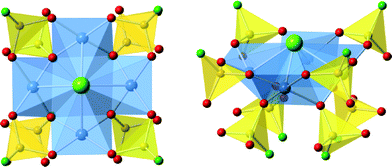
Copper(II) chlorofluorophosphate: a new layered square-net for intercalating amines†
Edward R.
Williams
,
Kayleigh
Marshall
and
Mark T.
Weller
*
Department of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK
First published on 7th November 2014
Abstract
Three copper(II) chlorofluorophosphates, synthesised in hydrofluorothermal conditions and characterised using single crystal X-ray diffraction, have been shown to adopt a new type of mixed-anion, layered structure. Identical two-dimensional, inorganic, square-nets, of the general formula [Cu4Cl(PO3(F,OH))4]−, exist in all three compounds. Three different cationic species, [NH4]+, [H-piperazine]+ and [H-1,4-diaminocyclohexane]+, can be incorporated between the inorganic layers and all three cations are oriented to hydrogen-bond to the anionic sheets. The [Cu4Cl(PO3(F,OH))4]− layers are constructed from a new type of secondary building unit formed from four ClCuO4 square-based pyramids, connected via a μ4 bridging chloride ion, which are further linked via PO3(F,OH) tetrahedra.
Introduction
Two-dimensional solid lattices that undergo intercalation reactions are of considerable scientific interest due to their important and diverse applications. For example they are widely used as electrode materials (both the anode and the cathode) in rechargeable Li-ion batteries,1,2 due to the reversible intercalation of Li-cations into the interlayer space. Further applications include their use as catalysts,3 pigments,4 in photochemistry5 and as hybrid functional inorganic–organic materials.6 A large variety of compositions for the inorganic layers in these compounds has been reported, and includes simple elemental forms, (e.g. graphite), metal chalcogenides (e.g. TaS2), FeOCl, clays and layer double hydroxides (LDHs), metal phosphates (e.g. α-Zr(HPO4)·H2O) and lead iodides, RPbI4.7–11 Interest in many of these layered inorganic systems has been heightened recently due to the ability to exfoliate the layers, for example as with graphene and many metal disulfides. These compounds all share a number of distinct structural and compositional features, including the ability to insert a variety of different-sized guest species between the layers of a crystalline host lattice. Ions and molecular species that have been successful entrapped as guests within these compounds, include alkali metal ions,12 organic molecules and molecular cations, such as alkylamines, ethanol, benzyl amine and pyridazine (yielding hybrid inorganic–organic materials),11 discrete organometallic species, including [Co(en)3]3+ and [Fe(C5H5)2],9,13 and even polymers (PEO).14Our recent investigations have concerned the incorporation of halide anions into complex copper phosphates with the aim of producing frameworks comprised of linked copper polyhedra, of composition [Cu(O,F,Cl)n], in combination with fluorophosphates/hydrogenphosphate tetrahedra, [P(O,OH,F)4]. Results from the AxCu6(P2O7)4Cl(x−6)(TX4) family of materials,15 demonstrate the ability to add chloride anions to copper-based oxopolyhedra; compounds with this general formula have a three-dimensional framework forming channels incorporating a variety of anions. In this article we report a new family of copper chloro-(fluorophosphates) with layered structures derived from linked CuO4(Cl,P(O,OH)4) and PO3(F,OH) polyhedra.
Experimental
Synthesis
All reactants were obtained from commercial suppliers at >99.5% purity and used as received. Reactions were undertaken in Parr® 23 ml Teflon-lined stainless steel autoclaves.Single crystal X-ray diffraction
Single-crystal X-ray diffraction (SXD) data were collected for all structures at 120 K on a Bruker Nonius KappaCCD diffractometer, using Mo Kα (λ = 0.71073 Å) radiation. Structures were solved using the WinGX suite of programmes,16 utilising XPREP17 and SHELX-2013,18 to solve the structure by direct methods.19 All non-hydrogen atoms were refined anisotropically, using the F2 least-squares method, details of the outcomes of the single crystal refinements are summarised in Table 1. Assignment of the terminal sites on the phosphate tetrahedra as a fluorine or oxygen (of OH) atom was undertaken where possible using a combination of the quality of the data fits (R-indices), charge balancing and bond valence sum (BVS) calculations as used previously.20–23 BVSs showed this site to have a −1 charge and lowest residuals were obtained for fluoride in all refinements but the potential for the presence, including partially, of hydroxide instead of fluoride existed in all these phases. Therefore, this group is represented as PO3F in the following discussions though this may include some level of PO3(F1−xOHx), with 0 ≤ x ≤ 1. CCDC numbers [NH4]Cu4Cl(PO3F)4, 1031087; [H-C6N2H14]Cu4Cl(PO3F)4, 1002029; [H-C4N2H10]Cu4Cl(PO3F)4, 1002030.| Structure | Formula weight | Crystal system | Space group | Unit cell dimensions (Å/°) | Z | R-Indices – all data | R-Indices – observed data | GooF |
|---|---|---|---|---|---|---|---|---|
| [NH4]Cu4Cl(PO3F)4 | 699.6 | Tetragonal | P4/nmm | a = 9.8043(10) | 2 | R 1 = 0.120 | R 1 = 0.139 | 1.183 |
| c = 7.4487(11) | wR2 = 0.227 | wR2 = 0.241 | ||||||
| [H-piperazine] Cu4Cl(PO3F)4 | 770.8 | Orthorhombic | Cmca | a = 13.9096(6) | 8 | R 1 = 0.135 | R 1 = 0.087 | 1.078 |
| b = 13.6136(5) | wR2 = 0.182 | wR2 = 0.158 | ||||||
| c = 19.7230(10) | ||||||||
| [H-trans-1,4-diaminocyclohexane] Cu4Cl(PO3F)4 | 880.1 | Orthorhombic | Cmca | a = 13.7220(6) | 8 | R 1 = 0.148 | R 1 = 0.091 | 1.09 |
| b = 13.7879(6) | wR2 = 0.172 | wR2 = 0.154 | ||||||
| c = 24.8892(9) |
Powder X-ray diffraction data were collected from approximately 10 mg of well ground material from each sample using a Bruker D8 diffractometer (CuKα1 radiation). Infra-red spectra were collected using a Perkin-Elmer Spectrum 100 FT-IR spectrometer operating in the frequency range 4000–550 cm−1.
Results
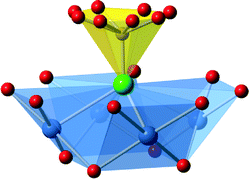
Three new compounds of this family have been synthesized as good to high purity phases. Individual crystals, of blue-green coloration, adopted square tabular morphologies, with typical dimension of 100 μm parallel to the plane directions, and 20 μm perpendicular to the direction of the plane. Crystallographic information of each compound is summarized in Table 1, for simplicity the fluorophosphate tetrahedra are described as PO3F units, as indicated from the SXD structure refinement though a proportion of OH substitution for F is likely to occur in these materials i.e. M[Cu4X(PO3(F,OH))4]; based on residual electron/hole density when assigning these anion sites as fluoride the level of OH is estimated to be relatively small (<25%). The structures of these compounds, M[Cu4X(PO3F)4], M = NH4, [H-piperazine] and [H-trans-1,4-diaminocyclohexane], and X = Cl or the mixture (Cl,HPO4) comprise of identical inorganic two-dimensional layers, of composition [Cu4X(PO3F)4]−, separated by the M+ molecular cations. In the case of M = NH4 and [H-piperazine] X is purely chloride ions while for M = [H-trans-1,4-diaminocyclohexane] Cl− is partially substituted by [HPO4]2−; for simplicity in the following sections describing the structure X is written using just X = Cl−.The inorganic layers are built from copper-centred tetramers as secondary building units (SBUs), these are formed from four CuO4Cl square-based pyramids, with each CuO4Cl unit linked to each two neighbouring species at right-angles through sharing the μ4-bridging chloride ions at the apex and one equatorial O-atom. This new type of SBU, based on metal centres linked through oxygen and chlorine, is depicted in detail in Fig. 1. Each corner of a [(CuO4)4Cl] tetrameric SBU is linked to the next through two bridging PO3F tetrahedra, each tetrahedron sharing three oxygen atoms with copper atoms (Fig. 1 and 2). This produces in a square network of the formula [Cu4Cl(PO3F)4]−. As observed previously in fluorophosphates and hydrogenphosphate materials,20 the fluoro- (hydrogen-)phosphate tetrahedra are orientated such that their fluoride(hydroxide) anions are directed into the interlayer regions and so can take part as an acceptor site in hydrogen bonding to non-framework species. In each pair of connecting PO3F tetrahedra (or possibly partially PO3OH) one fluoride (hydroxide) is directed above the [Cu4Cl(PO3F)4]− and the other below The [(CuO4)4Cl] SBUs display similar behaviour in that these units are orientated within each layers such that the chloride anion is directed into the inter-layer space and alternate SBUs in the square network have the chloride anions orientated in opposite directions along the c-axis.
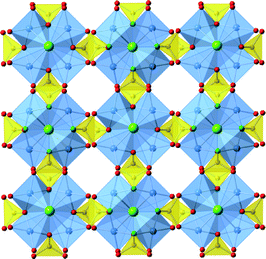 | ||
| Fig. 2 View down the c-axis of one inorganic layer in the M[Cu4Cl(PO3F)4] series of compounds. Key as Fig. 1. | ||
In all three compounds the monocationic guest species occupies the inter-layer space and is orientated such that the protonated amino-group of the molecular species is directed towards the inorganic layers. This permits hydrogen bonding between the protonated amine groups and oxygen and/or fluorine atoms within the inorganic layers, with NH⋯O, donor–acceptor, distances for the different interlayer cationic species between 2.704 and 2.984 Å. Infrared spectra of each compound, see ESI,† showed absorptions characteristic of the polyhedral layers and the individual intercalated cations. The interactions between the [Cu4Cl(PO3F)4]− and their respective interlayer cationic species and small differences in the individual structures are now discussed in detail.
[NH4]Cu4Cl(PO3F)4
The inorganic layers in [NH4]Cu4Cl(PO3F)4 are perfectly aligned with the Cu-centred SBUs lying directly above each other in adjacent layers and orientated with the Cu–Cl units pointing in the same direction. The ammonium cations lie between the layers positioned between eight (fluoro-) phosphate groups such that each N–H forms a difurcated hydrogen bond to two oxygen atoms, of two different PO3F tetrahedra, with both NH⋯OP distances at 2.227 Å, Fig. 3. Adjacent to each interlayer ammonium ion within the plane are four sites at a distance of a/2 = 4.9 Å bordered by the chloride anion of one layer and four fluorine atoms of the adjacent [Cu4Cl(PO3F)4] layer. A small level of residual electron density (maximum 1.4 e Å−3) was found in the final Fourier difference across this volume probably associated with low levels of disordered water molecules in this site.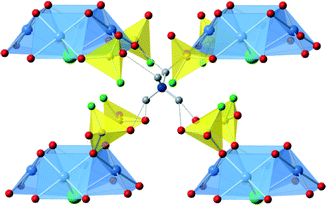 | ||
| Fig. 3 The ammonium cation position in [NH4]Cu4Cl(PO3F)4. Key as Fig. 1 and nitrogen – dark blue sphere and hydrogen-grey sphere. Hydrogen bonds are shown as dotted lines. | ||
[H-piperazine] [Cu4Cl(PO3F)4]
[H-piperazine][Cu4Cl(PO3F)4] contains monoprotonated piperazinium cations between the [Cu4Cl(PO3F)4]− layers, Fig. 4. Neighbouring layers are shifted by 1.262 Å in the b-direction relative to each other which has the effect of transforming the unit cell to an orthorhombic system. The origin of the effect seems to lie in maximising hydrogen bonding interactions between inorganic and organic components and the chair shaped geometry adopted by the piperizine ring. In this conformation hydrogen atoms at both ends of the piperizine form hydrogen bonds with oxygen atoms of the [Cu4Cl(PO3F)4] layer with NH⋯O distances in the range 2.0–2.25 Å. As with the ammonium system a small level of residual electron density was found in the region between the terminal halide ions which may be associated with disordered anions, vide infra, or water molecules.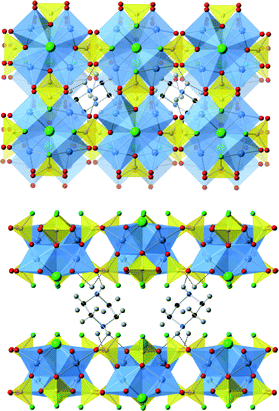 | ||
| Fig. 4 The structure of [H-piperazine] [Cu4Cl(PO3F)4]. Top view from above the layers (down the c-axis, with the a-axis from left to right) and highlighting the offset layers. Bottom view is parallel to the layers (along the b-axis with a-axis from left to right). Key as Fig. 3 plus C-black sphere. Hydrogen bonds are shown as dotted lines. | ||
[H-trans-1,4-diaminocyclohexane][Cu4(Cl,H2PO4)(PO3F)4]
Similar to [H-piperazine] [Cu4Cl(PO3F)4] the orthorhombic [H-trans-1,4-diaminocyclohexane]+-containing structure has the [Cu4Cl(PO3F)4] layers offset in the b-direction, though this is to a significantly larger degree at 1.649 Å, Fig. 5.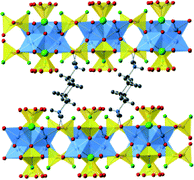 | ||
| Fig. 5 The structure of [H-trans-1,4-diaminocyclohexane] [Cu4Cl(PO3F)4] viewed, side on to the layers, along the a-axis with the c-axis vertical. Key as Fig. 4 hydrogen bonds are shown as dotted lines. | ||
This arrangement accommodates the larger dimensions of the protonated amine so that it hydrogen bonds to framework oxygen atoms in two adjacent layers, in pockets formed of fluoro-/hydrogenphosphate groups. One additional feature of this material found in its refined structure, see Fig. 6, is the high level of replacement of the Cl− by a phosphate group, probably present as either dihydrogen phosphate or hydrogenphosphate; the degree of protonation can be balanced by that of the trans-1,4-diaminocyclohexane unit. Indeed the refined level of phosphate modelled at his site, using two phosphate positions at different rotations around the O3PO–(Cu)4 axis was 0.84 PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.16 Cl. One reason that this substitution occurs in this compound might be that a hydrogen bond can form between the amine and this hydrogenphosphate group (NH⋯O = 2.43 Å). A small level of water may be present in the cavity delineated by the four terminal fluorides when chloride is present at the apical site as indicated in the crystallographic analyses of the related structures.
0.16 Cl. One reason that this substitution occurs in this compound might be that a hydrogen bond can form between the amine and this hydrogenphosphate group (NH⋯O = 2.43 Å). A small level of water may be present in the cavity delineated by the four terminal fluorides when chloride is present at the apical site as indicated in the crystallographic analyses of the related structures.
Thermal analysis
Simultaneous thermogravimetric (TGA) and differential thermal (DTA) analyses were undertaken for [H-trans-1,4-diamino-cyclohexane][Cu4(Cl,H2PO4)(PO3F)4] and [NH4][Cu4Cl(PO3F)4] both of which were obtained as single phase products. A 10.5 mg sample of [H-trans-1,4-diamino-cyclohexane][Cu4Cl(PO3F)4] was heated in an alumina crucible to 500 °C at 2 °C min−1 under a flow of nitrogen gas, with a data point collected every 2 s, Fig. 7. The relatively low maximum temperature was chosen to allow decomposition of the templating species but prevent any significant evolution of HF through decomposition of the framework. A small weight loss was observed up to 250 °C probably corresponding to surface species and a small level of halide cavity water molecules, see structure description. Above 260 °C a 15.5% mass loss was recorded. This corresponds to the loss of one mole of trans-1,4-diaminocyclohexane (theoretical value 14.6%); a sharp strong endothermic peak observed in the DTA trace confirms a significant structural change is associated with this weight loss.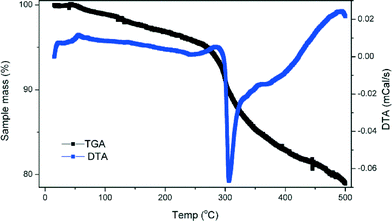 | ||
| Fig. 7 TGA and DTA plots for [H-trans-1,4-diaminocyclohexane][Cu4Cl(PO3F)4], showing the one step decomposition of the organic template molecule. | ||
An identical thermal analysis experiment was undertaken for a 5.8 mg sample of [NH4][Cu4Cl(PO3F)4. This material undergoes a stepwise decomposition, with individual steps beginning at 79, 177 and 242 °C and corresponding masses of 4.7%, 4.3% and 10.4% respectively. The initial step is attributed to a small level of water molecules present in the cavities formed by the terminal fluoride and chloride groups as discussed previously. The weight loss near 177 °C can be assigned to the loss of 1 mole of NH3, (expected weight loss 2.8%) and formation of [H][Cu4Cl(PO3F)4]. The final weight loss can be attributed to the destructive decomposition of the inorganic framework with evolution of a mixture of gases such as Cl2 (0.5 moles, 5.0% weight loss) and F2/HF/O2/H2O (2–4 moles ~10.9% mass loss, CARE! F2 and HF are highly toxic). This was confirmed by examination of the TGA product by PXD, which showed it to be Cu2P2O7.
Conclusions
A family of new layered copper chlorofluorophosphates of the general formula [R+][Cu4Cl(PO3F)4]− incorporating a range of amine cations, [R+] between individual [Cu4Cl(PO3F)4]− sheets has been synthesised. The copper chlorofluorophosphate layers are constructed of a novel SBU formed from four square-based pyramidal CuO4Cl units joint at a single chloride ion vertex and further linked at the edges by PO3F tetrahedra. This crosslinking of the SBUs through the PO3F tetrahedra produces a square network with interleaved ammonium ions, though this layer is slightly puckered when protonated organic amines are present. A comparison of the three structures shows that the inter layer-separation reflects the size of the included molecular cations, ranging from ~3.2 Å for [NH4]+, ~5.7 Å for [H-piperazine]+ and ~8.0 Å for [H-trans-1,4-diaminocyclohexane]+. The parallel planes are slightly offset relative to each other for the two organic amine cationic molecules, which allows them to adopt a chair formation for the six membered ring while simultaneously having the protonated amine head-groups hydrogen bond to the phosphate oxygen atoms present in dips in the inorganic layers. This “ball-and-socket” arrangement allows a closer layer spacing with the organic amines than might be expected based solely on the cation size.One other unusual feature of these structures is the μ4 chloride ion which is mainly substituted by a μ4 –O(P(O,OH)3) anion in the trans-1,4-diamino-cyclohexane derivative. The Cu–Cl/(OP(O,OH)3) bond lengths are 2.642 Å (R = NH4+), 2.698 Å (R = [H-piperazine]) and 2.518 Å respectively, with the expected significantly shorter measured interaction to the smaller (prevalent) oxygen. While relatively long the Cu–Cl distances are not unusual for apical sites on square-based pyramidal and elongated octahedral coordination of Cu(II). However, the ability to replace this anion site with a hydrogenphosphate group is noteworthy as it may indicate that facile anion exchange is possible at this position. Such behaviour has been seen for the AxCu6(P2O7)4Cl(x−6)(TX4) family of materials,15 with a one dimensional channel system; in the M[Cu4X(PO3F)4] system described here a two dimensional access is possible with the added possibility of tuning access to the anion site through control of the interlayer spacing via different sized amines.
Acknowledgements
This research was supported by the EPSRC (EP/G068038/1).Notes and references
- D. Buchholz, A. Moretti, R. Kloepsch, S. Nowak, V. Siozios, M. Winter and S. Passerini, Chem. Mater., 2013, 25, 142 CrossRef CAS.
- M. Winter, J. O. Besenhard, M. E. Spahr and P. Novak, Adv. Mater., 1998, 10, 725 CrossRef CAS.
- S. P. Newman and W. Jones, New J. Chem., 1998, 22, 105 RSC.
- P. G. Tang, Y. J. Feng and D. Q. Li, Recent Pat. Nanotechnol., 2012, 6, 193 CrossRef CAS.
- T. Nakato, K. Kuroda and C. Kato, Chem. Mater., 1992, 4, 128 CrossRef CAS.
- P. Judeinstein and C. Sanchez, J. Mater. Chem., 1996, 6, 511 RSC.
- M. S. Dresselhaus and G. Dresselhaus, Adv. Phys., 1981, 30, 139 CrossRef CAS.
- G. A. Wiegers, Prog. Solid State Chem., 1996, 24, 1 CrossRef CAS.
- D. G. Billing and A. Lemmerer, CrystEngComm, 2007, 9, 236 RSC.
- A. I. Kahn and D. O'Hare, J. Mater. Chem., 2002, 12, 3191 RSC.
- G. Alberti, M. Casciola, U. Costantino and R. Vivani, Adv. Mater., 1996, 8, 291 CrossRef CAS.
- W. P. F. Omloo and F. Jellinek, J. Less-Common Met., 1970, 20, 121 CrossRef CAS.
- M. Holtz, T. R. Park, J. Amarasekera, S. A. Solin and T. J. Pinnavaia, J. Chem. Phys., 1994, 100, 3346 CrossRef CAS PubMed.
- P. Aranda and E. Ruizhitzky, Chem. Mater., 1992, 4, 1395 CrossRef CAS.
- E. R. Williams, R. M. Leithall, R. Raja and M. T. Weller, Chem. Commun., 2013, 49, 249 RSC.
- L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837 CrossRef CAS.
- G. Sheldrick, XPREP. Space Group Determination and Reciprocal Space Plots, 1991 Search PubMed.
- G. M. Sheldrick, Release 97-2, University of Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- E. R. Williams, S. A. Morris and M. T. Weller, Dalton Trans., 2012, 41, 10845 RSC.
- J. A. Armstrong, E. R. Williams and M. T. Weller, J. Am. Chem. Soc., 2011, 133, 8252 CrossRef CAS PubMed.
- J. A. Armstrong, E. R. Williams and M. T. Weller, Dalton Trans., 2012, 41, 14180 RSC.
- J. A. Armstrong, E. R. Williams and M. T. Weller, Dalton Trans., 2013, 42, 2302 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: IR spectra and powder X-ray diffraction patterns for all compounds, Fig. S1a–c and S2a–c. Crystal structure data, CIFs, for all compounds. CCDC 1031087, 1002029, 1002030. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ce01126k |
| This journal is © The Royal Society of Chemistry 2015 |