Open Access Article
Open Access ArticleIn situ X-ray diffraction study on the formation of α-Sn in nanocrystalline Sn-based electrodes for lithium-ion batteries†
Nikolas
Oehl
*a,
Guido
Schmuelling
*b,
Martin
Knipper
a,
Richard
Kloepsch
 b,
Tobias
Placke
b,
Joanna
Kolny-Olesiak
a,
Thorsten
Plaggenborg
a,
Martin
Winter
b and
Juergen
Parisi
a
b,
Tobias
Placke
b,
Joanna
Kolny-Olesiak
a,
Thorsten
Plaggenborg
a,
Martin
Winter
b and
Juergen
Parisi
a
aUniversity of Oldenburg, Energy and Semiconductor Research Laboratory, Institute of Physics, Carl-von-Ossietzky-Str. 9-11, 26129 Oldenburg, Germany. E-mail: nikolas.oehl@uni-oldenburg.de
bUniversity of Muenster, MEET Battery Research Center, Institute of Physical Chemistry, Corrensstr. 46, 48149 Muenster, Germany
First published on 8th October 2015
Abstract
In situ X-ray diffraction (XRD) was performed to study the formation of the α-Sn structure in nanocrystalline Sn-based electrodes during electrochemical lithium insertion and extraction at room temperature. Therefore, pure β-Sn nanoparticles were synthesised and further processed into electrodes. The lithiation and de-lithiation process of the β-Sn nanoparticles follows the formation of discrete lithium–tin phases which perfectly fits the voltage plateaus in the charge/discharge diagram. However, unlike bulk electrodes, where no α-Sn is formed, we observed the formation of the semiconducting α-modification at 870 mV vs. Li within the first de-lithiation process. This observation explains earlier reports of an increasing internal resistance of such an electrode. Additionally, our study supports earlier suggestions that predominantly small tin crystallites are transformed from the β-Sn phase into the α-Sn phase, while larger crystallites retain their metallic β-Sn structure.
Introduction
In order to increase the specific energy (Wh kg−1) and energy density (Wh L−1) of lithium-ion batteries, there is a need for high-capacity anode materials to replace graphitic carbons as state-of-the-art negative electrode materials. Apart from silicon,1 tin is a promising anode material due to its high gravimetric (959 mAh g−1) and volumetric (2000 mAh cm−3) capacities.2 Tin nanoparticles can be transformed from the metallic β-Sn into the semiconducting α-Sn structure at room temperature when cycled in a lithium-ion battery.3–7 Kim et al.3 reported the existence of cubic Sn (α-Sn) in small (3–8 nm) nanoparticles after 30 cycles in a lithium-ion cell. Furthermore, Xu et al.4 investigated the mechanical damage of 10 nm sized Sn nanocrystals during electrochemical reactions with lithium using ex situ high-resolution transmission electron microscopy (HR-TEM). Their findings show that the Sn nanocrystals were transformed from the tetragonal β-Sn phase to the cubic α-Sn after lithium insertion and extraction. The authors concluded that the stability of the polymorphs α-/β-Sn could be affected by the size of the crystallites. Such size-dependent phase transformations are well-documented in the literature, especially for oxides like TiO2 or Al2O3.8,9 Im et al.5 studied the phase evolution of Sn nanocrystals (7 nm) embedded in a carbon matrix during long-term cycling. They pointed out that the α-/β-Sn ratio increases with respect to the cycle number. Finally, Oehl et al.7 investigated at what crystallite size the Sn nanoparticles are transformed from the β structure to the α structure. The critical size was determined to be 17(4) nm for β-Sn nanocrystals at room temperature. However, these investigations did not address the question, when does the transformation occur? Furthermore, these studies did not deal with the lithiation of the α-Sn phase.In situ X-ray diffraction (XRD) measurements by Rhodes et al.10 on bulk Sn electrodes showed that the lithiation and de-lithiation process follows the formation of discrete lithium–tin phases. The presence of four phases including β-Sn, Li2Sn5, β-LiSn and Li22Sn5, which were formed during lithiation and de-lithiation, was identified and could be correlated to the voltage plateaus in the charge/discharge profile. In their work, no α-Sn phase formation was observed.
In this study, we investigated the first-cycle lithiation and de-lithiation process of Sn/SnOx core/shell nanoparticles using an in situ XRD technique in order to get an insight into the mechanism of the α-Sn formation. The in situ XRD measurements show that the lithiation/de-lithiation process follows the formation of lithium–tin phases similar to those in Sn bulk electrodes. However, in contrast to the bulk material, the α-Sn phase is formed at the end of the first de-lithiation process at 870 mV vs. Li. Furthermore, the measurements show that the α-Sn phase gets lithiated contemporaneous with the β-Sn phase, forming the same Li–Sn phases.
Experimental methods
Sn nanoparticles with a volume-weighted mean diameter of 24 nm (D4,3 = 24 nm) were synthesized following a method reported in one of our previous studies.11 Briefly, 10 g of polyvinylpyrrolidone (PVP, M.W. 40 000 g mol−1, Alfa Aesar) and 3.5 g of SnCl2 (99%, Sigma-Aldrich) were dissolved at room temperature in 900 mL of tetraethylene glycol (TEG, 99% Alfa Aesar). The stirred solution was heated up to 120 °C using the Schlenk technique under an argon atmosphere. A freshly prepared solution of 9 g of sodium borohydride (NaBH4, 99%, Acros) in 100 mL of TEG was added dropwise to the stirred SnCl2 solution. The nanoparticle solution was cooled down to room temperature and diluted with acetone in order to isolate the prepared nanoparticles. The nanoparticles were dispersed and stored in ethanol.XRD investigations were performed using a Bruker D8 Advance X-ray diffractometer (Bruker AXS GmbH) equipped with a copper target X-ray tube (λ = 1.54 Å). In situ XRD analysis of the Sn active material upon galvanostatic cycling was carried out using a self-designed in situ cell, which has been described previously.12 An electrode paste consisting of 80 wt% Sn nanoparticles, a 12 wt% conductive carbon agent Super C65 (Imerys) and 8 wt% sodium carboxymethylcellulose (Na-CMC) as a binder (Walocel CRT 2000 PPA 12, Dow Wolff Cellulosics) was applied onto a beryllium (Be) window serving as both the current collector and the X-ray transparent window. Metallic lithium foil (Rockwood Lithium) served as a counter electrode, Whatman glass fiber (grade GF/D) as a separator and 1 M LiPF6 in EC/DEC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (by weight) with 2 wt% VC (UBE) as an electrolyte. After resting, the cell was galvanostatically cycled at a current rate of C/15 (based on the tin active weight m = 6.52 mg, theoretical specific capacity = 950 mAh g−1) between 0.025 V and 1.5 V with a constant voltage step at the lower cut-off voltage (I ≤ C/20). Simultaneously, XRD measurements were performed in the 2θ angular range of 21–81° with a step size of 0.015 degrees and a step time of 0.7 s, resulting in 50 minutes per XRD scan at an accelerating voltage of 40 kV and a current of 40 mA. The Rietveld refinement of the diffraction pattern was applied with the Maud software 2.4.9 in the 2θ angular range of 21–47°. The crystal structure parameters for the β-Sn (03-065-7657) and the α-Sn (01-087-0794) phases were taken from the ICDD database. A polynomial of the fourth degree was used to refine the background; the incident intensity factor, lattice constants and crystallite size and strain were refined in the second and third steps.
7 (by weight) with 2 wt% VC (UBE) as an electrolyte. After resting, the cell was galvanostatically cycled at a current rate of C/15 (based on the tin active weight m = 6.52 mg, theoretical specific capacity = 950 mAh g−1) between 0.025 V and 1.5 V with a constant voltage step at the lower cut-off voltage (I ≤ C/20). Simultaneously, XRD measurements were performed in the 2θ angular range of 21–81° with a step size of 0.015 degrees and a step time of 0.7 s, resulting in 50 minutes per XRD scan at an accelerating voltage of 40 kV and a current of 40 mA. The Rietveld refinement of the diffraction pattern was applied with the Maud software 2.4.9 in the 2θ angular range of 21–47°. The crystal structure parameters for the β-Sn (03-065-7657) and the α-Sn (01-087-0794) phases were taken from the ICDD database. A polynomial of the fourth degree was used to refine the background; the incident intensity factor, lattice constants and crystallite size and strain were refined in the second and third steps.
Results and discussion
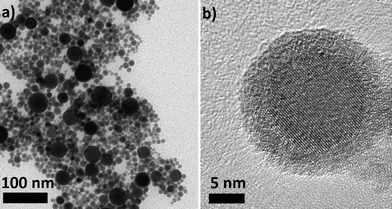
TEM and XRD measurements reveal that the as-prepared Sn nanoparticles are spherical (see Fig. 1a) and consist of a crystalline β-Sn core and a small amorphous SnOx shell (see Fig. 1b). The nanoparticles show a broad size distribution from 5 nm to 40 nm (see Fig. 1a). This size distribution is well suited for the study of the lithiation of β-Sn as well as the lithiation of the α-Sn phase which is already formed in the first cycle.The size distribution was determined by TEM measurements and reveals a mean diameter of 9.8 nm and a volume-weighted mean diameter of 24 nm. The volume-weighted mean diameter was calculated according to eqn (1):
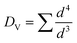 | (1) |
This size is large enough to get strong reflections for the in situ X-ray diffraction measurements and it is small enough to observe the desired β/α-phase transformation.
Rietveld refinement with the Maud software version 2.3.3 was applied in order to analyse the diffraction pattern of the as-prepared nanoparticles. The crystal structures of Sn and SnO were considered to calculate the diffraction pattern. Fig. 2 shows the result of the refinement.
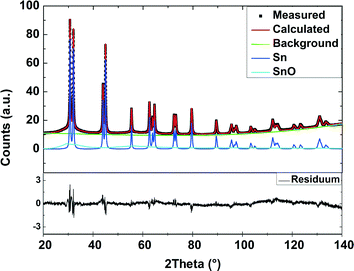 | ||
| Fig. 2 XRD measurement of the prepared Sn/SnOx nanoparticles and the result of the Rietveld refinement. The weighted R factor is 2.1%. | ||
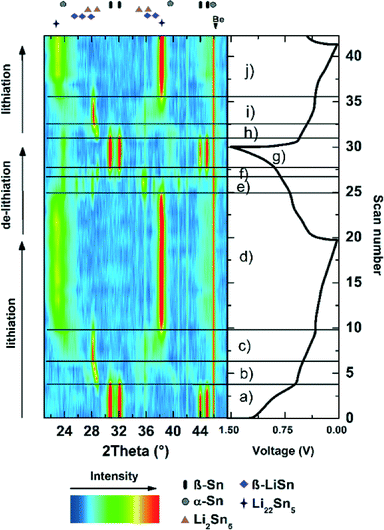
All strong reflections in this pattern could be assigned to the β-Sn phase. A broad peak at 2θ between 30° and 35° can be explained with the SnOx phase. This strong broadening results from the small crystalline domains of that phase. Therefore we conclude that the nanoparticles consist of a crystalline β-Sn core and a SnOx shell with only very small crystalline domains. This conclusion is supported by HR-TEM measurements (Fig. 1b) which show a highly crystalline core and an amorphous shell. The results from the refinement of the diffraction pattern of the as-prepared nanoparticles are in good agreement with the TEM measurements. Fig. 3 shows the volume-weighted size distribution of the as-prepared Sn nanoparticles. According to earlier observations, at a critical size of 17(4) nm, 33(7) wt% of the particles should be transformed into the α-modification after electrochemical lithium insertion and extraction. The X-ray diffraction patterns of the in situ XRD measurement and the corresponding voltage profile of the first charge/discharge cycle are displayed in Fig. 4. The results show that the lithiation process follows the formation of discrete lithium–tin phases, which is perfectly correlated to the voltage plateaus from the charge/discharge profile. The lithiation process follows the formation of the same lithium–tin phases which are formed in bulk Sn-based electrodes, i.e. Li2Sn5, LiSn and Li22Sn5.10 At the beginning, the assembled in situ cell has an initial open circuit voltage of 2.78 V and the diffraction pattern only shows the reflections from β-Sn (Fig. 4a) and the (100) reflection originating from the Be window at 2θ = 46.1°. The transformation of the SnOx shell into the Li2O phase is not observed in the X-ray diffraction measurements because of the small crystalline domain of the shell and the small scattering cross-section between X-rays and light elements. Nevertheless, a small voltage plateau at 0.9 V indicates such a transformation. When the cell voltage reaches the first plateau at 0.56 V, the reflections from the β-Sn phase vanish and a crystalline Li2Sn5 (Fig. 4b) phase is formed. Shortly after the voltage falls below 0.42 V, the reflections from Li2Sn5 disappear and the signals of the LiSn (Fig. 4c) phase appear. Once the voltage decreases below 0.31 V, the characteristic reflections13,14 (a broad reflection at 22° and a sharp one at 38°) of the highest lithiated phase Li22Sn5 (Fig. 4d) occur. In some literature reports, Li17Sn4 was identified as the highest lithiated phase.15 Nevertheless, the Li17Sn4 crystal structure does not fit the XRD pattern from the highest electrochemically lithiated Sn phase in our experiment.
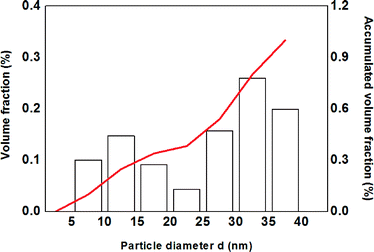 | ||
| Fig. 3 Volume-weighted size histogram of the prepared tin nanoparticles determined by TEM measurements. | ||
During de-lithiation (Fig. 4d–g), the same lithium–tin phases appear and vanish in reverse order and with more overlap between diffraction peaks representing the various phases. However, an uprising phase can still be correlated to a new plateau in the voltage profile. When the voltage exceeds 0.87 V, the reflections from β-Sn return, and additionally, the reflections from α-Sn (Fig. 4g) appear as well. In the following cycles (Fig. 4h–j), the signals from the α-Sn phase appear and vanish contemporaneous with the β-Sn phase, indicating that the α-Sn phase gets lithiated and de-lithiated in a similar way as the β-Sn phase. Fig. 5 shows the XRD patterns and the result of the structural Rietveld refinement for the measurement before and after the first charge/discharge cycle. Fig. 5a shows the XRD pattern before the lithiation process starts. All detectable reflections can be assigned to the β-Sn phase and the Be window. After one complete cycle (see Fig. 5b), three additional reflections appear. These reflections can be explained by the formation of the α-Sn phase. The results of the refinement are summarized in Table 1.
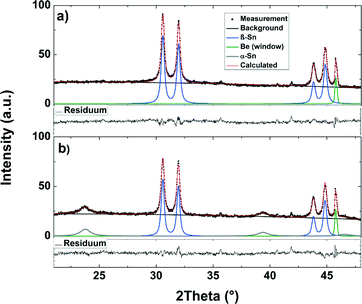 | ||
| Fig. 5 XRD measurement and refinement of the nano-Sn-based electrode before (a) and after (b) the first charge/discharge cycle. | ||
| β-Sn | α-Sn | w α/wβa | R wp (%) | ||||
|---|---|---|---|---|---|---|---|
| a (Å) | c (Å) | L (nm)c | a (Å) | L (nm) | |||
| a w α/wβ denotes the weight ratio of the α-phase/β-phase. b R wp denotes the weighted R factor. c L denotes the crystallite size. d In the de-lithiated state. | |||||||
| As prepared | 5.8388(1) | 3.186(1) | 24.6(9) | — | — | — | 3.5 |
| After the 1st cycled | 5.8393(1) | 3.188(1) | 26(2) | 6.467(1) | 7.1(4) | 0.23 | 3.4 |
The results of the refinement show that the crystallites of the α-Sn phase (L = 7.1(4) nm) are significantly smaller than the crystallites of the β-Sn phase (L = 26(2) nm). The mean crystallite size of the β-Sn nanoparticles increases. Therefore we believe that the original size distribution is split into α- and β-phase distributions. This observation is in conformity with earlier reports that the β-/α-phase equilibrium depends on the size of the crystallites.7 From the ratio of the peak intensities, we calculated the weight ratio. Therefore, after one complete cycle, the crystalline part of the Sn nanoparticles consists of 19(1) wt% α-Sn and 81(1) wt% β-Sn. On the assumption that only the smallest particles in this distribution are transformed into the α-phase, one could calculate a critical size of 11(3) nm for the transformation. Our measurements show for the first time that the α-Sn phase is already formed during the first charge/discharge cycle at 870 mV and simultaneously with the β-phase.
Although the size-dependent phase transformation is very common for nanoparticle crystal structures, the phase transformation for Sn nanocrystals is poorly studied so far. Im et al.5 used ab initio calculations to study the emergence of the α-phase. They concluded that once the α-phase is formed, it preserves its crystal structure, while the β-phase is easily transformed into an amorphous phase during lithiation. Nevertheless, the formation of the α-phase is still supposed to be a size-related effect, which is not fully understood, so far. Besides the contribution of the surface energy, the formation and the stability of α-Sn depends on other factors as well. Gallerneault et al.16 showed that small amounts (0.6 wt%) of Si can enhance the α-phase stability. Furthermore, a matrix is able to stabilize the α-Sn phase up to a melting point of 200 °C.17 However, there are no reports about the α-phase in Sn nanoparticles without the participation of lithium. Therefore, a lithium contribution to the α-phase formation should be considered as well. How the size of the crystallites, the incorporation of lithium and the matrix stabilization might affect the α-phase formation needs to be studied in future work.
Furthermore, the influence of the α-phase on the overall electrochemical performance of Sn-based anode materials needs to be examined more carefully. For example, Im et al.5 reported that crystalline α-Sn can lead to improved rate performance. However, α-Sn is a semiconductor and may lead to an increased internal resistance compared to metallic β-Sn. Studies by Kaghazchi18–20 showed that the insertion of Li in α-Sn is kinetically more favorable than that in β-Sn. Nevertheless, our measurements show that the α-phase gets lithiated contemporaneous with the β-Sn phase. Therefore the kinetic influence of Li insertion into the α-Sn phase seems to be negligible. Therefore, the insertion of lithium into the α-Sn phase and the effect of an increased electrical resistance need to be investigated more carefully and will be the topic of our future work.
Conclusions
In conclusion, we could show for the first time by an in situ X-ray diffraction study that the α-Sn phase is already formed within the first cycle in a nano-particulate tin anode. The α-phase is formed simultaneously with the β-Sn phase at a voltage of 870 mV. This α-phase gets lithiated in the next cycles similar to the β-Sn phase. Furthermore, earlier observations about a critical size of 17(4) nm could be confirmed within the experimental error. Although it is well known that unusual phase transformations in nanoparticles are size related, one should consider that lithium insertion/extraction affects the formation and stability of the α-phase. The impact of the α-phase on the electrochemical performance of tin-based anodes in a lithium-ion cell should be the topic of future research to gain a deeper insight into this only little studied phase transformation.Acknowledgements
The authors gratefully acknowledge the funding of the EWE-Nachwuchsgruppe by EWE AG Oldenburg. Guido Schmuelling gratefully acknowledges the funding of the project “Pouch-Zelle” (funding code: 64.65.69-EM 1024 C) by the state NRW within the NRW Ziel 2-Programm (EFRE), Germany.Notes and references
- W.-R. Liu, Y.-C. Yen, H.-C. Wu, M. Winter and N.-L. Wu, J. Appl. Electrochem., 2009, 39, 1643–1649 CrossRef CAS.
- M. Winter, J. O. Besenhard, J. H. Albering, J. Yang and M. Wachtler, Prog. Batteries Battery Mater., 1998, 17, 208–213 CAS.
- C. Kim, M. Noh, M. Choi, J. Cho and B. Park, Chem. Mater., 2005, 17, 3297–3301 CrossRef CAS.
- L. Xu, C. Kim, A. K. Shukla, A. Dong, T. M. Mattox, D. J. Milliron and J. Cabana, Nano Lett., 2013, 13, 1800–1805 CAS.
- H. S. Im, Y. J. Cho, Y. R. Lim, C. S. Jung, D. M. Jang, J. Park, F. Shojaei and H. S. Kang, ACS Nano, 2013, 7, 11103–11111 CrossRef CAS PubMed.
- G. Schmuelling, N. Oehl, M. Knipper, J. Kolny-Olesiak, T. Plaggenborg, H.-W. Meyer, T. Placke, J. Parisi and M. Winter, Nanotechnology, 2014, 25, 355401 CrossRef PubMed.
- N. Oehl, L. Hardenberg, M. Knipper, J. Kolny-Olesiak, J. Parisi and T. Plaggenborg, CrystEngComm, 2015, 17, 3695–3700 RSC.
- J. M. McHale, A. Auroux, A. J. Perrotta and A. Navrotsky, Science, 1997, 277, 788–791 CrossRef CAS.
- A. Navrotsky, ChemPhysChem, 2011, 12, 2207–2215 CrossRef CAS PubMed.
- K. J. Rhodes, R. Meisner, M. Kirkham, N. Dudney and C. Daniel, J. Electrochem. Soc., 2012, 159, A294–A299 CrossRef CAS PubMed.
- N. Oehl, P. Michalowski, M. Knipper, J. Kolny-Olesiak, T. Plaggenborg and J. Parisi, J. Phys. Chem. C, 2014, 118, 30238–30243 CAS.
- H. Jia, C. Stock, R. Kloepsch, X. He, J. P. Badillo, O. Fromm, B. Vortmann, M. Winter and T. Placke, ACS Appl. Mater. Interfaces, 2015, 7, 1508–1515 CAS.
- J. R. Dahn, I. A. Courtney and O. Mao, Solid State Ionics, 1998, 111, 289–294 CrossRef CAS.
- I. A. Courtney and J. R. Dahn, J. Electrochem. Soc., 1997, 144, 2045–2052 CrossRef CAS PubMed.
- C. Lupu, J.-G. Mao, J. W. Rabalais, A. M. Guloy and J. W. Richardson, Inorg. Chem., 2003, 42, 3765–3771 CrossRef CAS PubMed.
- W. M. T. Gallerneault, F. Vnuk and R. W. Smith, J. Appl. Phys., 1983, 54, 4200–4201 CrossRef CAS PubMed.
- M. F. Fyhn, J. Chevallier, A. N. Larsen, R. Feidenhans and M. Seibt, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 5770 CrossRef CAS.
- P. Kaghazchi, J. Phys.: Condens. Matter, 2013, 25, 382204 CrossRef PubMed.
- P. Kaghazchi, J. Chem. Phys., 2013, 138, 054706 CrossRef PubMed.
- S. Sabet and P. Kaghazchi, J. Chem. Phys., 2014, 140, 191102 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Cif data of β-Sn and α-Sn nanoparticle crystal structures. See DOI: 10.1039/c5ce01841b |
| This journal is © The Royal Society of Chemistry 2015 |