. Wicks, M. Chotkowski, A. Rebane, E. Gorecka and D. T. Gryko, Chem. Commun., 2013, 49, 8368–8370 RSC
.
- H. Ftouni, F. Bolze and J.-F. Nicoud, Dyes Pigm., 2013, 97, 77–83 CrossRef CAS PubMed
.
- H. Ftouni, F. Bolze, H. de Rocquigny and J.-F. Nicoud, Bioconjugate Chem., 2013, 24, 942–950 CrossRef CAS PubMed
.
- S. Luo, E. Zhang, Y. Su, T. Cheng and C. Shi, Biomaterials, 2011, 32, 7127–7138 CrossRef CAS PubMed
.
- R. Lenz and O. Wallquist, Surf. Coat. Int., Part B, 2002, 85, 19–26 CrossRef CAS
.
- G. M. Fischer, A. P. Ehlers, A. Zumbusch and E. Daltrozzo, Angew. Chem., Int. Ed., 2007, 46, 3750–3753 CrossRef CAS PubMed
.
- G. M. Fischer, M. Isomaki-Krondahl, I. Gottker-Schnettmann, E. Daltrozzo and A. Zumbusch, Chem. – Eur. J., 2009, 15, 4857–4864 CrossRef CAS PubMed
.
- G. M. Fischer, E. Daltrozzo and A. Zumbusch, Angew. Chem., Int. Ed., 2011, 50, 1406–1409 CrossRef CAS PubMed
.
- S. Wiktorowski, C. Rosazza, M. J. Winterhalder, E. Daltrozzo and A. Zumbusch, Chem. Commun., 2014, 50, 4755–4758 RSC
.
- S. Shimizu, T. Iino, Y. Araki and N. Kobayashi, Chem. Commun., 2013, 49, 1621–1623 RSC
.
- M. Grzybowski, E. Glodkowska-Mrowka, T. Stoklosa and D. T. Gryko, Org. Lett., 2012, 14, 2670–2673 CrossRef CAS PubMed
.
- H. Burckstummer, A. Weissenstein, D. Bialas and F. Wurthner, J. Org. Chem., 2011, 76, 2426–2432 CrossRef PubMed
.
- R. Beninatto, G. Borsato, O. De Lucchi, F. Fabris, V. Lucchini and E. Zendri, Dyes Pigm., 2013, 96, 679–685 CrossRef CAS PubMed
.
- G. Zhang, L. Song, S. Bi, Y. Wu, J. Yu and L. Wang, Dyes Pigm., 2014, 102, 100–106 CrossRef CAS PubMed
.
- M. V. R. Raju, P. Raghunath, M.-C. Lin and H.-C. Lin, Macromolecules, 2013, 46, 6731–6743 CrossRef CAS
.
- Y. Qu, Y. Wu, Y. Gao, S. Qu, L. Yang and J. Hua, Sens. Actuators, B, 2014, 197, 13–19 CrossRef CAS PubMed
.
- F. He, L. Liu and L. Li, Adv. Funct. Mater., 2011, 21, 3143–3149 CrossRef CAS
.
- S. Lin, S. Liu, H. Zou, W. Zeng, L. Wang, R. Beuerman and D. Cao, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3882–3889 CrossRef CAS
.
- S. Lin, S. Liu, F. Ye, L. Xu, W. Zeng, L. Wang, L. Li, R. Beuerman and D. Cao, Sens. Actuators, B, 2013, 182, 176–183 CrossRef CAS PubMed
.
- H. Shen, C. Kou, M. He, H. Yang and K. Liu, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 739–751 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |