Image molecular dipoles in surface enhanced Raman scattering†
Abstract
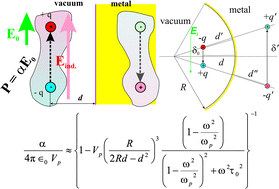
The surface enhanced Raman scattering (SERS) effect is explained using the interaction of a polarized molecule with its instantaneous image dipole in a metal surface. This model explains why SERS is obtained mostly on noble metals (Au, Ag), since these metals usually have lower inherent contamination as compared with other more reactive metals; thus, molecules may be found closer to the metal surface. It is shown how stronger SERS amplifications may be obtained using nanostructured surfaces, once the excited molecules are localized in concave sites. The dependence on the fourth power of the incoming radiation electric field is obtained by taking into account the dynamics of adsorption–desorption processes of molecules. The SERS effect is maximal when the excitation frequency is red-shifted with respect to the bulk plasmon resonance. Also, the SERS amplification factor may be dictated by the polarizability of the investigated molecule, α, in a much more critical way than just a power law α2 or even α4. By comparing the dipole induced charge density with the amplitudes of plasma waves, the domain of validity of the present theory is derived to be in the low separation regime, where the distance between molecules and metal substrates is below a few nanometres. Some data from the literature are analyzed in the framework of this model, namely the distance, frequency and temperature dependence of the SERS signal, all confirming the validity of the model.
- This article is part of the themed collection: Surface-enhanced spectroscopies

 Please wait while we load your content...
Please wait while we load your content...