Enhancement of hydrogen production using photoactive nanoparticles on a photochemically inert photonic macroporous support†
Robert
Mitchell
a,
Rik
Brydson
b and
Richard E.
Douthwaite
*a
aDepartment of Chemistry, University of York, Heslington, York, YO10 5DD, UK. E-mail: richard.douthwaite@york.ac.uk
bInstitute for Materials Research, School of Process, Environmental and Materials Engineering, University of Leeds, Leeds LS2 9JT, UK
First published on 10th November 2014
Abstract
The propagation of light in photonic materials can be modified to increase the probability of photon absorption. Here we report the synthesis of composite materials comprising a photochemically inert photonic macroporous ZrO2 support decorated with photocatalytically active CdS nanoparticles. The relative energies of valence and conduction bands restrict photon absorption and catalysis to the CdS nanoparticles. The generation of hydrogen from water under visible light illumination (>400 nm) has been studied as a function of the photonic support. A maximum 4.7 fold enhancement in hydrogen production is observed compared to a non-photonic support when the absorption band of the CdS nanoparticles partially overlaps with the blue edge of the photonic ZrO2 stop band. This general strategy supports the independent optimization of optical and photochemical processes to increase the overall conversion efficiency of solar to chemical energy.
Introduction
The conversion of solar to chemical energy could provide a significant contribution to future energy needs if a viable process can be developed.1–3 Many challenges remain, requiring the discovery of new materials and structures for light capture, charge separation and catalysis, and the integration of these phenomena into a functioning device.4,5 For light capture, the morphology of the absorbing material or structure supporting the light-active species is important to maximize absorption.63-Dimensional macroporous photonic structures exhibit potentially useful phenomena for increasing the efficiency of solar energy conversion.7,8 Their periodic structure results in an ordered modulation of the refractive index between the material and voids, causing the propagation of light to be modified for specific frequencies.9 Photonic materials are characterized by a stop band, which corresponds to frequencies that formally cannot propagate through the structure. The frequencies which respond to the photonic medium are determined by the composition and dimensions of the structure, providing the opportunity for predictable modification of the optical properties.9
In the context of solar energy conversion, macroporous photonic materials exhibit phenomena that have been shown to enhance the photon-to-electron conversion of photoelectrochemical devices at specific frequencies.10–16 For example, a macroporous film of TiO2 integrated into a dye-sensitized solar cell acts as a dielectric mirror to reflect light at the stop band and effectively increase the path length and probability of absorption.10,12 Enhancement can also result from a slowing of the photon group velocity at frequencies that overlap with the edges of the stop band, effectively increasing the path length.17 For example, a macroporous photoelectrode of WO3 exhibits over a 2-fold enhancement in photon-to-electron conversion at frequencies which overlap with the red edge of a stop band.13 With respect to photochemical reactions, photonic macroporous TiO2 has been shown to photocatalyze the degradation of an adsorbed dye monolayer.18,19 This work showed that materials with broad stop bands due to variations in periodicity can have practical application. Enhancement of photocatalytic hydrogen production from an aqueous methanol solution has also been demonstrated using platinum loaded macroporous TiO2 and a macroporous WO3 composite.20,21
The synthesis of photonic photocatalysts is challenging for all but the simplest binary oxides. Our motivation was to prepare photoactive nanocrystals incorporated into a photocatalytically inert macroporous photonic host material and use the composite to mediate a photochemical reaction (Fig. 1). This general strategy allows potentially any photoactive material to be incorporated into a photonic host and the loading to be controlled. By varying the periodicity of the photonic host, the optical stop band can be positioned predictably with respect to the absorption band of the photoactive nanocrystals. Predictable colocation of the photonic support stop band and photoactive nanocrystal absorption edge could lead to enhancement of, for example, hydrogen production from water under broad band visible light illumination, which is a key reaction for future solar fuel technology.
Experimental
Chemicals
Oleic acid (C18H34O2, 90%), styrene (C8H8, 99%), potassium persulfate (K2S2O8, 99%), titanium tetraisopropoxide (Ti(OC3H7)4, 97%), polyvinylpyrrolidone (average molecular weight 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 1-octadecene (C18H36, 90%), triethylamine (C6H15N, > 99%), zirconium acetate solution in dilute acetic acid (Zr[OCOCH3]4, 16% Zr), sodium sulfide nonahydrate (Na2S·9H2O, 98%), sodium sulfite (Na2SO3, 98%), tantalum oxide (Ta2O5, 99.99%), cadmium oxide (CdO, 99.99%) and sulphur (reagent grade) were purchased from Sigma Aldrich and used as received.
000), 1-octadecene (C18H36, 90%), triethylamine (C6H15N, > 99%), zirconium acetate solution in dilute acetic acid (Zr[OCOCH3]4, 16% Zr), sodium sulfide nonahydrate (Na2S·9H2O, 98%), sodium sulfite (Na2SO3, 98%), tantalum oxide (Ta2O5, 99.99%), cadmium oxide (CdO, 99.99%) and sulphur (reagent grade) were purchased from Sigma Aldrich and used as received.
Characterisation
SEM images were obtained using an FEI Sirion scanning electron microscope operating at 5–10 kV. Prior to imaging, samples were supported on an aluminium stub with an adhesive carbon tab and sputter coated with a 10 nm layer of carbon using an Agar auto carbon coater. Energy Dispersive Analysis of X-rays (EDX) was performed using an attached EDAX Phoenix X-ray spectrometer. TEM images were obtained using a JEOL 2011 transmission electron microscope operated at 200 kV accelerating voltage. TEM samples were ground, sonicated in methanol and a drop of the dispersion evaporated onto a holey carbon, copper grid. Electron diffraction and HAADF STEM imaging/EDX mapping were performed at the University of Leeds on a Tecnai TF20 FEGTEM. PXRD patterns were recorded on a Bruker-AXS D8 Advance instrument with Lynx eye detector, using Cu Kα radiation, scanning in the range 5–70° (2θ), with a 0.02° step size, and each data point collected for 0.05 or 0.1 s. Diffuse reflectance UV spectra were recorded between two glass slides on an Ocean Optics HR2000 + High Resolution Spectrometer with DH-2000-BAL Deuterium/Helium light source (200–1100 nm). TGA analysis of OA-ZrO2 samples was performed using a Rheometric Scientific STA-625 instrument. The weight loss of a sample was measured on heating to 600 °C at 10 °C min−1 under N2 atmosphere. Nitrogen adsorption porosimetry was performed on a Micromeritics Tristar 3000 surface analyser. The sample was degassed under N2 at 150 °C for 6 h to remove all adsorbed water prior to measurement. The volume of nitrogen adsorbed at 77.4 K per gram of material was measured as a function of the relative pressure P/P0 within the system. Surface area measurements were calculated from data points in the 0.05 to 0.3 P/P0 region using the Brunauer Emmett Teller (BET) adsorption isotherm.22Synthesis procedures
Polystyrene sphere templates were prepared using a modified literature procedure.†Photocatalysis
The apparatus used for photoreaction and analysis of products has been described previously (Fig. S6a, ESI†).26 For 1-CdS–5-CdS, reactions were performed in water (20 mL) containing Na2S (0.25 M) and Na2SO3 (0.35 M) and 30 mg of the photocatalyst. The apparatus was maintained at a temperature of 40 °C. For 1-TiO2–5-TiO2, reactions were performed in aqueous 10% MeOH with 23 mg photocatalyst to obtain comparable amounts of hydrogen as 1-CdS–5-CdS. Reactions were run three times giving data within 15%. In addition, a control reaction using Ta2O5 (30 mg) in aqueous 10% methanol was run periodically to monitor system calibration, which we have found is reproducible from batch to batch. Illumination was performed using a 300 W Xe lamp fitted with a 15 cm IR filter and a wideband AlMgF2 coated mirror (irradiance 900 mW cm−2 measured using an International Light Technologies photometer, over the wavelength range 200–1100 nm). For CdS containing samples, a 400 nm band pass filter was used. All other reactions were performed without the filter. Gas analysis was performed using a Shimadzu Corporation GC-2014. Gases were separated on a 25 cm long column packed with 5 Å molecular sieves and detection was performed using a thermal conductivity detector (TCD). The gas samples were analysed using the following conditions; 20 mL min−1 flow rate of Ar gas, 90 °C column temperature and 120 °C detector temperature. Under these conditions the retention time of H2 is 1.5 min.Results and discussion
Synthesis and characterisation
The choice of photonic support was based on consideration of the optical and electronic properties, and chemical reactivity with respect to the photochemical reaction. An ideal material would have a large refractive index, wide band gap and be photochemically inert. A high refractive index is required because the photonic properties are characteristic of the difference in the refractive index between the material and voids. A wide band gap material will not absorb photons corresponding to visible wavelengths. Furthermore, where the valence and conduction bands of the photon absorbing species are within the valence and conduction bands of the support, the probability of charge transfer between the excited species and the support will be reduced (Fig. 1). In this study ZrO2 was selected which has a refractive index of ca. 2.20 across visible wavelengths, a band gap of ca. 4 eV,11 and does not corrode or catalyse photochemical reactions of water on illumination with the 300 W Xe arc lamp used here. In addition, synthesis of a photonic macroporous morphology has been described previously.5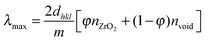 | (1) |
The macroporous materials described here exhibit the inverse opal structure and the position of optical stop bands are given by eqn (1) which allows predictable modification of the stop band position. λmax is the stop band maximum, n is the relative refractive indices of the void and wall materials, dhkl is the void lattice plane spacing, φ is the volume of the wall or ‘fill factor’, and m is the Bragg plane order. It is therefore possible to determine the periodicity and fill factors required to obtain stop bands in positions that may modify the photochemical behaviour of a photoactive material contained within the photonic structure.
CdS was chosen as the photocatalytically active material because the photophysics and chemistry are well described. CdS also exhibits photocatalytic activity for the reduction of water to dihydrogen under visible light illumination.27,28
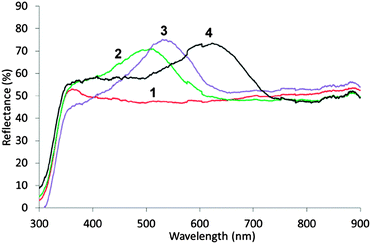
Initially, four samples of macroporous ZrO2 (mac-ZrO2), with varying periodicity, were prepared using an established polystyrene templating method.9 Scanning electron microscopy (SEM) shows an ordered macroporous structure (Fig. 2) with periodicities of 165 (1), 255 (2), 270 (3), and 320 nm (4), respectively. Powder X-ray diffraction (PXRD) (Fig. S4a, ESI†) indicates that the macroscopic structure comprises nanocrystallites of tetragonal ZrO2 approximately 5 nm in diameter and transmission electron microscopy (TEM) analysis gave 3.47 ± 0.33 nm. The surface areas determined from N2 adsorption isotherms gave very similar values of 25 m2 g−1 for 1–4 (Fig. S7, ESI†).
Diffuse reflectance UV-vis spectroscopy (DRUVS) (Fig. 3) showed characteristic stop bands, attributable to the 111 Bragg reflection, with λmax = 500 (2), 535 (3) and 625 nm (4), respectively which lead to distinct colour in reflection (1 = grey; 2 = blue; 3 = green; 4 = pale yellow) (Fig. S1b, ESI†). In air, the stop band of 1 is predicted to be in the UV and is not observed. However, on filling the voids with water, the stop bands shift to longer wavelength, 430 (1), 625 (2), 675 (3) and 770 nm (4), respectively (Fig. S5a, ESI†), reflective of the refractive index difference (nair = 1.00, nwater = 1.33) in accord with eqn (1). Fill factors φ = 16–17% for 1–4, are calculated using eqn (1), which is common for this class of material.
Prior to nanoparticle deposition, mac-ZrO2 was coated with oleic acid (OAmac-ZrO2).25 Thermogravimetric analysis of OAmac-ZrO2 showed a mass loss of 2.2% (Fig. S1a, ESI†), which corresponds to removal of a monolayer surface coating of oleic acid with an estimated footprint of 48 Å2.29 In the absence of an oleic acid coating, homogeneous nanoparticle deposition was not observed.
Oleic acid stabilized nanoparticles of CdS (OAnan-CdS) were prepared independently to give particles of 2.84 ± 0.30 nm diameter as judged by TEM (Fig. S3a, ESI†) and are readily dispersed in common organic solvents.23 Deposition of OAnan-CdS (9.5 wt% by CdS) from a toluene solution onto OAmac-ZrO2, followed by calcination at 450 °C gave the composites nan-CdS@mac-ZrO2 (1-CdS–4-CdS). Combustion analysis of the composites showed that no carbon is present indicating the oleic acid ligands are removed on calcination. All the composites exhibit shades of orange coloration (Fig. S1c, ESI†) due to outer surface coverage by nan-CdS. SEM and TEM (Fig. 4a and b) indicate that aggregation of the nanoparticles does not occur. Elemental mapping using scanning TEM/energy dispersive X-ray analysis (EDX) (Fig. 4d–h) shows that nanoparticle coverage throughout the macroporous structure is homogeneous. In addition, DRUVS spectra (Fig. S5b, ESI†) do not show a significant decrease in intensity which also reflects homogeneous coverage.
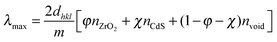 | (2) |
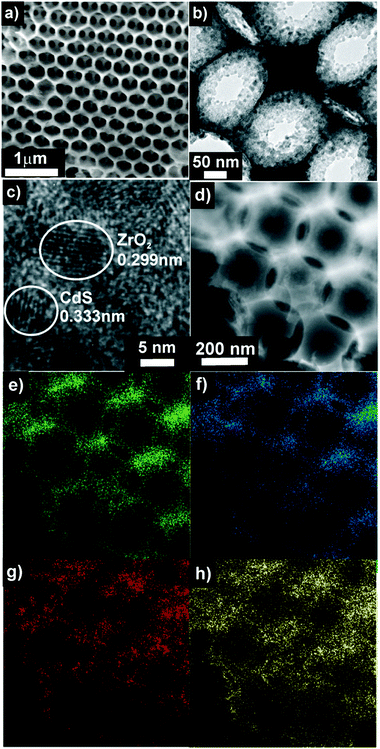 | ||
| Fig. 4 (a) SEM of 4-CdS; (b) TEM of 4-CdS; (c) lattice fringes of individual nanoparticles. (d)–(h) HAADF STEM image and EDX maps; (d) image; (e) zirconium; (f) oxygen; (g) cadmium; (h) sulfur. | ||
The addition of nan-CdS is predicted to cause a red shift of the stop band λmax commensurate with eqn (2), where χ is the volume fraction of nan-CdS.25 Red shifts of 6–11 nm are observed for 1-CdS–4-CdS which is consistent with the addition of 9.5 wt% of CdS (n = 2.4) to 1–4. The volume fraction χ = 1.9%, showing that the composite volume remains principally air as indicated by SEM (Fig. 4a).
For control experiments, a second series of composites containing anatase TiO2 nanoparticles (nan-TiO2) were deposited using an analogous method for 1-CdS–4-CdS to give 1-TiO2–5-TiO2. We have previously reported the detailed synthesis and characterisation of nan-TiO2@mac-ZrO2 composites.25 Red shifts in the DRUVS data for the samples prepared here (Fig. S5d and e, ESI†) confirm the addition of TiO2 and show that coverage is homogeneous, which was also supported by TEM.
Photocatalysis
Composites 1-CdS–4-CdS were tested for hydrogen production from water under visible light illumination (>400 nm), in an aqueous solution of Na2S and Na2SO3 as sacrificial reductants to prevent photocorrosion.27 It should also be noted that the addition of metal cocatalyst nanoparticles (typically Pt) is also commonly used to increase the activity in photocatalytic hydrogen production of semiconductors, including CdS. In this study a cocatalyst was avoided because distribution of a second quantity of nanoparticles would complicate analysis. Furthermore Pt is known to cause photoetching of CdS.27Data were compared against controls of 1–4, and a commercial non-porous powder of ZrO2 (5) decorated with 9.5 wt% nan-CdS (nan-CdS@ZrO2 (5-CdS)). As a wide band gap semiconductor (4 eV), tetragonal ZrO2 does not absorb significant photons above 400 nm and no hydrogen is evolved from 1–5. In addition, for all samples no hydrogen was observed in the absence of light. However, hydrogen production is mediated by 1-CdS–5-CdS under illumination with visible light (Fig. 5 and Table 1). For 2-CdS the reaction was continued to give 2 equivalents of hydrogen with respect to CdS, indicating the reaction is catalytic. After an initial induction period hydrogen production is constant and remains so for subsequent reuse of 1-CdS–5-CdS. For comparison, catalytic activity is often reported as hydrogen produced per gram of catalyst per hour (μmol g−1 h−1) although photoreactions are most commonly performed using much less than one gram of photocatalyst.28 Composites 1-CdS–5-CdS produce between 220 and 1083 μmol g−1 h−1 (Table 1) which is within the range reported for CdS photocatalysts of varying particle size and morphology including examples supported on metal oxides. Therefore the data in Table 1 supports the hypothesis that catalysis is limited to nan-CdS.28
| Catalyst | Activitya (μmol g−1 h−1) | Catalyst | Activityb (μmol g−1 h−1) |
|---|---|---|---|
a Conditions: 30 mg catalyst (2.6 mg nan-CdS), 20 mL Na2S (0.25 M) and Na2SO3 (0.35 M) in water, >400 nm.
b 23 mg catalyst (7.4 mg nan-TiO2), 20 mL 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 methanol 90 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, >325 nm. water, >325 nm.
|
|||
| 1-CdS | 450 | 1-TiO2 | 261 |
| 2-CdS | 1083 | 2-TiO2 | 221 |
| 3-CdS | 220 | 3-TiO2 | 260 |
| 4-CdS | 422 | 4-TiO2 | 326 |
| 5-CdS | 230 | 5-TiO2 | 200 |
The motivation for this work is the variation in hydrogen production of 1-CdS–5-CdS as a function of the photonic properties. However, any observed variation could feasibly be attributable to reactant or product diffusion, catalyst dispersion, and surface area. Diffusion throughout inverse opal macroporous solids is unrestricted30 and is irrelevant for powders such as 5-CdS. The data is therefore not consistent with diffusion-limited photocatalysis, where 5-CdS would be expected to exhibit the greatest activity. The dispersion of nan-CdS was determined using a combination of surface area measurements, TEM, and DRUVS. TEM and DRUVS data support homogeneous coverage (vide supra) and the surface area of 1–4 were found to be very similar (ca. 25 m2 g−1) and significantly greater than the commercial ZrO2 powder 5 (3 m2 g−1) (Fig. S7, ESI†). Previous work has shown that addition of nanoparticles to macroporous ZrO2 results in a small increase in surface area.25 The quantity of nanoparticles added to 1-CdS–4-CdS was identical, and therefore, the surface area and dispersion of nan-CdS does not account for the variation in hydrogen production.
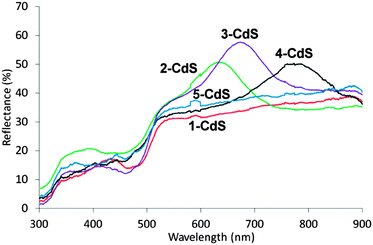
With respect to optical phenomena, the DRUVS spectra for 1-CdS–5-CdS after photocatalysis (Fig. 6) shows the onset of the absorption edge of nan-CdS at 520 nm, and stop bands from the photonic support. In comparison to 1–5 (Fig. 3) the position of the stop bands is consistent with the addition of 9.5 wt% nan-CdS and water to the pores as enumerated by eqn (2). An absorption edge of 520 nm is consistent with a band gap of 2.4 eV and indicates that nan-CdS does not exhibit quantum confinement behaviour that requires crystallites smaller than the Bohr radius of ca. 3 nm.31 TEM shows a CdS particle size on the macroporous support of 3.51 ± 0.36 nm for 1-CdS–5-CdS.
In the photocatalytic medium the composite 3-CdS exhibits a stop band at a longer wavelength than the absorption of nan-CdS (Fig. 6). Therefore the photonic structure of the mac-ZrO2 is not expected to significantly modify the photocatalytic behaviour of nan-CdS, and, as observed, in the absence of any other discriminating factors (vide supra) the photoactivity should be comparable to non-photonic 5-CdS.
In contrast, composites 1-CdS, 2-CdS and 4-CdS all show greater activity than 3-CdS and 5-CdS. For 2-CdS, the blue edge of the stop band partially overlaps the absorption edge of nan-CdS where slowing of the photons is expected to occur.17 The enhanced hydrogen production observed for 2-CdS is therefore consistent with a slowing of the photons in the region of overlap leading to increased absorption and greater photocatalytic activity. Composite 1-CdS, exhibits a stop band within the region of high absorption by nan-CdS. Photonic TiO2 and WO3 materials have shown enhancement of the photon-to-electron conversion efficiency where a stop band is within the absorption band.11,13 This is attributed to slow photons at the stop band edges and scattering effects arising from imperfections of the photonic structure arising from the particulate composition.18 However, enhancements less than a factor of 2 are typically found. A difference in enhancement is expected for photons slowed at the red and blue edges, respectively due to the opposing effects of greater transmission of low energy red photons17 and greater internal scattering of high energy blue photons. However, where absorption is commensurate with the stop band, reflection of photons occurs about the stop band centre, reducing absorption. Therefore any enhancement due to slow photons and scattering is offset by reflection. For semiconductor photocatalysts, which absorb all photons above a bandgap threshold, overlap between the stop band blue edge and absorption edge is therefore required to maximize enhancement. For 4-CdS the secondary 220 stop band centred at 470 nm (Fig. S5a, ESI†) would also exhibit similar phenomena attributable to 1-CdS, and hence comparable enhancement is observed.
As a further control, an alternative photocatalyst was identified which was not expected to exhibit significant enhancement attributable to the photonic structure of 1–4. We have previously reported the synthesis and characterisation of nan-TiO2@mac-ZrO2 composites,25 where TiO2 exhibits the anatase polymorph. TiO2 is considered the prototypical photocatalyst, however TiO2 is not photocatalytically active under visible light illumination. The absorption edge of anatase is at 380 nm and because the absorption edge is separated from the intense stop bands of 1–4, the photonic properties of 1–4 are not expected to significantly modify TiO2 light absorption. TiO2 is known to photocatalyse hydrogen production in water under UV illumination using methanol as a sacrificial reductant.32 Proton reduction and methanol oxidation redox reactions are not photoactivated, in common with the Na2S/Na2SO3 sacrificial oxidation reaction used for 1-CdS–5-CdS photoreactions (vide supra). Therefore only photon absorption by TiO2 could potentially be sensitive to the optical properties of the photonic host.
nan-TiO2 was coated onto 1–5 to give 1-TiO2–5-TiO2 and to obtain comparable amounts of hydrogen for the TiO2 and CdS composites a loading of 47 wt% TiO2 was used. Lower loadings led to excessive reaction times and collecting comparable amounts of hydrogen allows the variation in hydrogen production of 1-CdS–5-CdS and 1-TiO2–5-TiO2 to be conveniently compared. For 1-TiO2–5-TiO2 hydrogen production (Table 1 and Fig. S6b, ESI†) is shown to vary within a narrow range in contrast to 1-CdS–5-CdS and the variation observed for 1-TiO2–5-TiO2 is close to the estimated error (Fig. S6b, ESI†). The mismatch between the absorption edge of the TiO2 photocatalyst and stop band edges of the photonic host precludes variation of UV photon absorption due to the photonic effects, and possible enhancement of hydrogen production due to increased photon absorption.
Collectively, the data show that the photoactivity of nan-CdS is increased between a factor of 1.8 to 4.7 in comparison to 5-CdS depending on the relative position of nan-CdS absorption edge and mac-ZrO2 stop band. The variation in hydrogen production is predominantly attributable to the optical properties of the macroporous support.
Conclusions
The photochemical activity of nanoparticles can be modified within a chemically inert photonic host to increase the yield of hydrogen production under broad band illumination. The data are consistent with increased photon absorption due to the ‘slow photon’ effect induced by the photonic host. In these systems enhancement is maximized when the blue edge of the 111 stop band overlaps with the onset of the photocatalyst absorption. In contrast to photonic photocatalysts, decorating a photonic host with photoactive nanoparticles provides the opportunity to independently optimize optical enhancement and parameters more traditional to heterogeneous catalysis, including nanoparticle catalyst size and morphology,33,34 and support-nanoparticle interaction.35Acknowledgements
This work was supported by The White Rose Consortium and The Centre for Low Carbon Futures. Characterization data was enabled via support from the EPSRC-funded Leeds EPSRC Nanoscience and Nanotechnology Equipment Facility (LENNF) (EP/K023853/1).Notes and references
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729 CrossRef CAS PubMed.
- R. E. Blankenship, D. M. Tiede, J. Barber, G. W. Brudvig, G. Fleming, M. Ghirardi, M. R. Gunner, W. Junge, D. M. Kramer, A. Melis, T. A. Moore, C. C. Moser, D. G. Nocera, A. J. Nozik, D. R. Ort, W. W. Parson, R. C. Prince and R. T. Sayre, Science, 2011, 332, 805 CrossRef CAS PubMed.
- A. Harriman, Philos. Trans. R. Soc., A, 2013, 371, 16 CrossRef PubMed.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. X. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446 CrossRef CAS PubMed.
- I. McConnell, G. H. Li and G. W. Brudvig, Chem. Biol., 2010, 17, 434 CrossRef CAS PubMed.
- G. D. Scholes, G. R. Fleming, A. Olaya-Castro and R. van Grondelle, Nat. Chem., 2011, 3, 763 CrossRef CAS PubMed.
- F. Marlow, Muldarisnur, P. Sharifi, R. Brinkmann and C. Mendive, Angew. Chem., Int. Ed., 2009, 48, 6212 CrossRef CAS PubMed.
- A. Stein, B. E. Wilson and S. G. Rudisill, Chem. Soc. Rev., 2013, 42, 2763 RSC.
- R. C. Schroden, M. Al-Daous, C. F. Blanford and A. Stein, Chem. Mater., 2002, 14, 3305 CrossRef CAS.
- S. Nishimura, N. Abrams, B. A. Lewis, L. I. Halaoui, T. E. Mallouk, K. D. Benkstein, J. van de Lagemaat and A. J. Frank, J. Am. Chem. Soc., 2003, 125, 6306 CrossRef CAS PubMed.
- M. El Harakeh and L. Halaoui, J. Phys. Chem. C, 2010, 114, 2806 CAS.
- S. Guldin, S. Huttner, M. Kolle, M. E. Welland, P. Muller-Buschbaum, R. H. Friend, U. Steiner and N. Tetreault, Nano Lett., 2010, 10, 2303 CrossRef CAS PubMed.
- X. Q. Chen, J. H. Ye, S. X. Ouyang, T. Kako, Z. S. Li and Z. G. Zou, ACS Nano, 2011, 5, 4310 CrossRef CAS PubMed.
- L. W. Zhang, C. Baumanis, L. Robben, T. Kandiel and D. Bahnemann, Small, 2011, 7, 2714 CrossRef CAS PubMed.
- S. K. Karuturi, C. W. Cheng, L. J. Liu, L. T. Su, H. J. Fan and A. I. Y. Tok, Nano Energy, 2012, 1, 322 CrossRef CAS.
- K. Kim, M. J. Kim, S. I. Kim and J. H. Jang, Sci. Rep., 2013, 3, 8 Search PubMed.
- A. Imhof, W. L. Vos, R. Sprik and A. Lagendijk, Phys. Rev. Lett., 1999, 83, 2942 CrossRef CAS.
- J. I. L. Chen, G. von Freymann, V. Kitaev and G. A. Ozin, J. Am. Chem. Soc., 2007, 129, 1196 CrossRef CAS PubMed.
- J. I. L. Chen, E. Loso, N. Ebrahim and G. A. Ozin, J. Am. Chem. Soc., 2008, 130, 5420 CrossRef CAS PubMed.
- J. Liu, G. L. Liu, M. Z. Li, W. Z. Shen, Z. Y. Liu, J. X. Wang, J. C. Zhao, L. Jiang and Y. L. Song, Energy Environ. Sci., 2010, 3, 1503 CAS.
- X. Cui, Y. Wang, G. Jiang, Z. Zhao, C. Xu, Y. Wei, A. Duan, J. Liu and J. Gao, RSC Adv., 2014, 4, 15689 RSC.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
- W. W. Yu and X. G. Peng, Angew. Chem., Int. Ed., 2002, 41, 2368 CrossRef CAS.
- P. D. Cozzoli, A. Kornowski and H. Weller, J. Am. Chem. Soc., 2003, 125, 14539 CrossRef CAS PubMed.
- R. Mitchell, R. Brydson and R. E. Douthwaite, Nanoscale, 2014, 6, 4043 RSC.
- N. S. Hondow, Y. H. Chou, K. Sader, R. Brydson and R. E. Douthwaite, J. Phys. Chem. C, 2010, 114, 22758 CAS.
- N. Buhler, K. Meier and J. F. Reber, J. Phys. Chem., 1984, 88, 3261 CrossRef.
- K. Zhang and L. J. Guo, Catal. Sci. Technol., 2013, 3, 1672 CAS.
- I. Langmuir, Proc. Natl. Acad. Sci. U. S. A., 1917, 3, 251 CrossRef CAS.
- T. Cherdhirankorn, M. Retsch, U. Jonas, H. J. Butt and K. Koynov, Langmuir, 2010, 26, 10141 CrossRef CAS PubMed.
- A. D. Yoffe, Adv. Phys., 1993, 42, 173 CrossRef CAS.
- X. B. Chen, S. H. Shen, L. J. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503 CrossRef CAS PubMed.
- B. R. Cuenya, Acc. Chem. Res., 2013, 46, 1682 CrossRef PubMed.
- S. Fukuzumi and Y. Yamada, ChemSusChem, 2013, 6, 1834 CrossRef CAS PubMed.
- S. Schauermann, N. Nilius, S. Shaikhutdinov and H. J. Freund, Acc. Chem. Res., 2013, 46, 1673 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental and additional characterising data. See DOI: 10.1039/c4cp04333b |
| This journal is © the Owner Societies 2015 |