 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct 17O NMR experimental evidence for Al–NBO bonds in Si-rich and highly polymerized aluminosilicate glasses†
Aleksander
Jaworski
,
Baltzar
Stevensson
and
Mattias
Edén
*
Physical Chemistry Division, Department of Materials and Environmental Chemistry, Arrhenius Laboratory, Stockholm University, SE-106 91 Stockholm, Sweden. E-mail: mattias.eden@mmk.su.se
First published on 23rd June 2015
Abstract
By using solid-state 17O NMR spectroscopy, we provide the first direct experimental evidence for bonds between Al and non-bridging oxygen (NBO) ions in aluminosilicate glasses based on rare-earth (RE) elements, where RE = {Lu, Sc, Y}. The presence of ∼10% Al–NBO moieties out of all NBO species holds regardless of the precise glass composition, at odds with the conventional structural view that Al–NBO bonds are absent in highly polymerized and Si-rich aluminosilicate glass networks.
Owing to their importance for both materials and earth sciences, vast efforts have been spent to improve the structural understanding of aluminosilicate (AS) glasses.1 Ternary M(2)O–Al2O3–SiO2 glasses normally involve a monovalent alkali (M+) or divalent alkaline-earth (M2+) metal cation. The glass networks comprise SiO4 and AlO4 tetrahedra that are cornershared by bridging oxygen (BO) atoms. The additional negative charge of each AlO−4 moiety (relative to SiO4) requires nearby cations for attaining local charge-balance, while the remaining Mz+ cations depolymerize the glass network by converting BO (O[2]) atoms into non-bridging oxygen (NBO; O[1]) species.1 The relative BO/NBO speciations in melts and glasses dictate many properties, such as viscosity, conductivity, and thermal expansion.1,2
The following three features of the structural understanding of AS glasses have prevailed for decades, all building around the consequences of the excess negative charge of the AlO4 groups:1,2 (i) Both Si and Al are four-fold coordinated (Si[4] and Al[4]) by O, except if the network-modifier content is insufficient for balancing the entire Al speciation as Al[4]; then, higher-coordination AlO5 and AlO6 polyhedra form whenever znM < nAl or nSi < nAl,1,2 where nE denotes the stoichiometric amount of element E in the glass and z is the charge of Mz+. (ii) To avoid local negative charge-accumulation in the structure, there is a strong preference for Si[4]–O–Al[4] linkages, whereas those of Al[4]–O–Al[4] are absent (the “Loewenstein rule”3). (iii) Moreover, there is a dominance of Si–NBO contacts relative to Al–NBO, implying that all NBO species are accommodated by SiO4 in silica-rich AS glasses.1,4
However, over the past decade, violations of properties (i)–(ii) are well documented for AS glasses based on mono/di-valent cations, where several studies reveal minor fractional populations of AlO5 groups (a few %) in fully charge-balanced (nM = nAl/z = nSi/z) “tectosilicate” AS glasses4c,5 (notwithstanding that glasses formed at high pressure reveal significant AlO5/AlO6 populations6). Moreover, while Loewenstein's rule holds strictly for crystalline AS phases featuring nAl ≤ nSi (such as zeolites and minerals1a), minor deviations thereof are reported for M(2)O–Al2O3–SiO2 glasses.7 Yet, whereas the early literature identified the preference of Si–NBO over Al–NBO associations, the existence of the latter were often deduced from circumstantial evidence.2 Nevertheless, property (iii) is nowadays assumed to apply universally for any SiO2-dominated M(2)O–Al2O3–SiO2 glass. Direct experimental evidence for Al–NBO contacts only exist for amorphous M–Al–O aluminate phases, or AS glasses that are simultaneously rich in network-modifiers and Al2O3, while SiO2 is a minor component (<40 mol%).4
However, the presence of trivalent rare-earth (RE3+) cations in RE2O3–Al2O3–SiO2 glasses8 leads to markedly higher configurational and chemical disorder, as mirrored in the following structural features: (1) significant AlO5/AlO6 populations prevail throughout the entire range of RE AS compositions, i.e., not only for those featuring nAl > nSi and/or insufficient modifier contents.9 The relative amounts of higher-coordination polyhedra were demonstrated to grow for decreasing SiO2 content,9d–f and particularly when the RE3+ cation field-strength, CFS = z/R2, is increased,9b–e,g where R is the ionic radius. The markedly more cross-linked AS glass network stemming from the higher-coordination Al[p] species was recently employed for explaining the progressively enhanced Vickers hardness observed for RE–Al–Si–O glasses with growing CFS according to La3+ < Y3+ < Lu3+ < Sc3+.9e,10 (2) The high positive charge of the RE3+ cations implies clear violations of the Loewenstein rule, reflected in a pronounced Al/Si atomic disorder for RE AS glass networks, as demonstrated by 29Si and 27Al NMR,9f,11 as well as by molecular dynamics (MD) simulations.9f,h Noteworthy, the properties (1) and (2) apply generally to all AS glasses examined thus far from the RE = {La, Y, Lu, Sc} systems, regardless of the precise RE/Al/Si composition.9–11
Regarding the potential presence of Al[p]–NBO contacts, i.e., violation of property (iii) of the prevailing structural picture of (Si-rich) AS glasses, we have recently presented circumstantial experimental evidence by 29Si NMR of a significant BO/NBO intermixing among SiO4/AlO4 groups in La2O3–Al2O3–SiO2 structures,11 whereas MD-simulations of Y and Lu bearing glasses reveal that significant fractions (20–50%) of all NBO species are accommodated by AlOp groups.9e Here we provide the first direct experimental proof of significant Al–NBO contacts in SiO2-rich RE2O3–Al2O3–SiO2 glasses with RE = {Y, Lu, Sc}, by utilizing magic-angle spinning (MAS) 17O NMR. Each specimen was prepared with ≈20% 17O-enrichment and is denoted REab(r), where a and b represent the nominal Al2O3 and SiO2 contents in mol%, respectively, and r = nO/(nSi + nAl) conveys the glass network polymerization degree.1c All glasses feature 42–65 mol% SiO2 and n(RE2O3) < n(Al2O3); see Table 1. The ESI† describes all sample preparation and basic characterization procedures, as well as the NMR experimentation discussed below.
| Glass compositionsa | Oxygen populationsb | NBO populationsc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glass | aRE2O3 (mol%) | bAl2O3 (mol%) | cSiO2 (mol%) | n Al/nSi | x [0] | x [1] | x [2] | x [3] | x [1] | x [1]Si | x [1]Al |
| a Nominal aRE2O3–bAl2O3–cSiO2 glass composition with a + b + c = 100 mol%. b MD-derived fractional populations of oxygen coordinations {x[p]} with p = {0, 1, 2, 3}, where only bonds to Si and Al are counted to define the coordination number p. c Fractional populations of NBO species (x[1]) obtained by 17O MAS NMR, shown together with the contributions from Si–NBO (x[1]Si) and Al–NBO (x[1]Al) species, where x[1] = x[1]Si + x[1]Al. Values within parentheses are the corresponding MD-derived data. The uncertainties are ±0.015 and ±0.010 for the populations derived from NMR and MD simulations, respectively. | |||||||||||
| Y2855(2.21) | 17.05 | 27.70 | 55.25 | 1.00 | 0.010 | 0.226 | 0.653 | 0.109 | 0.221 (0.226) | 0.206 (0.154) | 0.015 (0.072) |
| Y3742(2.21) | 21.16 | 37.34 | 41.50 | 1.80 | 0.014 | 0.232 | 0.578 | 0.172 | 0.210 (0.232) | 0.186 (0.119) | 0.024 (0.113) |
| Lu2165(2.21) | 14.50 | 20.97 | 64.53 | 0.65 | 0.009 | 0.223 | 0.684 | 0.084 | 0.241 (0.223) | 0.224 (0.166) | 0.016 (0.056) |
| Lu2855(2.21) | 17.05 | 27.70 | 55.25 | 1.00 | 0.013 | 0.230 | 0.641 | 0.115 | 0.261 (0.230) | 0.236 (0.154) | 0.025 (0.076) |
| Lu2551(2.45) | 23.75 | 25.43 | 50.82 | 1.00 | 0.030 | 0.329 | 0.560 | 0.079 | 0.369 (0.329) | 0.329 (0.212) | 0.040 (0.117) |
| Sc3649(2.07) | 14.77 | 36.21 | 49.02 | 1.48 | 0.008 | 0.160 | 0.610 | 0.216 | 0.162 (0.160) | 0.144 (0.091) | 0.018 (0.069) |
| Sc2955(2.21) | 16.96 | 28.48 | 54.56 | 1.04 | 0.017 | 0.233 | 0.628 | 0.121 | 0.183 (0.233) | 0.170 (0.151) | 0.014 (0.082) |
Fig. 1 displays 17O MAS NMR spectra recorded from various RE–Al–Si–O glasses with RE = {Y, Lu, Sc} and variable cation compositions, as well as average network polymerization degrees. All NMR spectra manifest two main groups of resonances: one from 17O[2] species—whose peak-maximum ranges between 31–72 ppm and depending primarily on the nAl/nSi molar ratio—and one from NBO ions located at the SiO4 groups; Si–17O[1] (∼137–158 ppm). While the 17O[2] NMR signal dominates, that from Si–17O[1] grows concurrently with r, i.e., when the glass-network polymerization decreases. Moreover, a weak but significant 17O resonance appears in the high-ppm region (∼175–250 ppm) of all NMR spectra in Fig. 1: it is assigned to Al–17O[1] motifs. Incidentally, such a signal was previously reported by Schaller and Stebbins in the 17O MAS NMR spectrum from one Y2O3–Al2O3–SiO2 glass.9b However, despite noting that the “NBO peak may include oxygens bonded to AlO4 or SiO4 groups” (then referring to the peak herein assigned to Si–O[1] groups), they tentatively attributed the high-ppm signal to “NBO species with more yttrium neighbors” than those contributing to the more intense 17O[1] resonance. This 17O NMR peak appears to be a general feature of high-CFS RE-based AS glasses, but we did not detect it from La2O3–Al2O3–SiO2 glasses (data not shown), in accordance with observations made in ref. 9b.
Note that neither the 17O MAS NMR spectra (Fig. 1) nor their 27Al counterparts may directly inform about the presence of Al–NBO contacts. The 27Al NMR spectra were recently reported for Y,9e Lu,9e and Sc9g AS glasses, all revealing coexisting AlO4, AlO5, and AlO6 groups. Yet, unambiguous evidence for the assignment of the high-shift 17O resonance to Al–17O[1] species is provided by the 17O{27Al} TRAPDOR NMR12 data presented in Fig. 2. Here the 17O–27Al dipolar interaction is recoupled by applying a strong radio-frequency (rf) pulse for τrec = 2.5 ms. For all 17O sites in close spatial proximity to 27Al, an attenuated integrated 17O NMR signal intensity [S(τrec)] results relative to that observed in the absence of 17O–27Al recoupling by using a spin-echo [S0(τrec)]. Indeed, due to the presence of Si–O–Al and Al–O–Al structural motifs, the BO-deriving 17O NMR signals manifest a significant signal dephasing. This also applies to the weak 17O resonance ∼175–250 ppm in Fig. 2 (assigned to Al–17O[1] bonds), as is evidenced by its high dephasing ratio ΔS/S0 = [S0(τrec) – S(τrec)]/S0(τrec) obtained by deconvoluting the net 17O NMR peakshapes, as exemplified for the S0(τrec) spectra in Fig. 2(b, d and f).
 | ||
| Fig. 2 (a, c, e) 17O NMR spectra recorded at 9.4 T and 14.0 kHz MAS from the as-indicated Y and Lu bearing AS glasses by employing 17O{27Al} TRAPDOR NMR.12 The spectra labelled by S(τrec) and S0(τrec) were obtained in the presence and absence of dipolar dephasing, respectively (τrec = 2.5 ms). The difference between the black and red traces reflects the degree of 17O–27Al contacts among the BO, Al–NBO and Si–NBO species, with the number on top of each signal representing the dephasing degree, ΔS/S0 (uncertainty ±0.03). (b, d, f) Experimental S0(τrec) NMR spectra (black traces) displayed together with the component peaks (grey traces) obtained by spectra deconvolution. The curves beneath each NMR spectrum in (b, d, f) represents the difference between the experimental and best-fit results. | ||
In contrast, the “primary” NBO-stemming resonance reveals no dephasing within the experimental/deconvolution uncertainties; the deconvolution results of Fig. 2(b, d and f) verify that the apparent reduction of this signal stems exclusively from its overlap with the (indeed dephasing) 17O[2] resonances. This strongly suggests that the main 17O[1] NMR peak originates exclusively from Si–NBO fragments, also verifying the absence of contributions from Al–NBO moieties to this signal, as corroborated further by the additional TRAPDOR NMR data shown in Fig. 3. Fig. 3 also includes 17O NMR spectra recorded by the 27Al→17O RAPT-CP technique.13 In this experiment, solely 17O species in close proximity to 27Al are detected. Indeed, while the 17O resonance-range stemming from BO structural sites is very similar to that observed directly by using central-transition (CT) selective single pulses, no significant NMR-signal intensity is observed in the region ≳125 ppm that is primarily associated with Si–17O[1] moieties (note that the weak 27Al→17O polarization-transfer efficiency coupled with the low abundance of Al–17O[1] groups precludes their observation).
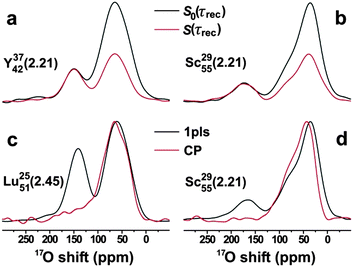 | ||
| Fig. 3 17O NMR spectra recorded at 14.1 T from the as-indicated RE AS glasses by employing (a, b) 17O{27Al} TRAPDOR12 and (c, d) 27Al → 17O RAPT-CP13 NMR experiments, the latter shown together with results obtained by CT-selective pulses (“1pls”). The data were collected at MAS rates of (a, b) 24.0 kHz and (c, d) 14.0 kHz, by using 3.2 mm and 4.0 mm triple-resonance MAS probeheads, respectively. The two NMR spectra in each of (a, b) are shown on the same absolute intensity scale, whereas those in (c, d) are scaled to display equal peak-maxima of the 17O[2] resonances. | ||
Each MAS NMR spectrum of Fig. 1 was deconvoluted into signal contributions from 17O[2], Si–17O[1], and Al–17O[1] moieties (see the ESI†). The fractional populations are presented in Table 1, together with MD-derived O speciations (see ref. 9e,h). 17O NMR reveals fractional populations x[1]Al ≈ 0.015–0.04 of Al–O[1] species. An overall good agreement is observed between experiments and simulations for the total NBO population (x[1] = x[1]Si + x[1]Al), the main discrepancy being clearly over-estimated Al–NBO contacts in the glass models. The latter also reveal non-negligible populations of oxygen triclusters (x[3]) and “free O2− ions” (x[0]), as discussed further in ref. 9e,f,h. The attribution of the weak NMR signal to Al–17O[1] motifs is consistent with the following trends/observations:
(i) As expected from Al–NBO fragments, there is a concomitant increase of x[1]Al with the total NBO content in the glass structure (compare the results for Lu2855(2.21) with Lu2551(2.45) in Table 1), as well as with the Al content [compare Lu2165(2.21) and Lu2855(2.21)].
(ii) The isotropic chemical shifts associated with the various 17O[1] sites vary significantly with the nature of the RE3+ cation, as is also evident from the NMR spectra of Fig. 1. Yet, the Al–17O[1] isotropic shifts remain consistently ∼60–100 ppm higher than their Si–17O[1] counterparts, in qualitative accordance with reported trends of Ca-based aluminate and Si-poor/Ca-rich AS glasses (we stress, however, that no Al–NBO signals were observed for Ca AS glasses exhibiting >20 mol% SiO2).4a,b
(iii) The low (average) quadrupolar products CQη = CQ(1 + η2/3)1/2 ≈ 2.6 MHz observed for the Si–17O[1] sites are consistent with previous reports from AS glasses,1b whereas the Al–17O[1] species reveals CQη ≈ 1.7 MHz (obtained by spectra deconvolution; see the ESI†). This is to our knowledge the first estimate of quadrupolar products for Al–17O[1] sites. We note that its lower value relative to Si–17O[1] is expected from the higher ionic character of the Al–17O[1] bond and consistent with the well-established trend observed for 17O[2] sites: CQη(Al–O–Al) < CQη(Al–O–Si) < CQη(Si–O–Si).1b The NMR parameters of the various 17O species will be discussed in detail elsewhere.
To conclude, we have provided the first direct experimental evidence for Al–NBO contacts in highly polymerized RE2O3–Al2O3–SiO2 glasses with variable RE/Al/Si contents. The results are corroborated by MD simulations. Given the following MD-derived propensity trends of AlOp groups to associate with NBO species, AlO4 > AlO5 > AlO6,9h we attribute most of the Al-associated NBO species to be located at AlO4 tetrahedra. Notwithstanding a strong preference for SiO4 groups to accommodate the NBO ions, the presence of Al–NBO moieties of ≲4% out of the total O speciation (7–11% of all NBO) appears to be a general feature of AS glasses that incorporate trivalent cations with high field strength: apparently they stabilize otherwise energetically disfavoured structural motifs.
While the relative Al–NBO populations grow concurrently with the Al content of the glass, they persist in SiO2-rich networks (at least up to ≈65 mol% SiO2), despite that their net NBO population remain relatively low (x[1] ≈ 0.22). This is in stark contrast to AS glasses based on low-CFS mono/divalent cations, where non-negligible Al–NBO contacts have hitherto only been observed directly for fragmented networks rich in modifiers (≳50 mol% M(2)O) and simultaneously featuring low SiO2 (≲30 mol%) contents and high molar ratios n(Al2O3)/n(SiO2) > 2.4a
This work was supported by the Carl Trygger Foundation, the Magn. Bergvall Foundation, and the Swedish Research Council (contract VR-NT 2010-4943). We gratefully acknowledge NMR equipment grants from the Swedish Research Council and the Knut and Alice Wallenberg Foundation.
Notes and references
- (a) G. Engelhardt and D. Michel, High-resolution solid-state NMR of silicates and zeolites, John Wiley, Chichester, UK, 1987 Search PubMed; (b) K. J. D. MacKenzie and M. E. Smith, Multinuclear solid-state NMR of inorganic materials, Pergamon Press, Amsterdam, 2002 Search PubMed; (c) M. Edén, Annu. Rep. Prog. Chem., Sect. C: Phys. Chem., 2012, 108, 177 RSC.
- (a) B. O. Mysen, D. Virgo and I. Kushiro, Am. Mineral., 1981, 66, 678 CAS; (b) J. B. Murdoch, J. F. Stebbins and I. S. E. Carmichael, Am. Mineral., 1985, 70, 332 CAS; (c) C. I. Merzbacher, B. L. Sherriff, J. S. Hartman and W. B. White, J. Non-Cryst. Solids, 1990, 124, 194 CrossRef CAS.
- W. Lowenstein, Am. Mineral., 1954, 39, 92 Search PubMed.
- (a) J. R. Allwardt, S. K. Lee and J. F. Stebbins, Am. Mineral., 2003, 88, 949 CAS; (b) S. K. Lee and J. F. Stebbins, Geochim. Cosmochim. Acta, 2006, 70, 4275 CrossRef CAS PubMed; (c) D. R. Neuville, L. Cormier and D. Massiot, Chem. Geol., 2006, 229, 173 CrossRef CAS PubMed.
- (a) J. F. Stebbins, S. Kroeker, S. K. Lee and T. J. Kiczenski, J. Non-Cryst. Solids, 2000, 275, 1 CrossRef CAS; (b) M. J. Toplis, S. C. Kohn, M. E. Smith and I. J. F. Poplett, Am. Mineral., 2000, 85, 1556 CAS.
- S. K. Lee, Solid State Nucl. Magn. Reson., 2010, 38, 45 CrossRef CAS PubMed.
- (a) S. K. Lee and J. F. Stebbins, J. Phys. Chem. B, 2000, 104, 4091 CrossRef CAS; (b) S. K. Lee and J. F. Stebbins, J. Non-Cryst. Solids, 2000, 270, 260 CrossRef CAS.
- (a) A. Makishima, M. Kobayashi, T. Shimohira and T. Nagata, J. Am. Ceram. Soc., 1982, 65, C210 CrossRef CAS PubMed; (b) J. T. Kohli and J. E. Shelby, Phys. Chem. Glasses, 1991, 32, 67 CAS.
- (a) J. T. Kohli, J. E. Shelby and J. S. Frye, Phys. Chem. Glasses, 1992, 33, 73 CAS; (b) T. Schaller and J. F. Stebbins, J. Phys. Chem. B, 1998, 102, 10690 CrossRef CAS; (c) S. Iftekhar, J. Grins, P. N. Gunawidjaja and M. Edén, J. Am. Ceram. Soc., 2011, 94, 2429 CrossRef CAS PubMed; (d) P. Florian, N. Sadiki, D. Massiot and J. P. Coutures, J. Phys. Chem. B, 2007, 111, 9747 CrossRef CAS PubMed; (e) S. Iftekhar, B. Pahari, K. Okhotnikov, A. Jaworski, B. Stevensson, J. Grins and M. Edén, J. Phys. Chem. C, 2012, 116, 18394 CrossRef CAS; (f) A. Jaworski, B. Stevensson, B. Pahari, K. Okhotnikov and M. Edén, Phys. Chem. Chem. Phys., 2012, 14, 15866 RSC; (g) B. Pahari, S. Iftekhar, A. Jaworski, K. Okhotnikov, K. Jansson, B. Stevensson, J. Grins and M. Edén, J. Am. Ceram. Soc., 2012, 95, 2545 CrossRef CAS PubMed; (h) K. Okhotnikov, B. Stevensson and M. Edén, Phys. Chem. Chem. Phys., 2013, 15, 15041 RSC.
- B. Stevensson and M. Edén, J. Non-Cryst. Solids, 2013, 378, 163 CrossRef CAS PubMed.
- S. Iftekhar, E. Leonova and M. Edén, J. Non-Cryst. Solids, 2009, 355, 2165 CrossRef CAS PubMed.
- C. P. Grey and W. S. Veeman, Chem. Phys. Lett., 1992, 192, 379 CrossRef CAS.
- M. Edén, J. Grins, Z. Shen and Z. Weng, J. Magn. Reson., 2004, 169, 279 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and numerical procedures; examples of best-fit 17O NMR spectra; additional NMR spectra obtained by strong rf pulses. See DOI: 10.1039/c5cp02985f |
| This journal is © the Owner Societies 2015 |

