Spin-crossover in phenylazopyridine-functionalized Ni–porphyrin: trans–cis isomerization triggered by π–π interactions†
Gerard
Alcover-Fortuny
a,
Coen
de Graaf
*ab and
Rosa
Caballol
a
aDepartament de Química Física i Inorgànica, Universitat Rovira i Virgili, Marcel.lí Domingo s/n, 43007 Tarragona, Spain. E-mail: coen.degraaf@urv.cat
bInstitució Catalana de Recerca i Estudis Avançats (ICREA), Passeig Lluis Companys 23, 08010 Barcelona, Spain
First published on 3rd November 2014
Abstract
Reversible, room-temperature light-induced spin-crossover has been reported in a Ni–porphyrin functionalized with a phenylazopyridine (PAPy) ligand (Venkataramani et al., Science, 2011, 331, 445). Upon light irradiation (500 nm), the azopyridine moiety induces a change in the Ni(II) coordination sphere from square planar (n = 4) to square pyramid (n = 5), leading to a change in the total spin of the molecule from S = 0 to S = 1. The trans–cis isomerization in the azopyridine ligand has been proposed to trigger the spin-crossover effect. However, the radiation used to induce the HS state is about 135 nm red-shifted with respect to the radiation used for trans–cis isomerization of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond in other compounds. To elucidate the light-induced spin-crossover mechanism of this Ni(II) compound, a combined DFT/CASSCF/CASPT2 study has been performed to determine the most stable cis and trans conformers with n = 4 or n = 5, and to characterize the excitation that triggers the SCO process. π–π interactions between porphyrin and PAPy are shown to play an essential role in the spin crossover.
N double bond in other compounds. To elucidate the light-induced spin-crossover mechanism of this Ni(II) compound, a combined DFT/CASSCF/CASPT2 study has been performed to determine the most stable cis and trans conformers with n = 4 or n = 5, and to characterize the excitation that triggers the SCO process. π–π interactions between porphyrin and PAPy are shown to play an essential role in the spin crossover.
1 Introduction
Many first row transition metal complexes present fascinating magnetic properties due to the presence of unpaired electrons in the 3d-shell. Transition metal ions with d4 to d7 electronic configurations are highly interesting because high-spin and low-spin configurations can be very close in energy depending on the nature of the coordinating ligands. Changes in the population of these configurations induced by external perturbations can lead to magnetic bistability when spin crossover (SCO) is accompanied by geometrical rearrangements in the complex. Particularly interesting for technical purposes are materials with SCO at room temperature.1 These materials have attracted attention due to their potential application as sensors, optical switches, energy and information storage or energy transformation devices.2–5SCO can be induced by environmental changes, such as temperature, pressure, external magnetic fields or light. Among these possibilities, the latter variant is probably the most interesting one. The prototype SCO complex consists of a (quasi-)octahedral FeN6 core, where the nitrogen atom can be part of a monodentate or a multidentate ligand.6–8 At low temperatures, these complexes present a singlet ground state in most cases. The standard mechanism of light-induced SCO involves the excitation of an electron from the 3d orbitals of the Fe(II) ion into a higher lying orbital, for instance the π antibonding orbital of the coordinating ligand through a metal to ligand charge transfer (MLCT) excitation. This excitation is followed by a relaxation process through intersystem crossings and internal conversions to the metastable HS state.6,9–21 However, this is not the only way to induce SCO by light. The HS state can also be populated through photoisomerization reactions in the ligands (light-driven ligand-induced spin change, LD-LISC). When two isomers of a certain ligand exert a different ligand-field on the metal center, switching by light from one form to the other can invert the thermodynamic stability of the LS and HS states.22–24 A more robust variant of this mechanism is based on a photoinduced change of the coordination number of the metal center. Isomerization can change an n-dentate ligand into an (n + 1)-dentate ligand or vice versa,25,26 and modify the ligand field strength in such a way that a spin change is induced.
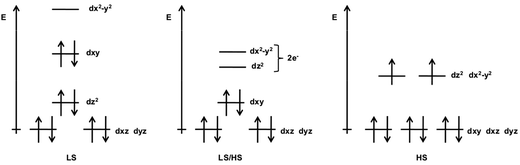
Ni(II) complexes do not belong to the traditional class of SCO compounds. The sixfold, quasi-octahedral coordination mode strongly favors an S = 1 spin coupling of the 3d8 configuration and complexes with square-planar (sqp) fourfold coordination usually have an S = 0 spin state. Square pyramidal (spy) pentacoordinated complexes can be found in both LS and HS states, but only recently a classical SCO system with a pentacoordinated Ni(II) has been identified.27 In square-planar Ni(II) n = 4 complexes, the absence of axial ligands stabilizes the 3dz2 orbital with respect to the 3dx2−y2 one. The 3dxy, 3dxz and 3dyz are also more stable than the 3dx2−y2 orbital, causing the pairing of all eight metal electrons and these complexes have a singlet ground state, as shown in Fig. 1 on the left. On the other extreme, (quasi-)octahedral n = 6 complexes have (nearly-)degenerate 3dz2 and 3dx2−y2 orbitals, favoring single occupancy and therefore a triplet ground state (S = 1), see Fig. 1 on the right.28 Square pyramidal n = 5 Ni(II) complexes can be either high-spin or low-spin depending on the nature of the axial ligand. In comparison to the square-planar coordination, the axial ligand stabilizes the 3dxy and 3dx2−y2 orbitals with respect to the orbitals with a z-component. Depending on the gap between the 3dz2 and 3dx2−y2 orbitals, the electrons occupy the 3dz2 orbital to form a S = 0 state or both orbitals are singly occupied leading to an S = 1 state, see Fig. 1 middle.
Transition metal (TM) porphyrins are planar structures that can easily incorporate axial ligands and therefore are good candidates for LD-LISC processes guided by changes in the coordination of the TM. Recently, room-temperature SCO has been reported for Ni–tetrakis(pentafluorophenyl)porphyrin (NiTPP) functionalized with a phenylazopyridine (PAPy) arm,26 see Fig. 2. Under standard conditions, the NiTPP–PAPy complex is low-spin (S = 0) and NMR experiments, among other characterization techniques, indicate a tetracoordinated Ni(II) in a square planar geometry with a trans configuration of the PAPy arm. Upon irradiation at 500 nm, the compound becomes paramagnetic and rearranges to a pentacoordinated complex where the pyridine moiety coordinates axially to Ni(II), which becomes high-spin. The SCO process in this Ni–porphyrin is reversible upon irradiation at 435 nm, while the thermal back reaction is very slow.
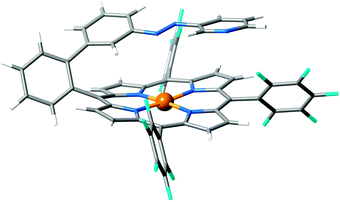 | ||
| Fig. 2 Ni–tetrakis(pentafluorophenyl)porphyrin with a phenylazopyridine functionalized axial arm (NiTPP–PAPy). | ||
The authors interpret that the SCO process is triggered by the trans–cis isomerisation of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the PAPy arm. Irradiation at 500 nm induces a π–π* excitation on the azo group and the PAPy arm evolves to the cis isomer. This orients the non-bonding electron pair of pyridine nitrogen perpendicularly to the Ni–porphyrin plane, making possible the coordination of the arm to Ni. The assignment of the paramagnetic product as a pentacoordinated Ni ion with the PAPy arm in the cis form was based on NMR Overhauser experiments, indicating that the Ni–H distance of the two hydrogens adjacent to the nitrogen of the pyridine ring is identical for both H atoms and significantly smaller than in the initial complex. The pentacoordination of the Ni(II) ion induces a spin change to S = 1, further stabilized by the electron withdrawing pentafluorophenyl groups attached to the porphyrin ring that lower the energy of the 3dx2−y2 orbital and decrease the gap with the 3dz2 orbital (see Fig. 1, middle).28 Upon irradiation with visible light (435 nm) or by heating the sample, the PAPy arm recovers the initial trans conformation and the pyridine group dissociates from Ni(II), which turns back to its initial low-spin configuration.
N bond of the PAPy arm. Irradiation at 500 nm induces a π–π* excitation on the azo group and the PAPy arm evolves to the cis isomer. This orients the non-bonding electron pair of pyridine nitrogen perpendicularly to the Ni–porphyrin plane, making possible the coordination of the arm to Ni. The assignment of the paramagnetic product as a pentacoordinated Ni ion with the PAPy arm in the cis form was based on NMR Overhauser experiments, indicating that the Ni–H distance of the two hydrogens adjacent to the nitrogen of the pyridine ring is identical for both H atoms and significantly smaller than in the initial complex. The pentacoordination of the Ni(II) ion induces a spin change to S = 1, further stabilized by the electron withdrawing pentafluorophenyl groups attached to the porphyrin ring that lower the energy of the 3dx2−y2 orbital and decrease the gap with the 3dz2 orbital (see Fig. 1, middle).28 Upon irradiation with visible light (435 nm) or by heating the sample, the PAPy arm recovers the initial trans conformation and the pyridine group dissociates from Ni(II), which turns back to its initial low-spin configuration.
However, this description of the LD-LISC leaves a few points open to discussion. The most important one is the fact that the irradiation used to induce the HS state is significantly red-shifted (∼135 nm) with respect to the wavelength normally used for cis–trans isomerization of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds. In fact, the 500 nm irradiation used in the experiments is typical for the Q band absorption of porphyrins. A second point is related to the indirect characterization of the cis isomer of the arm in the paramagnetic product, which is based on measurements on the diamagnetic Zn-analogue of the NiTPP–PAPy complex. Furthermore, the PBE functional used in the density functional theory (DFT) geometry optimizations may not be the most appropriate choice given the large π-systems of the porphyrin ring and the PAPy arm. The interaction between π-systems is in general not accurately described with standard GGA functionals.
N double bonds. In fact, the 500 nm irradiation used in the experiments is typical for the Q band absorption of porphyrins. A second point is related to the indirect characterization of the cis isomer of the arm in the paramagnetic product, which is based on measurements on the diamagnetic Zn-analogue of the NiTPP–PAPy complex. Furthermore, the PBE functional used in the density functional theory (DFT) geometry optimizations may not be the most appropriate choice given the large π-systems of the porphyrin ring and the PAPy arm. The interaction between π-systems is in general not accurately described with standard GGA functionals.
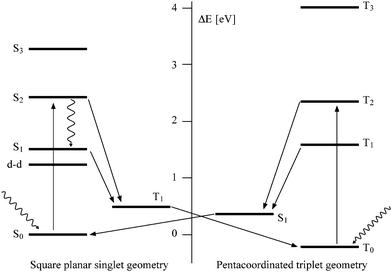
Light induced SCO in simpler Ni(II) porphyrins in basic solvents such as pyridine has been observed before. The mechanism is well established and goes through the 500 nm excitation in the Q band from the LS ground state (S0) to an excited singlet state (S2). This transition involves π and π* orbitals of the porphyrin system. The S2 state relaxes via an intersystem crossing directly to T1 or via S1 through internal conversion followed by an intersystem crossing as schematically depicted in Fig. 3. A solvent molecule is then axially coordinated to Ni(II) and the pentacoordinated species relaxes to the T0 state.29–32
The aim of the present work is to gain more insight into the details of the LD-LISC mechanism of NiTPP–PAPy. We clarify the origin of the 500 nm irradiation that triggers the SCO and discuss the geometries and relative stabilities of several cis and trans isomers of the complex in LS and HS configurations. For this purpose, we apply DFT/M06-2X calculations to optimize the geometries combined with CASPT2 calculations for accurate energetics.
2 Computational information
Dispersion forces are poorly described with standard DFT schemes. Since the π–π interactions between porphyrin and the PAPy arm can be determinant for the relative stabilities of the different confomers, we compare standard BP8633,34 results with those obtained with the dispersion corrected functional M06-2X.35 The DFT calculations are performed with the Gaussian09 package.36 A triple zeta valence + polarization (TZVP)37 basis set was used for the Ni(II) ion and basis sets of split-valence + polarization (SVP) quality38 for the rest of the atoms. The optimized geometries were characterized as minima on the potential energy surface by frequency calculations. Solvent effects were included by means of the COSMO model39,40 using the dielectric constant of dimethyl sulfoxide (ε = 46.7) to mimic the experimental conditions.To address the energetics of the ground and excited states in the different isomers, we apply multiconfigurational wave function based methods. Although computationally much more demanding, they provide accurate, well-balanced descriptions of ground and excited states of different spin multiplicity in TM complexes. Among the different options, we opt for a standard strategy consisting of the complete active space self-consistent field (CASSCF) approach followed by a second-order perturbation (CASPT2) treatment of the remaining electron correlation effects. Calculations are performed with the Molcas 7 package.41,42 A Cholesky decomposition of the two-electron integrals is used to reduce the computational cost. The basis sets centered on the different atoms are of the atomic natural orbitals type (ANO-RCC).43–45 Apart from being advantageous to recover the maximum of the correlation energy, these basis sets are also optimized for scalar relativistic effects and core correlation. The most relevant atoms in the NiTPP–PAPy and Ni–porphyrin systems (Ni and N) are described with a TZVP basis set, while the more peripheral atoms (C, F and H) have a basis set of double-zeta quality. In the PAPy arm, the basis set for carbon atoms is also of TZVP quality.
The size of the active space to construct the reference CASSCF wave function for CASPT2 is critical to obtain reliable results. The standard active space in the CASSCF calculations on the complexes with Ni (NiTPP–PAPy, and Ni–porphyrin + pyridine) contains 10 electrons distributed in all possible ways over eleven orbitals, [10,11]-CASSCF. Ten of the active orbitals are localized on Ni. From these, five orbitals show mainly contributions from the Ni-3d basis functions and the other five are built from more diffuse d-functions. This double d-shell is essential to account for the large correlation effects in the crowded Ni-d shell. The remaining active orbital is essentially localized on the N-atoms of the porphyrin that coordinate to Ni, describing the lone-pairs interacting with the electrons in the 3dx2−y2 orbital of Ni. This active space suffices to calculate the relative stability of the fundamental singlet and triplet states in the tetra- and pentacoordinated species. For the study of the vertical excitation energies in NiTPP–PAPy a [8,10]-CASSCF wave function is considered. The nature of the active orbitals will be specified in the presentation of the results. CASSCF calculations on the PAPy arm were done with an active space of 12 electrons and 9 orbitals (π, π*, and N lone pair orbitals), further details on the shape of the orbitals will be given when discussing the vertical spectrum. The absorption spectrum of Ni–porphyrin was calculated with an active space of 14 electrons and 16 orbitals.
3 Results and discussion
3.1 Conformational analysis
To rationalize the SCO process, Venkataramani et al.26 performed DFT calculations on a series of potentially suitable molecular structures. They used the PBE functional46,47 and found several minima for cis and trans tetra-coordinated and pentacoordinated NiTPP–PAPy. For this last coordination only a stable structure was found with the cis configuration of the PAPy arm. As explained in the computational information, the present work compares the optimization process by means of the BP86 functional, which is supposed to give similar results to PBE, to those obtained with dispersion corrected functionals. In this way we can access the importance of the π–π interactions and locate the minimum structure of the pentacoordinated complex with the PAPy arm in trans.Table 1 reports M06-2X relative energies and the nickel–nitrogen distance, d(Ni–Npyridine), of the different minima found for both cis and trans PAPy arm configurations. The BP86 optimized metal–nitrogen distance and relative energies are also reported for comparison. All sqp structures have a singlet ground state and the spy complexes are triplet. The M06-2X optimized structures are represented in Fig. 4. The trans-sqp conformer is the most stable singlet structure, and the pentacoordinated cis-spy S = 1 structure is the absolute minimum of the whole set. As a general feature, the axial coordination slightly enlarges the distance between nickel and the porphyrin–nitrogens, from 1.99 to 2.06 Å. This lengthening of the bond can be easily related to the single occupancy of the 3dx2−y2 orbital (of Ni–N antibonding character) in the HS state, being empty in the LS state. Geometry optimizations of trans-sqp and cis-spy with a larger basis set (TZVP on all atoms) and with the B97D functional (Grimme dispersion corrected) give practically the same results; most distances and angles do not differ by more than 0.1 Å and 5 degrees in both cases. The largest change is observed in the Ni–Npyridine distance in trans-sqp. The 4.36 Å predicted by the M06-2X functional changes to 4.21 Å with B97D. This 0.15 Å reduction reflects the relatively flat potential of the PAPy arm when it is not coordinated to the Ni atom.
| Configuration | Ni–Npyridine distance (Å) | Relative energy (eV) | ||
|---|---|---|---|---|
| BP86 | M06-2X | BP86 | M06-2X | |
| trans-sqp | 6.99 | 4.36 | −0.81 | 0.42 |
| trans-sqp open | 12.34 | 11.52 | −0.81 | 0.72 |
| cis-sqp | 4.30 | 2.62 | −0.25 | 0.91 |
| cis-sqp open | 9.44 | 9.39 | −0.23 | 1.32 |
| trans-spy | 2.08 | 2.17 | 0.15 | 0.28 |
| cis-spy | 2.08 | 2.16 | 0.00 | 0.00 |
 | ||
| Fig. 4 M06-2X geometries of the NiTPP–PAPy conformers listed in Table 1. | ||
The role of dispersion forces is particularly noticeable in the tetracoordinated trans-sqp minimum, where the pyridine ring is parallel to the porphyrin unit and the π–π interaction is important. The distance between Ni and the N of the pyridine is shortened by 2.6 Å when the dispersion is included in the calculation. The influence of long-range interactions is also evident in the cis-sqp conformer, where the relatively short Ni–Npyridine distance suggests an incipient pentacoordination, albeit with a singlet ground state. In this case the nitrogen lone pair is not conveniently oriented to Ni(II), since the angle between the pyridine and porphyrin planes is 79°. Hence, the gap between the 3dz2 and 3dx2−y2 orbitals stays large and the singlet remains the ground state.
However, the most important consequence of including long-range interactions in the calculations of the present system is the strong stabilization of the trans-spy triplet. BP86 predicts a very high energy (0.96 eV) for this pentacoordinated structure with respect to the tetracoordinated trans conformers, while M06-2X predicts it to be 0.14 eV more stable than trans-sqp. Besides being close in energy, the square pyramid cis and trans isomers have also similar Ni–Npyridine distances, 2.16–2.17 Å, compatible with the experimentally estimated value of 2.1 Å. Recalling that the cis configuration of the product was assigned from Nuclear Overhauser experiments on a diamagnetic Zn analog, we conclude that the trans structure cannot be excluded yet as a possible candidate of the pentacoordinated paramagnetic product and that alternative mechanisms not involving azopyridine trans–cis isomerization as the key step of the SCO process should not be completely discarded.
Finally, we mention that the differences between the geometry optimizations in a vacuum and those with solvent effects through the PCM model lead to virtually the same results. Neither the energies, nor the geometrical parameters show any significant changes when the complex is treated in a DSMO solvent. All the results reported below are obtained in a vacuum.
3.2 Axial coordination in cis and trans NiTPP–PAPy
The standard active space for an accurate determination of the relative stability of different spin states in (quasi-)octahedral TM-3dn (n ≥ 5) complexes consists of (n + 4)-electrons and 12 orbitals.48 In addition to the TM-3d orbitals and the extra set of d-orbitals to account for the double shell effect, one should also include the two ligand-σ orbitals of eg-like symmetry in the active space. However, in the present case this choice necessarily leads to an unbalanced description of the four- and five-coordinated species. The in-plane σ-orbital of the Nporph atoms is easily included in the active space for both species, but the second σ orbital corresponding to the axial ligand can only be included in the five-coordinated complexes. The corresponding orbital in the four-coordinated species is the lone pair orbital of the N atom on the pyridine ring, which in itself contributes very little to the electron correlation, and hence, does not stay in the active space. Therefore, we calculate the relative stability of the singlet and triplet state of the different NiTPP–PAPy species with a [10-11]-CASSCF wave function. The stability of the results was checked by comparing the singlet–triplet splitting of different five-coordinated species to the results obtained with the [12,12]-CASSCF calculations. The shape of the active orbitals is given in the ESI.† CASSCF single point calculations were performed using the DFT optimized structures of most stable conformers: trans-sqp, cis-spy and trans-spy.The relative energies listed in Table 2 indicate that both CASSCF and CASPT2 correctly predict a triplet ground state for the pentacoordinated confomers. However, CASSCF fails to establish a singlet ground state for the four coordinated sqp complex, which is repaired by including dynamic correlation through CASPT2. The results with the [12,12] active space are only slightly different to those obtained with the smaller active space. Interestingly enough, CASPT2 places the triplet of the trans-spy isomer energetically close to the initial singlet state of the square planar conformation, which does not discard the possibility of a SCO process without trans–cis isomerization.
| Spin | [10,11] | [12,12] | ||
|---|---|---|---|---|
| CASSCF | CASPT2 | CASSCF | ||
| trans-sqp | Singlet | 0.94 | 0.26 | — |
| Triplet | 0.70 | 0.39 | — | |
| cis-spy | Singlet | 1.34 | 1.07 | 1.39 |
| Triplet | 0.00 | 0.00 | 0.00 | |
| trans-spy | Singlet | 1.67 | 1.32 | 1.73 |
| Triplet | 0.36 | 0.29 | 0.36 | |
To further settle the question about the mechanism, we have calculated triplet energies along the dissociation path of the PAPy arm from the Ni ion. This provides information about the thermal stability of the light induced pentacoordinated product in the cis and trans forms. The points along the scan were generated with M06-2X geometry optimizations fixing the Ni–Npy distances at different values. As shown in Fig. 5, the release of the pyridine group of the PAPy arm in the trans-spy form is nearly barrierless, while a steep rise in the energy is observed when the Ni–N distance is increased in the cis-spy isomer. The energy along this path reaches a maximum at d(Ni–Nazo) = 3.3 Å, where it is 0.84 eV higher than the triplet of the pentacoordinated cis isomer. This means that a hypothetical triplet pentacoordinated NiTPP–PAPy in trans form will release the axial ligand very rapidly at room temperature and that this process is slow for the cis form. Finally, we mention that the relative stability of the singlet trans-sqp and the triplet cis-spy states is opposite to what may be expected. This can at least partially be ascribed to the limited basis set that we use in our calculation. It is well-known that larger basis sets (especially on the metal) are needed to obtain correct high-spin/low-spin energy differences in spin crossover processes.48–50 However, such lowering of the singlet state(s) will not affect any of the arguments discussed above, and hence, the only plausible mechanism to explain the LD-LISC in this compound is via trans–cis isomerization. However, note that the 500 nm irradiation does not directly cause a πazo → πazo* transition in PAPy and similar systems and the nature of the excitation causing the isomerization needs further clarification.
3.3 Vertical excitation energies of PAPy
In order to locate the πazo → πazo* excitation in the absorption spectrum and to characterize the 500 nm band of the NiTPP–PAPy complex, we first calculated the vertical excitation spectrum of the PAPy arm. The minimal active space to correctly study the vertical excitation spectrum of the PAPy arm should contain the π and π* orbitals of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and the lone pair orbitals (nazo) of the nitrogen atoms, extended with two pairs of π and π* orbitals of the phenyl and pyridine side groups (πside and πside*), and the lone pair orbital of the pyridine nitrogen. This active space with 12 electrons and 9 orbitals was used to generate the reference wave function for the subsequent CASPT2 calculations. Active orbitals are graphically represented in the ESI,† and the results are summarized in Table 3.
N bond and the lone pair orbitals (nazo) of the nitrogen atoms, extended with two pairs of π and π* orbitals of the phenyl and pyridine side groups (πside and πside*), and the lone pair orbital of the pyridine nitrogen. This active space with 12 electrons and 9 orbitals was used to generate the reference wave function for the subsequent CASPT2 calculations. Active orbitals are graphically represented in the ESI,† and the results are summarized in Table 3.
| trans 3-PAPy | CASSCF | CASPT2 | Oscillator strength | Exp.51 |
|---|---|---|---|---|
| nazo → πazo* | 3.15 | 2.75 (450 nm) | 6.22 × 10−8 | 2.76 |
| πside → πazo* | 5.96 | 3.48 (356 nm) | 0.32 | 3.91 |
| πazo → πazo* | 7.41 | 6.99 (177 nm) | 0.28 |
The effect of electron correlation on the excitation energies is large, especially for the πside → πazo* transitions. The very high CASSCF energy of these states (∼6 eV) is lowered by approximately 2.5 eV by the CASPT2 treatment of the electron correlation. The CASPT2 transition energies are in good agreement with the experimental values for all states. The most important features of the excitation spectrum are a low-lying nazo → πazo* excitation around 2.75 eV (450 nm) and a high intensity πside → πazo* transition appearing at 3.48 eV (356 nm). The lowest CASSCF root that involves πazo and πazo* orbitals is located at an energy of 7.41 eV and CASPT2 lowers the transition energy to 6.99 eV (177 nm), much higher in energy than the reported 500 nm wavelength to induce SCO.
3.4 Vertical excitation energies of NiTPP–PAPy
An important question that remains to be answered is the nature of the 500 nm excitation used to induce the SCO in the NiTPP–PAPy complex. The CASPT2 results of the PAPy subsystem place the π → π* excitation at higher energies, in concordance with the findings in other azobenzene derivatives, but the interaction of the PAPy arm with the porphyrin system may shift the π → π* transition to lower energies as suggested by Venkataramani.26 Hence, to obtain a complete description of the optical transitions of the NiTPP–PAPy system, the π system of the porphyrin ring should also be considered. The most basic description of the optical absorption of porphyrin systems involves at least four orbitals as established by numerous studies that go back to the original four-orbital model of Gouterman.52–54 Adding these four orbitals (πporph) to the lone pair orbitals (nazo), πazo and πazo* of the PAPy arm gives a [10,8]-CASSCF wave function. The shape of the 8 active orbitals is given in the ESI.† This active space does not describe any excited state involving the electrons in the Ni-3d orbitals, but the metal-centered excitations have a low oscillator strength and the charge transfer excitations appear at higher energy. Whereas the inclusion of the Ni-3d and 3d′ orbitals is essential to obtain accurate estimates of the relative stability of the different species, this is rather unimportant for the vertical spin allowed excitations from the closed shell n = 4 ground state.
Table 4 lists the lowest calculated transition energies and the corresponding oscillator strengths. The states are labeled according to the most important electron replacements involved in the excitation. The lowest two transitions have intermediate oscillator strength and can be associated with the Q bands observed at around 523 nm in the experimental work. The π orbitals involved in these transitions are well localized on the porphyrin ring, but as is shown in Fig. 6 the π* orbitals have a non-negligible contribution from the antibonding combination of the N-2p orbitals of the azo bond of the PAPy arm. Hence, the Q band is not simply a porphyrin centered excitation but also causes a certain degree of occupation of the πazo* orbital. This weakens the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and could very well trigger trans–cis isomerization. It is interesting to compare these orbitals with those of the trans-sqp optimized geometry obtained with the BP86 functional (see ESI†). The PAPy arm is more separated from the porphyrin ring in this geometry and the orbitals are either pure πporph* or πazo*, without any mixing. This suggests a determinant role of the π–π interactions in this SCO mechanism, revealed by the M06-2X functional. The important shortening of the porphyrin–pyridine distance (Table 1) induces the delocalization and the subsequent possibility of populating the π* orbital at lower energy.
N bond and could very well trigger trans–cis isomerization. It is interesting to compare these orbitals with those of the trans-sqp optimized geometry obtained with the BP86 functional (see ESI†). The PAPy arm is more separated from the porphyrin ring in this geometry and the orbitals are either pure πporph* or πazo*, without any mixing. This suggests a determinant role of the π–π interactions in this SCO mechanism, revealed by the M06-2X functional. The important shortening of the porphyrin–pyridine distance (Table 1) induces the delocalization and the subsequent possibility of populating the π* orbital at lower energy.
| trans NiTPP–PAPy | CASSCF | CASPT2 | Oscillator strength | Exp.26 |
|---|---|---|---|---|
| πporph → πazo+porph* | 3.55 | 2.59 (478 nm) | 3.70 × 10−2 | 523 |
| πporph → πazo+porph* | 3.57 | 2.65 (467 nm) | 5.60 × 10−2 | 523 |
| nazo → πazo* | 3.60 | 3.23 (383 nm) | 9.00 × 10−6 | 406 |
| nazo → πazo*/πporph → πazo* | 5.29 | 4.57 (271 nm) | 5.90 × 10−4 | 322 |
 | ||
| Fig. 6 Graphical representation of the orbital that is occupied in the lowest two vertical excitations of NiTPP–PAPy. | ||
Severe intruder state problems hinder the accurate description of the πazo → πazo* excitation in the large complex. However, the similarity of the SA-CASSCF excitation energy (7.25 eV) to the one obtained for the PAPy arm (see Section 3.3) situates the πazo → πazo* excitation in NiTPP–PAPy at significant higher energy than 2.5 eV (500 nm) used in the experiments to induce the SCO.
While the Q band is well reproduced by our calculations, the experimental absorption spectrum of NiTPP–PAPy shows a Soret band of high intensity at 406 nm. At this region a nazo → πazo* excitation is found, but the low oscillator strength cannot explain the experimentally observed intense peak. Probably, this low intensity band is hidden by the optically allowed πporph → πporph* excitations of the porphyrin moiety which cannot be reproduced with the present [10,8]-CAS.55
3.5 Vertical excitation energies of Ni–porphyrin
Previous studies show that for an accurate description of the absorption spectrum of porphyrin and its derivatives, the basic Gouterman model is not good enough, and more orbitals have to be included in the active space of the CASSCF calculations. The “minimal” active space for correctly addressing the vertical spectrum of porphyrins consists of 8 π and 6 π* orbitals. Hence, a complete account of the vertical absorption spectrum of NiTPP–PAPy requires a [20,18]-CAS (14 porphyrin orbitals, π, π* and two lone pair orbitals of the azo-group on the PAPy arm). This is clearly beyond the limits of CASSCF and the spectrum has to be calculated by parts. On the one hand, the 8 orbital and 10 electron active space used in Section 3.4 allows us to calculate the Q band. On the other hand, an active space consisting of 8 π and 6 π* orbitals of the porphyrin is used to characterize the Soret band, which is expected to be a πporph → πporph* excitation.55 A [16,14]-CASPT2 is cumbersome for NiTPP–PAPy, and therefore, this calculation has been carried out on the Ni–porphyrin system (see Fig. 7). Ni–porphyrin presents D2h point group symmetry. Two pairs of π–π* orbitals with b2g and b3g characters, a pair of π–π* orbitals with au character and a set of 3 π and 1 π* orbitals with b1u character are taken to form the 14 orbitals active space. The shape of the active orbitals is shown in the ESI.†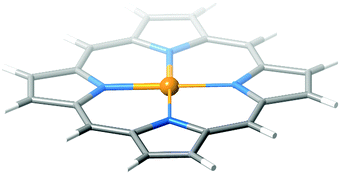 | ||
| Fig. 7 Ni–porphyrin model used to study the contribution of the porphyrin moiety to the absorption spectrum of NiTPP–PAPy. | ||
Table 5 lists the four lowest π → π* excitations of the Ni–porphyrin system. The most intense band is due to the transition to the second 1B2u state and appears at 335 nm. The deviation with respect to the experimental Soret band of NiTPP–PAPy (406 nm) can be attributed to the geometrical differences between the whole system and the Ni–porphyrin model. Furthermore, the calculated oscillator strength for this excitation shows that this band is of high intensity compared to the bands obtained in Section 3.4. One can conclude that for the NiTPP–PAPy spectrum, the Q band corresponds to πporph → πazo+porph* and the Soret band to πporph → πporph* excitations not relevant for the SCO mechanism.
| State | CASSCF | CASPT2 | Oscillator strength |
|---|---|---|---|
| 1B2u | 3.72 | 2.66 (465 nm) | 1.45 × 10−2 |
| 1B3u | 3.09 | 2.73 (453 nm) | 1.58 × 10−2 |
| 1B2u | 5.54 | 3.70 (335 nm) | 1.18 |
| 1B3u | 4.42 | 4.08 (303 nm) | 0.22 |
4 Summary and conclusion
Although the original proposal of trans–cis isomerization of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond induced by a π → π* excitation offers a plausible explanation for reversible room-temperature spin crossover observed in NiTPP–PAPy, the large red-shift of 150 nm of the excitation energy leaves some room for improvement. SCO in standard Ni–porphyrins is induced by a π–π* excitation of 2.5 eV (500 nm) on the porphyrin ring. The same wave length was used in the experiments on NiTPP–PAPy and therefore we first investigated the possibility of SCO induced by a π–π* excitation on the porphyrin ring without the need for trans–cis isomerization. The inclusion of the effect of dispersion forces in the geometry optimization using the M06-2X functional leads to cis and trans isomers with four- and five-coordinated Ni ions in a relatively small energy window. Single point CASPT2 calculations on the optimized geometries predicts a near degeneracy for the low-spin state of the four-coordinated complex and the high-spin state of the five-coordinated complex with the PAPy arm in trans conformation. However, the absence of a barrier along the dissociation path of the PAPy arm in a hypothetical all-trans mechanism is incompatible with the experimental stability of the high-spin state. In contrast, a significant barrier was found for the dissociation of the PAPy-arm in the cis conformation.
N double bond induced by a π → π* excitation offers a plausible explanation for reversible room-temperature spin crossover observed in NiTPP–PAPy, the large red-shift of 150 nm of the excitation energy leaves some room for improvement. SCO in standard Ni–porphyrins is induced by a π–π* excitation of 2.5 eV (500 nm) on the porphyrin ring. The same wave length was used in the experiments on NiTPP–PAPy and therefore we first investigated the possibility of SCO induced by a π–π* excitation on the porphyrin ring without the need for trans–cis isomerization. The inclusion of the effect of dispersion forces in the geometry optimization using the M06-2X functional leads to cis and trans isomers with four- and five-coordinated Ni ions in a relatively small energy window. Single point CASPT2 calculations on the optimized geometries predicts a near degeneracy for the low-spin state of the four-coordinated complex and the high-spin state of the five-coordinated complex with the PAPy arm in trans conformation. However, the absence of a barrier along the dissociation path of the PAPy arm in a hypothetical all-trans mechanism is incompatible with the experimental stability of the high-spin state. In contrast, a significant barrier was found for the dissociation of the PAPy-arm in the cis conformation.
Subsequently, we used CASSCF/CASPT2 calculations to clarify the character of the excitation that induces the trans–cis isomerization at 500 nm. The π–π* excitation on the azo group was found at very high energy and the excitation from the π orbitals of the six-membered rings bonded to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group into the N
N group into the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N π* orbital occurs at 350 nm, also too high to be assigned to the experimental excitation. The CASPT2 results identify the 500 nm transition as an excitation involving a bonding π orbital of the porphyrin ring and an antibonding π* orbital with important contributions from both the porphyrin system and the N
N π* orbital occurs at 350 nm, also too high to be assigned to the experimental excitation. The CASPT2 results identify the 500 nm transition as an excitation involving a bonding π orbital of the porphyrin ring and an antibonding π* orbital with important contributions from both the porphyrin system and the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group. The occupation of this anti-bonding orbital of mixed character weakens the N
N group. The occupation of this anti-bonding orbital of mixed character weakens the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and leads to trans–cis isomerization.
N bond and leads to trans–cis isomerization.
Hence, the combination of DFT and multiconfigurational wave function calculations is able to firmly establish the trans–cis isomerism in the PAPy arm as a mechanism for the spin crossover in NiTPP–PAPy. The structures obtained with DFT/M06-2X show that the arm is closer to the porphyrin ring than in the structure without taking into account the dispersion. The character of the excitation that triggers the spin crossover is identified and involves the electron replacement from a bonding π orbital on the porphyrin to an antibonding orbital with contributions to the porphyrin and the azo group.
Acknowledgements
Financial support has been provided by the Spanish Administration (Project CTQ2011-23140), the Generalitat de Catalunya (Projects 2014SGR199 and Xarxa d'R+D+I en Química Teòrica i Computacional, XRQTC) and the European Union (COST Actions CODECS CM1002 and ECOSTBio CM1305).References
- A. Bleuzen, V. Marvaud, C. Mathonière, B. Sieklucka and M. Verdaguer, Inorg. Chem., 2009, 48, 3453 CrossRef CAS PubMed.
- O. Sato, J. Photochem. Photobiol., C, 2004, 5, 203 CrossRef CAS PubMed.
- J.-F. Létard, P. Guionneau and L. Goux-Capes, Spin Crossover in Transition Metal Compounds III, Springer-Verlag, Berlin, 2004, vol. 235, p. 221 Search PubMed.
- S. M. Aldoshin, J. Photochem. Photobiol., A, 2008, 200, 19 CrossRef CAS PubMed.
- K. Szaciłowski, Chem. Rev., 2008, 108, 3481 CrossRef PubMed.
- P. Gütlich, Y. Garcia and H. A. Goodwin, Chem. Soc. Rev., 2000, 29, 419 RSC.
- J.-F. Létard, P. Guionneau, O. Nguyen, J. Sánchez Costa, S. Marcén, G. Chastanet, M. Marchivie and L. Goux-Capes, Chem. – Eur. J., 2005, 11, 4582 CrossRef PubMed.
- A. Hauser, C. Enachescu, L. M. Lawson Daku, A. Vargas and N. Amstutz, Coord. Chem. Rev., 2006, 250, 1642 CrossRef CAS PubMed.
- J. J. McGarvey and I. Lawthers, J. Chem. Soc., Chem. Commun., 1982, 906 RSC.
- S. Decurtins, P. Gütlich, C. P. Köhler, H. Spiering and A. Hauser, Chem. Phys. Lett., 1984, 105, 1 CrossRef CAS.
- A. Hauser, J. Chem. Phys., 1991, 94, 2741 CrossRef CAS PubMed.
- J. K. McCusker, K. N. Walda, R. C. Dunn, J. D. Simon, D. Magde and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 298 CrossRef CAS.
- P. Gütlich, A. Hauser and H. Spiering, Angew. Chem., Int. Ed. Engl., 1994, 33, 2024 CrossRef.
- A. Hauser, Spin Crossover in Transition Metal Compounds I, Springer-Verlag, Berlin, 2004, vol. 233, p. 49 Search PubMed.
- H. Ando, Y. Nakao, H. Sato and S. Sakaki, J. Phys. Chem. A, 2007, 111, 5515 CrossRef CAS PubMed.
- A. L. Smeigh, M. Creelman, R. A. Mathies and J. K. McCusker, J. Am. Chem. Soc., 2008, 130, 14105 CrossRef CAS PubMed.
- A. Cannizzo, C. J. Milne, C. Consani, W. Gawelda, C. Bressler, F. van Mourik and M. Chergui, Coord. Chem. Rev., 2010, 254, 2677 CrossRef CAS PubMed.
- M. Chergui, Dalton Trans., 2012, 41, 13022 RSC.
- N. Huse, T. K. Kim, L. Jamula, J. K. McCusker, F. M. F. de Groot and R. W. Schoenlein, J. Am. Chem. Soc., 2010, 132, 6809 CrossRef CAS PubMed.
- C. de Graaf and C. Sousa, Chem. – Eur. J., 2010, 16, 4550 CrossRef CAS PubMed.
- C. Sousa, C. de Graaf, A. Rudavskyi, R. Broer, J. Tatchen, M. Etinski and C. M. Marian, Chem. – Eur. J., 2013, 19, 17541 CrossRef CAS PubMed.
- C. Roux, J. Zarembowitch, B. Gallois, T. Granier and R. Claude, Inorg. Chem., 1994, 33, 2273 CrossRef CAS.
- J. S. Kolb, M. D. Thomson, M. Novosel, K. Sénéchal-David, E. Rivière, M.-L. Boillot and H. G. Roskos, C. R. Chim., 2007, 10, 125 CrossRef CAS PubMed.
- M. L. Boillot, J. Zarembowitch and A. Sour, Spin Crossover in Transition Metal Compounds II, Springer-Verlag, Berlin, 2004, vol. 234, p. 261 Search PubMed.
- Y. Hasegawa, S. Kume and H. Nishihara, Dalton Trans., 2009, 280 RSC.
- S. Venkataramani, U. Jana, M. Dommaschk, F. D. Sönnichsen, F. Tuczek and R. Herges, Science, 2011, 331, 445 CrossRef CAS PubMed.
- H. Ma, J. L. Petersen, V. G. Young, Jr., G. T. Yee and M. P. Jensen, J. Am. Chem. Soc., 2011, 133, 5644 CrossRef CAS PubMed.
- S. Thies, H. Sell, C. Schütt, C. Bornholdt, C. Näther, F. Tuczek and R. Herges, J. Am. Chem. Soc., 2011, 133, 16243 CrossRef CAS PubMed.
- D. Kim, C. Kirmaier and D. Holten, Chem. Phys., 1983, 75, 305 CrossRef CAS.
- D. Kim and D. Holten, Chem. Phys. Lett., 1983, 98, 584 CrossRef CAS.
- J. Rodriguez and D. Holten, J. Chem. Phys., 1990, 92, 5944 CrossRef CAS PubMed.
- L. X. Chen, X. Zhang, E. C. Wasinger, K. Attenkofer, G. Jennings, A. Z. Muresan and J. S. Lindsey, J. Am. Chem. Soc., 2007, 129, 9616 CrossRef CAS PubMed.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822 CrossRef.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, 2009 Search PubMed.
- N. Godbout, D. R. Salahub, J. Andzelm and E. Wimmer, Can. J. Chem., 1992, 70, 560 CrossRef CAS.
- T. H. Dunning and P. J. Hay, Methods of Electronic Structure Theory, Plenum, New York, 1977, vol. 3, p. 1 Search PubMed.
- A. Klamt and G. Schüürmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799 RSC.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999 CrossRef CAS PubMed.
- G. Karlström, R. Lindh, P.-Å. Malmqvist, B. O. Roos, U. Ryde, V. Veryazov, P.-O. Widmark, M. Cossi, B. Schimmelpfennig, P. Neogrady and L. Seijo, Comput. Mater. Sci., 2003, 28, 222 CrossRef.
- F. Aquilante, L. De Vico, N. Ferré, G. Ghigo, P.-Å. Malmqvist, P. Neogrady, T. B. Pedersen, M. Pito
![[n with combining breve]](https://www.rsc.org/images/entities/char_006e_0306.gif) ák, M. Reiher, B. O. Roos, L. Serrano-Andrés, M. Urban, V. Veryazov and R. Lindh, J. Comput. Chem., 2010, 31, 224 CrossRef CAS PubMed.
ák, M. Reiher, B. O. Roos, L. Serrano-Andrés, M. Urban, V. Veryazov and R. Lindh, J. Comput. Chem., 2010, 31, 224 CrossRef CAS PubMed. - P.-O. Widmark, P.-Å. Malmqvist and B. O. Roos, Theor. Chim. Acta, 1990, 77, 291 CrossRef CAS.
- B. O. Roos, R. Lindh, P.-Å. Malmqvist, V. Veryazov and P.-O. Widmark, J. Phys. Chem. A, 2004, 108, 2851–2858 CrossRef CAS PubMed.
- B. O. Roos, R. Lindh, P.-A. Malmqvist, V. Veryazov and P.-O. Widmark, J. Phys. Chem. A, 2005, 109, 6575 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396 CrossRef CAS.
- K. Pierloot and S. Vancoillie, J. Chem. Phys., 2006, 125, 124303 CrossRef PubMed.
- B. Ordejón, C. de Graaf and C. Sousa, J. Am. Chem. Soc., 2008, 130, 13961–13968 CrossRef PubMed.
- M. Kepenekian, V. Robert, B. Le Guennic and C. de Graaf, J. Comput. Chem., 2009, 30, 2327–2333 CAS.
- E. V. Brown and G. R. Granneman, J. Am. Chem. Soc., 1975, 97, 621 CrossRef CAS.
- M. Gouterman, J. Chem. Phys., 1959, 30, 1139 CrossRef CAS PubMed.
- M. Gouterman, G. H. Wagnière and L. C. Snyder, J. Mol. Spectrosc., 1963, 11, 108 CrossRef CAS.
- C. Weiss, H. Kobayashi and M. Gouterman, J. Mol. Spectrosc., 1965, 16, 415 CrossRef CAS.
- L. Serrano-Andrés, M. Merchán, M. Rubio and B. O. Roos, Chem. Phys. Lett., 1998, 295, 195 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Coordinates and total energies of the M06-2X optimized geometries of the NiTPP–PAPy conformers used in this study. Graphical representations of the active orbitals of NiTPP–PAPy, PAPy and Ni–porphyrin. See DOI: 10.1039/c4cp04402a |
| This journal is © the Owner Societies 2015 |