Nitrogen doped TiO2–CuxO core–shell mesoporous spherical hybrids for high-performance dye-sensitized solar cells
Enyan
Guo
and
Longwei
Yin
*
Key Laboratory for Liquid-Solid Structural Evolution and Processing of Materials, Ministry of Education, School of Materials Science and Engineering, Shandong University, Jinan 250061, P. R. China. E-mail: yinlw@sdu.edu.cn; Fax: +86 531 88396970; Tel: +86 531 88396970
First published on 5th November 2014
Abstract
We report on high-performance dye-sensitized solar cells (DSSCs) based on nitrogen doped anatase TiO2–CuxO core–shell mesoporous hybrids synthesized through a facile and controlled combined sol–gel and hydrothermal process in the presence of hexadecylamine as the structure-directing agent. The matching of band edges between CuxO and TiO2 to form a semiconductor heterojunction plays an important role in effective separation of light induced electrons and holes, providing a promising photoanode for DSSCs because of its wide absorption spectrum, high electron injection efficiency, and fast electron transference. DSSCs based on the mesoporous TiO2–CuxO core–shell hybrids show a high short-circuit current density of 9.60 mA cm−2 and a conversion efficiency of 3.86% under one sun illumination. While DSSCs based on the N-doped mesoporous TiO2–CuxO hybrids exhibit the higher short-circuit current density of 13.24 mA cm−2 and a conversion efficiency of 4.57% under one sun illumination. In comparison with un-doped TiO2–CuxO hybrids, the doping of nitrogen into the lattice of TiO2 can extend the light absorption in the ultraviolet range to the visible light region and effectively decrease the recombination rate of photo-generated electrons and holes. The presented N-doped mesoporous TiO2–CuxO hybrids as photoanodes could find potential applications for high performance DSSCs.
1. Introduction
Dye-sensitized solar cells (DSSCs) are attracting considerable attention because of their diverse advantages such as low cost, environmentally benign process and high efficiency.1,2 Among the various types of oxide semiconductors, titanium oxide (TiO2) is widely recognized as the most promising and versatile material for photocatalysis and solar cell applications due to its outstanding physical and chemical properties, including chemical stability, photostability, non-toxicity, inexpensiveness, and appropriate electronic band structure.3,4 In the past decades, considerable efforts have been concentrated on practical applications for DSSCs based on varieties of nanostructured TiO2, such as nanocrystals,5,6 nanofibers,7,8 nanotubes,9 inversed opals10 and mesoporous beads.11,12 Especially, mesoporous TiO2 spherical nanostructures have received significant research attention due to their abundant mesopores (2–50 nm), providing a high surface area to maximize the uptake of dye molecules, and enhancing the light-harvesting capability, thereby giving rise to a large current density and high photon-to-current conversion efficiency for the TiO2 based DSSCs.13,14Unfortunately, one of the deficiencies of TiO2 is its low efficiency of optical absorption in the visible light region due to its intrinsic wide band gap (Eg = 3.2 eV). Furthermore, the photo-generated electrons and holes can easily recombine, resulting in a large recombination rate for the photo-generated electron–hole pairs. To improve the photoelectron conversion energy efficiency, a variety of strategies have been developed to improve the optical response properties and photoelectron energy efficiency, such as coupling with low band gap semiconductors like PbS,15 CdTe,16 CdS,17,18 CdSe19 and PbSe,20 combining with noble metal nanoparticles, doping with non-metal21,22 and metal ions,4,23,24 and dye sensitization.25 Especially, narrow band gap semiconductors acting as sensitizers can effectively facilitate the electron transfer to the conduction band of large band gap TiO2 in the hybrids of semiconductor/TiO2 heterojunction, thereby efficiently separating photogenerated charge carriers. As a result, visible light can be efficiently utilized and the separation rate of photo-generated electron–hole pairs can be substantially increased in the hybrids of narrow band gap semiconductor/TiO2.
Monoclinic CuO and cubic Cu2O, as the two main lattice structures of p-type copper oxide, display narrow band gap energy of 1.2–1.85 eV and 2.1 eV, respectively.26,27 Because of various superiorities of copper oxides, such as low cost, low toxicity, abundance, and ability to be coupled with a wide band gap semiconductor, copper oxide compounds can be coupled with TiO2 for application of photocatalysis,28,29 hydrogen production,30–32 sensors,33,34 and solar cells.35,36 It is of great importance to fabricate hybrids of copper oxide nanoparticles sensitized mesoporous anatase TiO2 with a large surface area for their application in the field of solar cells.
In this work, we reported on nitrogen doped mesoporous TiO2–CuxO core–shell hybrids as photoanodes of DSSCs. In comparison with pure TiO2, the doping of nitrogen into the lattice of TiO2 can extend the light absorption in the ultraviolet range to the visible light region and effectively decrease the recombination rate of photo-generated electrons and holes, and the formation of mesoporous TiO2 and CuxO can provide a large surface area for dye loading, sufficient light harvesting and efficiently separated photogenerated charge carriers. DSSCs based on the as-synthesized core–shell hybrids of mesoporous TiO2–CuxO core–shell hybrids show a high short-circuit current density of 9.60 mA cm−2 and a conversion efficiency of 3.86% under one sun illumination. While DSSCs based on the mesoporous N-doped TiO2–CuxO core–shell hybrids exhibit the higher short-circuit current density of 13.24 mA cm−2 and a conversion efficiency of 4.57% under one sun illumination.
2. Experimental
2.1 Preparation of N-doped TiO2–CuxO hybrids
Amorphous precursor TiO2 beads were synthesized according to literature procedures.11,37 At room temperature, 2.69 g of hexadecylamine was dissolved in 400 mL of ethanol under vigorous stirring, followed by the addition of 1.6 mL of 0.1 M KCl solution in the reaction solution. Then, 8.8 mL of tetraisopropyl titanate was dropwise added into the solution keeping constant the stirring speed at ambient temperature. The white TiO2 suspension was kept still at the same temperature for 18 h. Finally, the TiO2 beads were collected on a filter, washed with ethanol three times and dried at room temperature.TiO2–CuxO hybrids were synthesized through a solvothermal process in the presence of copper acetate monohydrate. 0.4 g of precursor TiO2 was dispersed in the solution containing 10 mL of ultrapure water and 20 mL of 0.025, 0.05 and 0.1 M concentrations of copper acetate monohydrate in ethanol solution (the corresponding TiO2–CuxO hybrids are denoted S2, S3 and S4 samples, respectively). Afterwards, 1.2 mL of 25 wt% ammonia solution was added to each one. For comparison, the pure mesoporous anatase TiO2 beads were also obtained. 1.6 g of precursor TiO2 beads was added into a 30 mL ethanol–water mixture (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) containing 0.5 mL of 25 wt% ammonia solutions (denoted sample S1). This mixed solution was stirred for 10 min. After that, the mixture was transferred into a Teflon-lined autoclave and heated to 160 °C for 16 h. The resulting precipitates were collected by centrifugation, washed with ethanol and dried in air at room temperature. Finally, the products were calcined at 550 °C for 2 h in air to remove the organic components and produce the good crystallinity for characterization.
1 by volume) containing 0.5 mL of 25 wt% ammonia solutions (denoted sample S1). This mixed solution was stirred for 10 min. After that, the mixture was transferred into a Teflon-lined autoclave and heated to 160 °C for 16 h. The resulting precipitates were collected by centrifugation, washed with ethanol and dried in air at room temperature. Finally, the products were calcined at 550 °C for 2 h in air to remove the organic components and produce the good crystallinity for characterization.
Nitrogen doped mesoporous TiO2–CuxO core–shell nanostructures were synthesized according to the above-mentioned experimental procedure. The only change is adding 1.8 mL of hydrazine hydrate solution after 8.8 mL of tetraisopropyl titanate was dropwise added into the mixed solution, the others are unchanged. The N-doped TiO2–CuxO hybrids prepared in 0.025, 0.05 and 0.1 M concentrations of copper acetate monohydrate in ethanol solution are denoted NS2, NS3 and NS4 samples, respectively.
2.2 Characterization
X-ray diffraction (XRD) patterns were obtained using a Philips Rigaku D/Max-kA X-ray diffractometer equipped with a Cu Kα source at 40 kV and 30 mA. The surface microstructure and chemical components of the products were analyzed using a SU-70 field-emission scanning electron microscope (FESEM) and an attached X-ray energy dispersive spectrometer (EDS). The absorption spectrum and the UV/visible diffuse-reflectance spectra (DRS) were recorded using a UV-vis spectrophotometer (TU-1900). Nitrogen adsorption–desorption isotherms were determined at 77 K using a Gold APP V-Sorb 2800P surface area and porosity 60 analyzer. The surface area measurements were performed according to the Brunauer–Emmett–Teller (BET) method, while the pore size distribution was obtained from the adsorption branch of isotherm using the corrected form of the Kelvin equation by means of the Barrett–Joyner–Halenda (BJH) method. Dye uptake per unit area (1 cm2) was investigated using a UV-Vis spectroscope (TU-1900) by the dissolution of dye adsorbed onto the sample film membrane in 0.2 M NaOH in water and ethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) solution. The selected-area electron diffraction (SAED) characterization and microstructural analyses were carried out on a Philips Tecnai 20U-Twin high-resolution transmission electron microscope at an acceleration voltage of 200 kV. The cells were tested using a solar simulator (Newport, Class 3A, 94023A) at one sun (AM1.5G, 100 mW cm−2) using a Keithley 2420 sourcemeter equipped with a calibrated Si-reference cell (certified by NREL). The incident photon to current efficiency (IPCE) measurement was carried out using a QEX 10 system (PV measurement). A reference Si photodiode calibrated for spectral response was used for the monochromatic power-density calibration. The electrochemical impedance spectra (EIS) were recorded using a Princeton Parstate 2273A in a two-electrode design; the sample films served as a working electrode and the Pt-coated ITO or FTO glass as a counter electrode at an applied bias of the open circuit voltage under one-sun irradiation. The frequency range was 10 mHz to 100 kHz; the magnitude of the alternating potential was 20 mV. The EIS data were analyzed using an appropriate equivalent circuit using simulation software.
50, v/v) solution. The selected-area electron diffraction (SAED) characterization and microstructural analyses were carried out on a Philips Tecnai 20U-Twin high-resolution transmission electron microscope at an acceleration voltage of 200 kV. The cells were tested using a solar simulator (Newport, Class 3A, 94023A) at one sun (AM1.5G, 100 mW cm−2) using a Keithley 2420 sourcemeter equipped with a calibrated Si-reference cell (certified by NREL). The incident photon to current efficiency (IPCE) measurement was carried out using a QEX 10 system (PV measurement). A reference Si photodiode calibrated for spectral response was used for the monochromatic power-density calibration. The electrochemical impedance spectra (EIS) were recorded using a Princeton Parstate 2273A in a two-electrode design; the sample films served as a working electrode and the Pt-coated ITO or FTO glass as a counter electrode at an applied bias of the open circuit voltage under one-sun irradiation. The frequency range was 10 mHz to 100 kHz; the magnitude of the alternating potential was 20 mV. The EIS data were analyzed using an appropriate equivalent circuit using simulation software.
2.3 Fabrication of solar cells
For the working electrode, the fabrication process of the sample paste was described in detail as follows.38 0.12 g of ethyl cellulose powder (Aladdin-reagent, China) was dissolved in ethanol to yield a 10 wt% solution. The obtained mixture was added into 0.2 g of the calcined sample and 0.8 g of terpineol (Aladdin-reagent, China) which was diluted with 1.0 mL of ethanol. The mixture was then stirred in a magnetic field and sonicated by an ultrasonic horn three consecutive times. At last, the obtained paste was spin-coated onto a FTO glass substrate using spin coating apparatus at 2000 rpm for several seconds to obtain a film of required thickness repetitively several times. In order to remove the polymer template and organic compounds, the TiO2 photoelectrode was dried at 125 °C, and gradually heated under flowing air at 325 °C for 5 min, at 375 °C for 5 min, at 450 °C for 15 min and at 500 °C for 15 min.After calcination, the TiO2 electrodes with an active working area of 0.4 × 0.4 = 0.16 cm2 were immersed into a 0.5 mM dye N-719 ethanol solution in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of acetonitrile (Aladdin-reagent, China) and tert-butanol (Aladdin-reagent, China) at room temperature for 24 h. Subsequently, the dye loaded photoanode and the Pt-counter electrode (Dyesol) were assembled into a sandwich type cell and sealed with a spacer of 25 μm thickness (Surlyn, DuPont). The internal space of the cell was filled with a liquid electrolyte, which consisted of 0.6 M 1-methy-3-propylimidazolium iodide (PMII), 0.05 M LiI, 0.05 M I2, and 0.5 M 4-tertbutyl pyridine (TBP) in a (85
1 (v/v) mixture of acetonitrile (Aladdin-reagent, China) and tert-butanol (Aladdin-reagent, China) at room temperature for 24 h. Subsequently, the dye loaded photoanode and the Pt-counter electrode (Dyesol) were assembled into a sandwich type cell and sealed with a spacer of 25 μm thickness (Surlyn, DuPont). The internal space of the cell was filled with a liquid electrolyte, which consisted of 0.6 M 1-methy-3-propylimidazolium iodide (PMII), 0.05 M LiI, 0.05 M I2, and 0.5 M 4-tertbutyl pyridine (TBP) in a (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 vol%) mixture of acetonitrile (Aladdin-reagent, China) and valeronitrile (Aladdin-reagent, China).
15 vol%) mixture of acetonitrile (Aladdin-reagent, China) and valeronitrile (Aladdin-reagent, China).
3. Results and discussion
3.1 Structural characterization
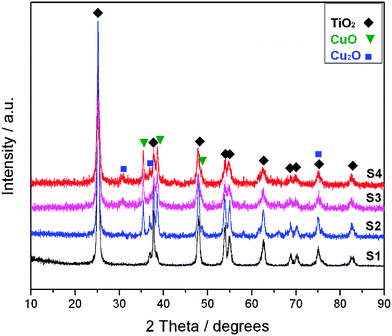
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), respectively. The average size of CuO and Cu2O nanoparticles in the as-prepared nanomaterials is estimated from the Debye–Scherrer formula39 to be about 23.50 ± 1.0 nm and 19.6 ± 1.0 nm, respectively. For the complex of copper oxides composed of Cu2O and CuO phases, it can be denoted CuxO in the TiO2–CuxO hybrids.
m), respectively. The average size of CuO and Cu2O nanoparticles in the as-prepared nanomaterials is estimated from the Debye–Scherrer formula39 to be about 23.50 ± 1.0 nm and 19.6 ± 1.0 nm, respectively. For the complex of copper oxides composed of Cu2O and CuO phases, it can be denoted CuxO in the TiO2–CuxO hybrids.
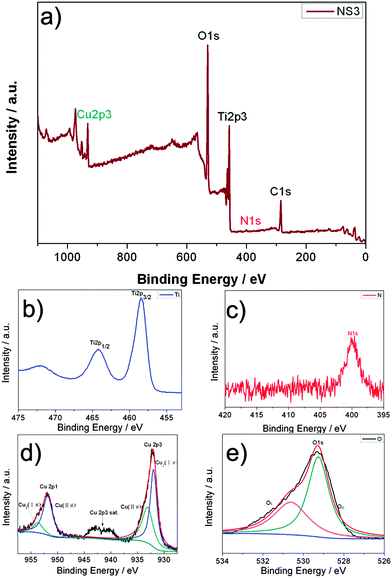
For this reason, we choose hydrazine as the nitrogen source to dope mesoporous TiO2 into the TiO2–CuxO core–shell hybrid photoanodes for DSSCs. XPS characterization is conducted to clarify the chemical composition component and the chemical bonding state of the N-doped TiO2–CuxO core–shell hybrids (NS3). As shown in Fig. 2a, the general survey spectrum of the N-doped TiO2–CuxO nanoparticles contains C, N, Cu, Ti, and O elements. The carbon could have resulted from adventitious hydrocarbons from the XPS instrument itself and can be taken as the standard signal for the correction of other peaks. For the Ti 2p spectrum (Fig. 2b), two main peaks of Ti 2p3/2 and 2p1/2 at bonding energies of 458.5 and 464.3 eV, respectively, reveal that Ti ions exist in the form of Ti4+ in the lattice of TiO2.41,45Fig. 2c depicts a significant N 1s peak at 400.9 eV, which can be attributed to the formation of Ti–O–N or Ti–N–O bonding.46 In the present work, hydrazine was used as the nitrogen source.47Fig. 2d shows the representative XPS spectra of Cu 2p3 and Cu 2p1. Two fitting peaks for Cu 2p3 at around 933.2 and 932 eV can be assigned to the Cu(II)48 state and the Cu(I)49 state, respectively. In addition, the shakeup satellite peaks around 942.7 and 940.5 eV suggest the existence of fully oxidized CuO and incompletely oxidized Cu2O.50 The XPS results above obtained for Cu reveal that two types of phases of CuO or Cu2O coexist for the copper oxides, which can be denoted CuxO, in good agreement with the XRD results. Fig. 2e displays core-level high-resolution XPS spectra of O 1s for the representative nitrogen doped TiO2–CuxO hybrids. The XPS spectral peak deconvolution of O 1s shows a large peak at 529.3 eV, indicating O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonding linked to the Ti–O or Cu–O structure, and two smaller shoulders located at around 530.6 eV (Ti–O) and 229.2 eV (Cu–O).
O bonding linked to the Ti–O or Cu–O structure, and two smaller shoulders located at around 530.6 eV (Ti–O) and 229.2 eV (Cu–O).
3.2 Microstructure characterization
 | ||
| Fig. 3 FE-SEM images of TiO2–CuxO mesoporous beads prepared using different concentrations of copper acetate monohydrate: (a and b) S2, (c and d) S3 and (e and f) S4. | ||
The elemental energy-dispersive spectroscopy (EDS) mapping characterization was used to investigate the chemical composition component and elemental distribution of the TiO2–CuxO core–shell nanostructures (S4). Fig. 4a shows a low FESEM image of the TiO2–CuxO core–shell nanostructures. The EDS elemental mapping of Ti, O and Cu elements in Fig. 4b–d shows that Ti, O and Cu elements are homogenously distributed among the whole TiO2–CuxO core–shell nanostructures, suggesting that CuxO nanoparticles are grown homogeneously on the surfaces of mesoporous anatase TiO2 matrices. A typical EDS spectrum (Fig. 4e) shows that the products are composed of O, Ti and Cu elements.
TEM examination was carried out on the sample S4 to investigate the effects of concentration of copper acetate monohydrate on the microstructures of the TiO2–CuxO hybrid samples (Fig. 6). It can be seen that the sample S4 displays a typical core–shell structure with mesoporous anatase TiO2 acting as the core section and the CuxO as the shell section (Fig. 6a), and this is consistent with the FESEM images in Fig. 3f. Fig. 6b depicts a high magnification TEM image taken from the shell's edge of TiO2–CuxO core–shell nanostructures, showing CuxO nanoparticles with the size ranging from 20 to 30 nm to form a CuxO shell with a thickness of 150 nm. Fig. 6c demonstrates a HRTEM lattice image of an anatase TiO2 nanocrystal and a monoclinic CuO nanoparticle. The marked d-spacing of 0.35 nm and 0.35 nm correspond well to (−101) and (01−1) planes of anatase TiO2. The marked d-spacing of 0.20 nm and 0.16 nm correspond well to (1−1−2) and (02−1) planes of monoclinic CuO. Fig. 6d displays a typical SAED pattern of the samples. The diffraction rings are in agreement with (101), (200) and (211) planes of anatase TiO2 and (111) planes of monoclinic CuO, respectively.
In order to further improve the photoelectron conversion efficiency (η) of solar cells, we synthesized mesoporous N-doped TiO2–CuxO core–shell hybrids. Fig. 7c shows the UV-visible diffuse reflectance spectra (DRS) demonstrating the optical properties of the N-doped TiO2–CuxO hybrids of (a) NS1, NS2, NS3, and NS4 samples. It is observed that the N-doped TiO2–CuxO hybrids show continuous absorption in the visible range. The absorption edge for pure TiO2 is observed at 385 nm (∼3.2 eV), while the absorption edge of the N-doped TiO2 sample (NS1) is at around 415 nm (∼3.0 eV). Compared to un-doped TiO2–CuxO core–shell hybrids (S2, S3, and S4 samples), a drastic red-shift takes place towards the visible spectral range in the N-doped TiO2–CuxO core–shell hybrids (NS2, NS3 and NS4 samples). It could be ascribed to the combined effect of the doping-induced mid-gap electronic states and the lattice disorder effects due to the nitrogen doping. In the N–TiO2 system, the visible-light response arises due to the occupied localized N 2p states above the valence band. The doping also creates localized states below the conduction band edge.51,52
The band gap energy of the samples can be roughly confirmed according to the plots in Fig. 7d, which is obtained via the transformation based on the Kubelka–Munk function (F(R∞) = (1 − R)2/(2R), where R is the reflection coefficient).53 The N-doped TiO2 sample exhibits a smooth absorption edge at around 415 nm, corresponding to the band gap energy of 3.0 eV. The estimated band gap value of the N-doped TiO2–CuxO hybrids corresponds approximately to 3.00, 2.92, 2.81 and 2.70 eV for NS1, NS2, NS3, and NS4 samples, respectively.
 | ||
| Fig. 8 (a) N2 adsorption–desorption curves and (b) pore size distribution plots of TiO2, TiO2–CuxO and N-doped TiO2–CuxO samples (open symbols: adsorption; closed symbols: desorption). | ||
| Samples | BET surface area/m2 g−1 | Pore diameter/nm | Total pore volume/cm3 g−1 |
|---|---|---|---|
| S1 | 73.08 | 17.80 | 0.395 |
| S2 | 51.92 | 25.12 | 0.587 |
| S3 | 62.64 | 21.99 | 0.478 |
| S4 | 75.28 | 16.13 | 0.469 |
| NS1 | 68.56 | 27.29 | 0.612 |
| NS3 | 54.67 | 39.13 | 0.591 |
3.3 Performance of solar cells
| Samples | V oc (V) | J sc (mA cm−2) | Fill factor (%) | η (%) | Dye adsorption (nmol cm−2) |
|---|---|---|---|---|---|
| a η (%) = JscVocFF/Pin, where Pin = 100 mW cm−2 (AM 1.5). Each η is an average value obtained from 5 samples. | |||||
| P25 | 0.69 | 6.94 | 55.82 | 2.67 | 65.51 |
| S1 | 0.67 | 8.01 | 61.49 | 3.30 | 78.65 |
| S2 | 0.63 | 8.62 | 64.52 | 3.54 | 60.92 |
| S3 | 0.62 | 9.60 | 64.85 | 3.86 | 68.50 |
| S4 | 0.68 | 9.43 | 59.55 | 3.82 | 86.27 |
| NS1 | 0.64 | 11.70 | 56.55 | 4.23 | 70.45 |
| NS2 | 0.65 | 12.62 | 51.71 | 4.24 | 58.67 |
| NS3 | 0.66 | 13.24 | 52.26 | 4.57 | 59.40 |
| NS4 | 0.66 | 13.02 | 52.38 | 4.50 | 73.74 |
 | ||
| Fig. 10 J–V curves of solar cells based on (a) P25, (b) S1, (c) S2, (d) S3, (e) S4, (f) NS1, (g) NS2, (h) NS3, and (i) NS4. | ||
For the S2, S3 and S4 cells, the photoelectron conversion efficiency (η) can be maintained as 3.54%, 3.86%, 3.82%, respectively. It is suggested that for the CuxO sensitized TiO2 samples, the short-circuit current density (Jsc) and conversion efficiency (η) of the S2–S4 cells are higher than the S1 cell, which is attributed to the large amount of dye adsorption, sufficient light harvesting in the visible region, and fast charge transport. Firstly, the capacity of the adsorbed dye exerts a profound influence on the photocurrent density. In this regard, the amount of adsorbed N719 dyes can be estimated by measuring the eluted dye molecules using UV-vis absorption spectroscopy.40 The TiO2–CuxO core–shell hybrids display typical mesoporous characteristics with a large specific surface area and a narrow pore diameter (Table 1), which can adsorb a larger amount of dye molecules.
Secondly, after coupling CuxO nanoparticles with the mesoporous anatase TiO2, the TiO2–CuxO core–shell nanohybrids display a distinct red shift to the visible light region with a longer wavelength for the absorption edge (Fig. 7a). The S3 sample shows the mostly enhanced ability to absorb visible-light, and a stronger scattering is revealed from the diffuse reflectance measurement for the S3 sample in comparison with other samples (Fig. 7a), suggesting an improved light harvesting efficiency, and higher short circuit current Jsc.54–57
Finally, the electrochemical impedance spectroscopy (EIS) analysis of DSSCs fabricated based on P25 and TiO2–CuxO nanohybrids of S1, S2, S3, and S4 samples was performed to elucidate the characteristics of the charge transfer ability. As shown in Fig. 9, it is clearly shown that the sheet resistance (Rs) of the substrate for the three samples is almost the same (4 Ω). The R1 values of the samples are 28.7, 30.1, 29.9 Ω, however, the recombination resistance (R2) of the samples are 67.7, 67.9, 56.8 Ω, respectively. The EIS analysis suggested that as compared to pure TiO2 (P25), mesoporous TiO2 (S1 sample), the formation of mesoporous TiO2–CuxO core–shell hybrids facilitates the charge and electron transfer,58 which indicates that electrons are easier to move at the surface and contribute to the charge transport at the photoanode.
The incident photon to current efficiency (IPCE) measurement was carried out using a QEX 10 system (PV measurement). The corresponding incident monochromatic photon-to-electron conversion efficiency (IPCE) spectra of DSSCs based on P25, S1, S3, NS1, and NS3 samples are displayed in Fig. 11. The DSSC samples show a similar feature along the entire wavelength in the range of 400–750 nm. The IPCE results were consistent with the photovoltaic performance of the DSSC samples. Especially, the DSSC samples based on NS1 and NS3 exhibit a higher IPCE along the scanned wavelength in comparison with DSSCs based on S1, S2, and P25. The variation trend of IPCE spectra of DSSCs based on an N-doped photoanode clearly reveals the effect of the N doping on the photovoltaic performance of DSSCs based on the N-doped sample. It is shown that the photoelectric response of the N-doped photoanodes is enhanced and the electron density increases. Also, it is demonstrated that the IPCE of DSSCs based on the NS3 sample displays the highest IPCE over the whole wavelength among all the samples. The improvement of IPCE can be attributed to the decreased recombination rate of photogenerated electrons and holes, due to N-doping and heterostructure formation between TiO2 and copper oxide.
 | ||
| Fig. 11 IPCE spectra of solar cells based on samples of (a) P25, (b) S1, (c) S3, (d) NS1 and (e) NS3. | ||
A comparison of the J–V characteristics of DSSCs based on (e) NS1, (f) NS2, (g) NS3 and (i) NS4 film electrodes is shown in Fig. 10. The open-circuit photovoltage (Voc), corresponding short-circuit photocurrent density (Jsc), fill factor of the cell (FF), power conversion efficiency (η), and dye adsorption are listed in Table 2. Compared to the conversion efficiency of undoped TiO2–CuxO core–shell hybrids, the N-doped TiO2–CuxO hybrids display greatly improved solar cell performance, with a conversion efficiency of 4.23, 4.24, 4.57, and 4.5 for NS1, NS2, NS3, and NS4 samples, respectively. In comparison to the mesoporous TiO2–CuxO hybrids, the improved performance of the solar cells based on the N-doped TiO2–CuxO core–shell hybrids can be attributed to the higher light scattering ability, which enhances the utilization of solar light;59,60 the band gap of the nitrogen doped TiO2–CuxO which is more narrow than the pure TiO2–CuxO; the faster electron transport of the interfaces, which is confirmed by the electrochemical impedance spectroscopy (EIS) (Fig. 9); and the recombination resistance (R2) of S1, NS1, S3 and NS3 which is 67.9, 56.7, 56.8 and 36.9 Ω, respectively. It is well shown that NS3 has the smallest recombination resistance (R2); namely, NS3 has the highest open-circuit. The performance of the solar cell based on the N-doped TiO2–CuxO hybrid materials can be comparable to the related materials prepared via the other methods. For example, Sun38 reported that the optimal short-circuit photocurrent and EQE values of the CuxO modified TiO2 nanorod arrays can increase by more than five and nine times compared to the pristine TiO2, respectively. The performance of DSSCs based on the 0.3 wt% Cu2O–N-doped TiO2 hybrid is better than the undoped TiO2-based DSSCs by Koo.61
The highest power conversion efficiency of DSSCs based on the N-doped TiO2–CuxO material (NS3) photoanode is 4.57%, which is higher than 3.86% for S3, 3.30% for S1, and 2.67% for P25, respectively. A great enhancement in power conversion efficiency of the NS3 photoanode can be obtained compared to that of S3. Hence, the higher short-circuit current density and conversion efficiency of the solar cells based on N-doped TiO2–CuxO core–shell hybrids could be attributed to the larger surface area for adsorbing more dyes, and higher light scattering ability for enhancing the utilization of solar light, which were confirmed by the aforementioned dye adsorption, IPCE and UV-vis diffuse reflectance measurements. As excited by the incident photons, the photoelectrons in CuxO migrate to the conduction band of TiO2, and the holes gather in the valence band of CuxO. During this process, the lifetime of the charge carriers can increase, thus, the recombination of electron–hole pairs is further inhibited, finally resulting in an improved photoelectrical performance.38
4. Conclusions
We for the first time fabricated N-doped mesoporous TiO2–CuxO core–shell hybrids. The matching of band edges between CuxO and TiO2 to form a semiconductor heterojunction plays an important role in the effective separation of light induced electrons and holes, providing a promising photoanode for its wide absorption spectrum, high electron injection efficiency, and fast electron transference. DSSCs based on the mesoporous TiO2–CuxO core–shell hybrids show a high short-circuit current density of 9.60 mA cm−2 and a conversion efficiency of 3.86% under one sun illumination. Furthermore, DSSCs based on the N-doped mesoporous TiO2–CuxO hybrids exhibit the higher short-circuit current density of 13.24 mA cm−2 and a conversion efficiency of 4.57% under one sun illumination. The performance improvement of the solar cell based CuxO nanoparticles/mesoporous anatase TiO2 beads nanostructures can be attributed to the larger surface area adsorbing a large amount of dye molecules; the improved light harvesting efficiency by a distinct red shift moving to the visible light region and the electron transport facilitated by the heterojunction of TiO2–CuxO; and the decrease of the recombination rate of photogenerated electron–holes due to the N-doping into the lattice of TiO2 and the semiconductor heterostructure between TiO2 and CuxO.Acknowledgements
We acknowledge support from the National Natural Science Funds for Distinguished Young Scholars (No. 51025211), National Natural Science Foundation of China (No. 51272137), and the Tai Shan Scholar Foundation of Shandong Province.References
- B. O'Regan and M. Gratzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature, 1991, 353(6346), 737–740 CrossRef.
- X. Wu, Z. Chen, G. Q. M. Lu and L. Wang, Nanosized Anatase TiO2 Single Crystals with Tunable Exposed (001) Facets for Enhanced Energy Conversion Efficiency of Dye-Sensitized Solar Cells, Adv. Funct. Mater., 2011, 21(21), 4167–4172 CrossRef CAS.
- X. Chen and S. S. Mao, Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications, Chem. Rev., 2007, 107(7), 2891–2959 CrossRef CAS PubMed.
- Y. Li, H. Wang, Q. Feng, G. Zhou and Z.-S. Wang, Gold nanoparticles inlaid TiO2 photoanodes: a superior candidate for high-efficiency dye-sensitized solar cells, Energy Environ. Sci., 2013, 6(7), 2156–2165 CAS.
- X. Wu, Z. G. Chen, G. Q. Lu and L. Z. Wang, Nanosized Anatase TiO2 Single Crystals with Tunable Exposed (001) Facets for Enhanced Energy Conversion Efficiency of Dye-Sensitized Solar Cells, Adv. Funct. Mater., 2011, 21(21), 4167–4172 CrossRef CAS.
- J. W. Shiu, C. M. Lan, Y. C. Chang, H. P. Wu, W. K. Huang and E. W. G. Diau, Size-Controlled Anatase Titania Single Crystals with Octahedron-like Morphology for Dye-Sensitized Solar Cells, ACS Nano, 2012, 6(12), 10862–10873 CAS.
- S. H. Hwang, J. Roh and J. Jang, Nanosilver-decorated TiO2 nanofibers coated with a SiO2 layer for enhanced light scattering and localized surface plasmons in dye-sensitized solar cells, Chemistry, 2013, 19(39), 13120–13126 CrossRef CAS PubMed.
- X. Zhang, V. Thavasi, S. G. Mhaisalkar and S. Ramakrishna, Novel hollow mesoporous 1D TiO2 nanofibers as photovoltaic and photocatalytic materials, Nanoscale, 2012, 4(5), 1707–1716 RSC.
- G. K. Mor, K. Shankar, M. Paulose, O. K. Varghese and C. A. Grimes, Use of Highly-Ordered TiO2 Nanotube Arrays in Dye-Sensitized Solar Cells, Nano Lett., 2005, 6(2), 215–218 CrossRef PubMed.
- P. R. Somani, C. Dionigi, M. Murgia, D. Palles, P. Nozar and G. Ruani, Solid-state dye PV cells using inverse opal TiO2 films, Sol. Energy Mater. Sol. Cells, 2005, 87(1–4), 513–519 CrossRef CAS PubMed.
- D. H. Chen, F. Z. Huang, Y. B. Cheng and R. A. Caruso, Mesoporous Anatase TiO2 Beads with High Surface Areas and Controllable Pore Sizes: A Superior Candidate for High-Performance Dye-Sensitized Solar Cells, Adv. Mater., 2009, 21(21), 2206–2210 CrossRef CAS.
- M. Stefik, F. J. Heiligtag, M. Niederberger and M. Grätzel, Improved Nonaqueous Synthesis of TiO2 for Dye-Sensitized Solar Cells, ACS Nano, 2013, 7(10), 8981–8989 CrossRef CAS PubMed.
- C. J. Barbé, F. Arendse, P. Comte, M. Jirousek, F. Lenzmann, V. Shklover and M. Grätzel, Nanocrystalline Titanium Oxide Electrodes for Photovoltaic Applications, J. Am. Ceram. Soc., 1997, 80(12), 3157–3171 CrossRef PubMed.
- T. P. Chou, Q. Zhang, B. Russo, G. E. Fryxell and G. Cao, Titania Particle Size Effect on the Overall Performance of Dye-Sensitized Solar Cells, J. Phys. Chem. C, 2007, 111(17), 6296–6302 CAS.
- D. Cahen, G. Hodes, M. Grätzel, J. F. Guillemoles and I. Riess, Nature of Photovoltaic Action in Dye-Sensitized Solar Cells, J. Phys. Chem. B, 2000, 104(9), 2053–2059 CrossRef CAS.
- Y. Yang, W. Rodriguez-Cordoba, X. Xiang and T. Q. Lian, Strong Electronic Coupling and Ultrafast Electron Transfer between PbS Quantum Dots and TiO2 Nanocrystalline Films, Nano Lett., 2012, 12(1), 303–309 CrossRef CAS PubMed.
- X. Y. Lin, C. L. Wang, S. H. Xu and Y. P. Cui, Manipulation of inter-particle interactions between TiO2 and CdTe: an effective method to enhance the performance of quantum dot sensitized solar cells, J. Phys. D: Appl. Phys., 2014, 47(1) DOI:10.1088/0022-3727/47/1/015103.
- (a) S. C. Lin, Y. L. Lee, C. H. Chang, Y. J. Shen and Y. M. Yang, Quantum-dot-sensitized solar cells: assembly of CdS-quantum dots coupling techniques of self-assembled monolayer and chemical bath deposition, Appl. Phys. Lett., 2007, 90(14), 143517 CrossRef PubMed; (b) Z. M. Yuan and L. W. Yin, CdSe–CdS quantum dots co-sensitized ZnO hierarchical hybrids for solar cells with enhanced photo-electrical conversion efficiency, Nanoscale, 2014, 6(21), 13135–13144 RSC; (c) E. Y. Guo, L. W. Yin and L. Y. Zhang, CdS quantum dot sensitized anatase TiO2 hierarchical nanostructures for photovoltaic application, CrystEngComm, 2014, 16(16), 3403–3413 RSC.
- C.-H. Chang and Y.-L. Lee, Chemical bath deposition of CdS quantum dots onto mesoscopic TiO2 films for application in quantum-dot-sensitized solar cells, Appl. Phys. Lett., 2007, 91(5), 053503 CrossRef PubMed.
- Y. J. Shen and Y. L. Lee, Assembly of CdS quantum dots onto mesoscopic TiO2 films for quantum dot-sensitized solar cell applications, Nanotechnology, 2008, 19(4), 045602 CrossRef PubMed.
- Q. Shen, D. Arae and T. Toyoda, Photosensitization of nanostructured TiO2 with CdSe quantum dots: effects of microstructure and electron transport in TiO2 substrates, J. Photochem. Photobiol., A, 2004, 164(1–3), 75–80 CrossRef CAS PubMed.
- W. A. Tisdale, K. J. Williams, B. A. Timp, D. J. Norris, E. S. Aydil and X. Y. Zhu, Hot-electron transfer from semiconductor nanocrystals, Science, 2010, 328(5985), 1543–1547 CrossRef CAS PubMed.
- R. A. Naphade, M. Tathavadekar, J. P. Jog, S. Agarkar and S. Ogale, Plasmonic light harvesting of dye sensitized solar cells by Au-nanoparticle loaded TiO2 nanofibers, J. Mater. Chem. A, 2014, 2(4), 975–984 CAS.
- B. X. Lei, Q. P. Luo, X. Y. Yu, W. Q. Wu, C. Y. Su and D. B. Kuang, Hierarchical TiO2 flowers built from TiO2 nanotubes for efficient Pt-free based flexible dye-sensitized solar cells, Phys. Chem. Chem. Phys., 2012, 14(38), 13175–13179 RSC.
- X. Zhang, F. Liu, Q.-L. Huang, G. Zhou and Z.-S. Wang, Dye-Sensitized W-Doped TiO2 Solar Cells with a Tunable Conduction Band and Suppressed Charge Recombination, J. Phys. Chem. C, 2011, 115(25), 12665–12671 CAS.
- Z. Sun, R.-K. Zhang, H.-H. Xie, H. Wang, M. Liang and S. Xue, Nonideal Charge Recombination and Conduction Band Edge Shifts in Dye-Sensitized Solar Cells Based on Adsorbent Doped Poly(ethylene oxide) Electrolytes, J. Phys. Chem. C, 2013, 117(9), 4364–4373 CAS.
- W. Zhao, Y. L. Sun and F. N. Castellano, Visible-light induced water detoxification catalyzed by Pt-II dye sensitized titania, J. Am. Chem. Soc., 2008, 130(38), 12566–12567 CrossRef CAS PubMed.
- M. Vaseem, A. Umar, S. H. Kim and Y. B. Hahn, Low-temperature synthesis of flower-shaped CuO nanostructures by solution process: formation mechanism and structural properties, J. Phys. Chem. C, 2008, 112(15), 5729–5735 CAS.
- H. G. Yu, J. G. Yu, S. W. Liu and S. Mann, Template-free hydrothermal synthesis of CuO/Cu2O composite hollow microspheres, Chem. Mater., 2007, 19(17), 4327–4334 CrossRef CAS.
- B. Xu, L. Dong and Y. Chen, Influence of CuO loading on dispersion and reduction behavior of CuO/TiO2 (anatase) system, J. Chem. Soc., Faraday Trans., 1998, 94(13), 1905–1909 RSC.
- J. Zhang, H. Zhu, S. Zheng, F. Pan and T. Wang, TiO2 film/Cu2O microgrid heterojunction with photocatalytic activity under solar light irradiation, ACS Appl. Mater. Interfaces, 2009, 1(10), 2111–2114 CAS.
- M. Y. Wang, L. Sun, Z. Q. Lin, J. H. Cai, K. P. Xie and C. J. Lin, p-n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities, Energy Environ. Sci., 2013, 6(4), 1211–1220 CAS.
- G. K. Mor, O. K. Varghese, R. H. T. Wilke, S. Sharma, K. Shankar, T. J. Latempa, K. S. Choi and C. A. Grimes, p-type Cu-Ti-O nanotube arrays and their use in self-biased heterojunction photoelectrochemical diodes for hydrogen generation, Nano Lett., 2008, 8(7), 1906–1911 CrossRef CAS PubMed.
- K. Lalitha, G. Sadanandam, V. D. Kumari, M. Subrahmanyam, B. Sreedhar and N. Y. Hebalkar, Highly Stabilized and Finely Dispersed Cu2O/TiO2: A Promising Visible Sensitive Photocatalyst for Continuous Production of Hydrogen from Glycerol:Water Mixtures, J. Phys. Chem. C, 2010, 114(50), 22181–22189 CAS.
- Z. Y. Yin, Z. Wang, Y. P. Du, X. Y. Qi, Y. Z. Huang, C. Xue and H. Zhang, Full Solution-Processed Synthesis of All Metal Oxide-Based Tree-like Heterostructures on Fluorine-Doped Tin Oxide for Water Splitting, Adv. Mater., 2012, 24(39), 5374–5378 CrossRef CAS PubMed.
- S. Deng, V. Tjoa, H. M. Fan, H. R. Tan, D. C. Sayle, M. Olivo, S. Mhaisalkar, J. Wei and C. H. Sow, Reduced Graphene Oxide Conjugated Cu2O Nanowire Mesocrystals for High-Performance NO2 Gas Sensor, J. Am. Chem. Soc., 2012, 134(10), 4905–4917 CrossRef CAS PubMed.
- D. Barreca, G. Carraro, E. Comini, A. Gasparotto, C. Maccato, C. Sada, G. Sberveglieri and E. Tondello, Novel Synthesis and Gas Sensing Performances of CuO-TiO2 Nanocomposites Functionalized with Au Nanoparticles, J. Phys. Chem. C, 2011, 115(21), 10510–10517 CAS.
- Q. Sun, Y. Li, X. M. Sun and L. F. Dong, Improved Photoelectrical Performance of Single-Crystal TiO2 Nanorod Arrays by Surface Sensitization with Copper Quantum Dots, ACS Sustainable Chem. Eng., 2013, 1(7), 798–804 CAS.
- S. Ruhle, H. N. Barad, Y. Bouhadana, D. A. Keller, A. Ginsburg, K. Shimanovich, K. Majhi, R. Lovrincic, A. Y. Anderson and A. Zaban, Combinatorial solar cell libraries for the investigation of different metal back contacts for TiO2-Cu2O hetero-junction solar cells, Phys. Chem. Chem. Phys., 2014, 16(15), 7066–7073 RSC.
- M. Ye, X. Xin, C. Lin and Z. Lin, High Efficiency Dye-Sensitized Solar Cells Based on Hierarchically Structured Nanotubes, Nano Lett., 2011, 11(8), 3214–3220 CrossRef CAS PubMed.
- H. Tian, L. Hu, C. Zhang, W. Liu, Y. Huang, L. Mo, L. Guo, J. Sheng and S. Dai, Retarded Charge Recombination in Dye-Sensitized Nitrogen-Doped TiO2 Solar Cells, J. Phys. Chem. C, 2010, 114(3), 1627–1632 CAS.
- J. Yu, G. Dai, Q. Xiang and M. Jaroniec, Fabrication and enhanced visible-light photocatalytic activity of carbon self-doped TiO2 sheets with exposed 001 facets, J. Mater. Chem., 2011, 21(4), 1049–1057 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, Nitrogen and sulfur co-doped TiO2 nanosheets with exposed 001 facets: synthesis, characterization and visible-light photocatalytic activity, Phys. Chem. Chem. Phys., 2011, 13(11), 4853–4861 RSC.
- W. Ho, J. C. Yu and S. Lee, Synthesis of hierarchical nanoporous F-doped TiO2 spheres with visible light photocatalytic activity, Chem. Commun., 2006, 1115–1117 RSC.
- J. L. Cao, G. S. Shao, T. Y. Ma, Y. Wang, T. Z. Ren, S. H. Wu and Z. Y. Yuan, Hierarchical meso–macroporous titania-supported CuO nanocatalysts: preparation, characterization and catalytic CO oxidation, J. Mater. Sci., 2009, 44(24), 6717–6726 CrossRef CAS.
- T. C. Jagadale, S. P. Takale, R. S. Sonawane, H. M. Joshi, S. I. Patil, B. B. Kale and S. B. Ogale, N-Doped TiO2 Nanoparticle Based Visible Light Photocatalyst by Modified Peroxide Sol–Gel Method, J. Phys. Chem. C, 2008, 112(37), 14595–14602 CAS.
- N. S. Chaudhari, S. S. Warule, S. A. Dhanmane, M. V. Kulkarni, M. Valant and B. B. Kale, Nanostructured N-doped TiO2 marigold flowers for an efficient solar hydrogen production from H2S, Nanoscale, 2013, 5(19), 9383–9390 RSC.
- J. P. Espinós, J. Morales, A. Barranco, A. Caballero, J. P. Holgado and A. R. González-Elipe, Interface Effects for Cu, CuO, and Cu2O Deposited on SiO2 and ZrO2. XPS Determination of the Valence State of Copper in Cu/SiO2 and Cu/ZrO2 Catalysts, J. Phys. Chem. B, 2002, 106(27), 6921–6929 CrossRef.
- K. Borgohain, N. Murase and S. Mahamuni, Synthesis and properties of Cu2O quantum particles, J. Appl. Phys., 2002, 92(3), 1292–1297 CrossRef CAS PubMed.
- C. C. Chusuei, M. A. Brookshier and D. W. Goodman, Correlation of Relative X-ray Photoelectron Spectroscopy Shake-up Intensity with CuO Particle Size, Langmuir, 1999, 15(8), 2806–2808 CrossRef CAS.
- N. Serpone, Is the Band Gap of Pristine TiO2 Narrowed by Anion- and Cation-Doping of Titanium Dioxide in Second-Generation Photocatalysts?, J. Phys. Chem. B, 2006, 110(48), 24287–24293 CrossRef CAS PubMed.
- C. Di Valentin, G. Pacchioni and A. Selloni, Origin of the different photoactivity of N-doped anatase and rutile TiO2, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70(8), 085116 CrossRef.
- M. T. Uddin, Y. Nicolas, C. Olivier, T. Toupance, L. Servant, M. M. Muller, H. J. Kleebe, J. Ziegler and W. Jaegermann, Nanostructured SnO2-ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes, Inorg. Chem., 2012, 51(14), 7764–7773 CrossRef CAS PubMed.
- F. Shao, J. Sun, L. Gao, S. Yang and J. Luo, Template-free synthesis of hierarchical TiO2 structures and their application in dye-sensitized solar cells, ACS Appl. Mater. Interfaces, 2011, 3(6), 2148–2153 CAS.
- X. Chen and C. Burda, Photoelectron Spectroscopic Investigation of Nitrogen-Doped Titania Nanoparticles, J. Phys. Chem. B, 2004, 108(40), 15446–15449 CrossRef CAS.
- W. Guo, Y. Shen, G. Boschloo, A. Hagfeldt and T. Ma, Influence of nitrogen dopants on N-doped TiO2 electrodes and their applications in dye-sensitized solar cells, Electrochim. Acta, 2011, 56(12), 4611–4617 CrossRef CAS PubMed.
- H. Diker, C. Varlikli and E. Stathatos, N-doped titania powders prepared by different nitrogen sources and their application in quasi-solid state dye-sensitized solar cells, Int. J. Energy Res., 2014, 38(7), 908–917 CrossRef CAS.
- Y. K. Kho, A. Iwase, W. Y. Teoh, L. Mädler, A. Kudo and R. Amal, Photocatalytic H2 Evolution over TiO2 Nanoparticles. The Synergistic Effect of Anatase and Rutile, J. Phys. Chem. C, 2010, 114(6), 2821–2829 CAS.
- T. Ma, M. Akiyama, E. Abe and I. Imai, High-Efficiency Dye-Sensitized Solar Cell Based on a Nitrogen-Doped Nanostructured Titania Electrode, Nano Lett., 2005, 5(12), 2543–2547 CrossRef CAS PubMed.
- T. Lindgren, J. M. Mwabora, E. Avendaño, J. Jonsson, A. Hoel, C.-G. Granqvist and S.-E. Lindquist, Photoelectrochemical and Optical Properties of Nitrogen Doped Titanium Dioxide Films Prepared by Reactive DC Magnetron Sputtering, J. Phys. Chem. B, 2003, 107(24), 5709–5716 CrossRef CAS.
- H. S. Koo, D. T. Wang, Y. K. Yu, S. H. Ho, J. Y. Jhang, M. Chen and M. F. Tai, Effect of Cu2O Doping in TiO2 Films on Device Performance of Dye-Sensitized Solar Cells, Jpn. J. Appl. Phys., 2012, 51(10), NE18 Search PubMed.
| This journal is © the Owner Societies 2015 |