A highly enantioselective Biginelli reaction using self-assembled methanoproline–thiourea organocatalysts: asymmetric synthesis of 6-isopropyl-3,4-dihydropyrimidines†
Zhijun
Hang
a,
Jun
Zhu
a,
Xiang
Lian
a,
Peng
Xu
a,
Han
Yu
*ab and
Sheng
Han
*a
aSchool of Chemical and Environmental Engineering, Shanghai Institute of Technology, Shanghai 201418, P. R. China. E-mail: hanyu201212@gmail.com; hansheng654321@sina.com
bDepartment of Chemistry, Tsinghua University, Beijing 100084, P. R. China
First published on 16th October 2015
Abstract
An efficient self-assembled methanoproline–thiourea organocatalyst for the synthesis of optically active 6-isopropyl-3,4-dihydro-pyrimidines via an asymmetric Biginelli reaction was developed, which is superior to the individual precatalyst. A wide range of optically active 6-isopropyl-3,4-dihydropyrimidines with remarkable pharmacological interest was obtained in high yields with excellent enantioselectivities (up to 99% ee). A plausible transition state has been proposed to explain the origin of the activation and the asymmetric induction.
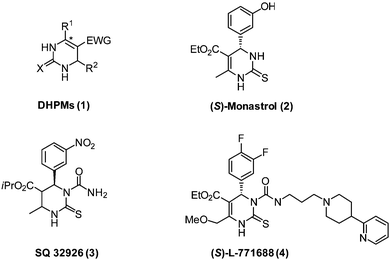
Chiral dihydropyrimidines (DHPMs) have found increasing applications in the synthesis of pharmaceutically relevant substances exhibiting a wide range of important pharmacological properties,1 including calcium channel modulation,2 α1a-adrenergic receptor antagonism,3 and mitotic kinesin inhibition.4 It has been recognized that the individual enantiomers exhibit different or in some cases even opposite biological activities.1 (Fig. 1 shows several representative examples.) Currently, the preparation of optically pure DHPMs in pharmaceutical research laboratories mainly relies on the resolution and chiral auxiliary-assisted asymmetric synthesis. Due to these important properties and applications, an efficient method for the preparation of optically pure DHPMs is highly desirable. Recent developments in this area have focused on asymmetric Biginelli reactions, which provide an important method for the straightforward synthesis of optically active 3,4-dihydro-pyrimidin-2-(1H)-ones and -thiones (DHPMs). In 2005, the breakthrough in the catalytic asymmetric Biginelli reaction was realized by Zhu and co-workers with a chiral ytterbium catalyst providing DHPMs in high yields with excellent enantioselectivities.5 One year later, Gong and co-workers developed an organocatalytic Biginelli reaction using a chiral BINOL-derived phosphoric acid catalyst, giving DHPMs with up to 97% ee.6 In 2008, Feng and Juaristi independently described an organocatalytic asymmetric Biginelli reaction using a combined catalyst system consisting of chiral secondary amines and a Brønsted acid.7 Subsequently, a variety of chiral DHPMs were obtained in good yields with excellent enantioselectivities via asymmetric Biginelli reactions,8 including primary amines,8b–f proline derivatives,7,8g pyrrolidinyl tetrazole,8h and ionic liquids.8i Although great success has been achieved in previous work, the development of more-effective asymmetric catalysts and substrate scope remains an interesting challenge.
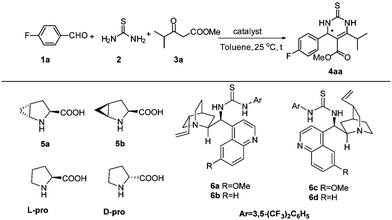
Most recently, there was considerable interest in applying self-assembled organocatalysts in catalytic reactions.9,10 For example, Zhao11 reported the first example of self-assembled organocatalysts from proline and quinidine thioureas which are highly efficient catalysts for enantioselective direct nitro-Michael addition of ketones and aldehydes to nitroalkenes, better than prolines. Subsequently, Demir,9e,f Hirose,9i Ramachary,9m and Zhao12 respectively reported similar self-assembled organocatalysts from proline and chiral or achiral thioureas, which could be used as efficient catalysts for Michael addition reactions, direct enantioselective aldol reactions, Mannich reactions and hetero-Diels–Alder reactions. Since self-assembled organocatalysts have undoubtedly been efficient catalysts in enamine-type reactions, and in light of the mechanism of the Biginelli reaction,13 herein, we wish to disclose a self-assembled methanoproline–thiourea organocatalyzed asymmetric Biginelli reaction, directly providing chiral 6-isopropyl-3,4-dihydropyrimidine compounds in high yields and with excellent enantioselectivities, which are very important intermediates of statin drugs and highly enantioselective synthesis of these compounds via asymmetric Biginelli reaction is yet to be reported.
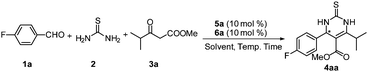
Initially, the asymmetric Biginelli reaction of 4-fluorobenzaldehyde 1a with thiourea 2 and methyl isobutyrylacetate 3a was adopted as the model reaction for optimizing the reaction conditions. As can be seen in Table 1, when trans-4,5-methano-L-proline 5a14 and quinidine thiourea 6a (10 mol% loading each) were used as the catalyst in toluene at 25 °C, the desired product was obtained in excellent yield (91%) and with high enantioselectivity (95% ee) (Table 1, entry 1). In contrast, when trans-4,5-methano-L-proline 5a, L-proline or quinidine thiourea 6a was used alone, low yield and low enantioselectivity were observed (Table 1, entries 2–4). These results clearly demonstrate that the self-assembled organocatalysts are superior to the individual precatalyst. When the catalyst combination is shuffled to be L-proline or D-proline and quinidine thiourea 6a, a slight mismatching of the catalyst was observed to deliver the product in 89% ee and 83% ee respectively (Table 1, entries 5 and 6). Replacing the quinidine thiourea 6a with hydro quinidine thiourea 6b in the catalyst combination of 5a/6b for asymmetric Biginelli reactions was not found to give superior results (Table 1, entry 7). Instead of trans-4,5-methano-L-proline, when cis-4,5-methano-L-proline 5b was used, the product was obtained with a similar yield and with a slightly lower enantioselectivity (Table 1, entry 8). The reaction catalyzed by the organocatalyst assembly of cis-4,5-methano-L-proline 5b and quinidine thiourea 6c yields the opposite enantiomer in 93% ee at 25 °C in toluene. Similar results were obtained for the assembly of cis-4,5-methano-L-proline 5b and quinidine thiourea 6d (Table 1, entries 9 and 10).
| Entry | Cat. (mol%) | t | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a Unless stated otherwise, all reactions were carried out with 4-fluorobenzaldehyde (1a; 0.4 mmol, 1.0 equiv.), thiourea (2; 0.48 mmol, 1.2 equiv.), methyl isobutyrylacetate (3a; 0.6 mmol, 1.5 equiv.), 5 and 6 (10 mol% each) in toluene (3 mL) at 25 °C. b Isolated yield after flash chromatography. c Determined by HPLC analysis by using a chiral column, and the configuration was assigned as S by comparison with the literature data.9d d The opposite configuration enantiomer value was obtained. | ||||
| 1 | 5a/6a (10:10) | 15 h | 91 | 95 |
| 2 | 5a (10) | 5 d | 25 | <10 |
| 3 | 6a (10) | 5 d | 17 | <10 |
| 4 | L-pro (10) | 5 d | 18 | <10 |
| 5 | L-pro/6a (10:10) | 22 h | 90 | 89 |
| 6 | D-pro/6a (10:10) | 27 h | 89 | 83 |
| 7 | 5a/6b (10:10) | 18 h | 90 | 93 |
| 8 | 5b/6a (10:10) | 20 h | 89 | 92 |
| 9d | 5b/6c (10:10) | 21 h | 87 | 93 |
| 10d | 5b/6d (10:10) | 22 h | 85 | 91 |
Having identified the assembly of trans-4,5-methano-L-proline 5a and quinidine thiourea 6a as the optimal catalyst, we studied the effects of solvent and temperature on this reaction. As summarized in Table 2, normal organic solvents were found to have only minimal influences on the enantioselectivity value, except that poor results were obtained with a very polar solvent DMF (Table 2, entry 6). When the reaction was carried out at 50 °C, it proceeded much faster, while there was a slight increase in the product ee value (Table 2, entry 7). When the temperature increased from 50 to 60 °C, the reaction yielded the product in 93% yield with a little compromise in the enantioselectivity of 96% ee (Table 2, entry 8). In addition, the catalyst loading was also surveyed. It was found that reducing the precatalyst loading to 5 mol% each did not affect the yield and enantioselectivity. However, further dropping the loading to 3 mol% each slowed down the desired reaction, and a drop in both the yield and enantioselectivity was found. Increasing the catalyst loading did not show a clear improvement in the catalytic performance (Table 2, entries 9–11). By screening a series of reaction conditions, operating with self-assembled 5a/6a (5 mol% loading each) in toluene at 50 °C was found to be the most favorable.
| Entry | Solvent | T (°C) | t (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Unless stated otherwise, all reactions were carried out with 4-fluorobenzaldehyde (1a; 0.4 mmol, 1.0 equiv.), thiourea (2; 0.48 mmol, 1.2 equiv.), methyl isobutyrylacetate (3a; 0.6 mmol, 1.5 equiv.), 5a and 6a (10 mol% each), solvent (3 mL). b Isolated yield after flash chromatography. c Determined by HPLC analysis by using a chiral column, and the configuration was assigned as S by comparison with the literature data.9d d The catalyst loading is 5 mol%. e The catalyst loading is 3 mol%. f The catalyst loading is 20 mol%. | |||||
| 1 | CH2Cl2 | 25 | 15 | 83 | 93 |
| 2 | Toluene | 25 | 15 | 91 | 95 |
| 3 | THF | 25 | 21 | 62 | 92 |
| 4 | CH3CN | 25 | 17 | 65 | 94 |
| 5 | 1,4-Dioxane | 25 | 20 | 57 | 91 |
| 6 | DMF | 25 | 48 | Trace | n.d. |
| 7 | Toluene | 50 | 15 | 92 | 98 |
| 8 | Toluene | 60 | 15 | 93 | 96 |
| 9d | Toluene | 50 | 15 | 92 | 99 |
| 10e | Toluene | 50 | 24 | 89 | 94 |
| 11f | Toluene | 50 | 15 | 93 | 97 |
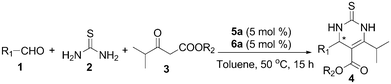
With the optimal reaction conditions in hand, we explored the generality of the self-assembled 5a/6a catalyzed asymmetric Biginelli reaction (Table 3). The scope of the aldehyde component was first investigated by reaction with thiourea (2) and methyl isobutyrylacetate (3a) (Table 3, entries 1–10). A variety of aromatic aldehydes bearing various types of substituents underwent the reaction to afford DHPMs in high yields (90–95%) with excellent enantioselectivities (92–99% ee). It appears that the electronic properties of the substituents on the aromatic aldehyde have a significant influence on the enantioselectivity of the reaction. All the reactions of para-substituted benzaldehydes with electron-withdrawing groups proceeded with excellent yields and afforded high enantioselectivities (Table 3, entries 1–4, 99% ee). Excellent enantio-selectivity was obtained when no-substituent benzaldehyde was employed (Table 3, entry 5). When aromatic aldehydes bearing electron-donating groups underwent the reaction, they also afforded high enantioselectivities ranging from 92 to 96% ee. In particular, 2,4,6-trimethylbenzaldehyde delivered a comparably lower yield and enantioselectivities may be attributed to the effect of steric hindrance (Table 3, entries 10 and 20). Furthermore, the scope of β-keto ester components in the organocatalytic asymmetric Biginelli reaction was examined next. The replacement of the R2 of the β-keto ester with the ethyl group with various aldehydes in the Biginelli reaction was carried out to give the corresponding 6-isopropyl DHPMs with up to 96% yield (Table 3, entries 11–20). The experimental results indicated that the variation of the R2 substituent of β-keto esters 3 could be tolerated and generally high enantioselectivities (91–99% ee) were provided for the reactions related to these substrates. The aliphatic aldehydes, such as n-butyraldehyde, were also reacted with β-keto ester 3 to generate the 6-isopropyl-3,4-dihydropyrimidine products with extremely high enantioselectivities (Table 3, entries 21 and 22, ee up to 94% and 95%, respectively).
| Entry | R1 | R2 | 4 | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Unless stated otherwise, all reactions were carried out with aldehyde (1; 0.4 mmol, 1.0 equiv.), thiourea (2; 0.48 mmol, 1.2 equiv.), β-keto ester (3; 0.6 mmol, 1.5 equiv.), 5a and 6a (10 mol% each) in toluene (3 mL) at 50 °C. b Isolated yield after flash chromatography, and the configuration was assigned as S by comparison with the literature data.9d c Determined by HPLC analysis by using a chiral column. | |||||
| 1 | 4-FPh | Me | 4aa | 92 | 99 |
| 2 | 4-ClPh | Me | 4ba | 93 | 99 |
| 3 | 4-CF3Ph | Me | 4ca | 94 | 99 |
| 4 | 4-NO2Ph | Me | 4da | 95 | 99 |
| 5 | Ph | Me | 4ea | 90 | 97 |
| 6 | 4-OHPh | Me | 4fa | 93 | 95 |
| 7 | 4-MePh | Me | 4ga | 92 | 96 |
| 8 | 4-OMePh | Me | 4ha | 92 | 95 |
| 9 | 4-CH(CH3)2Ph | Me | 4ia | 91 | 94 |
| 10 | 2,4,6-(CH3)3Ph | Me | 4ja | 90 | 92 |
| 11 | 4-FPh | Et | 4ab | 92 | 99 |
| 12 | 4-ClPh | Et | 4bb | 93 | 99 |
| 13 | 4-CF3Ph | Et | 4cb | 93 | 99 |
| 14 | 4-(NO2)Ph | Et | 4db | 96 | 99 |
| 15 | Ph | Et | 4eb | 92 | 98 |
| 16 | 4-OHPh | Et | 4fb | 94 | 95 |
| 17 | 4-MePh | Et | 4gb | 92 | 96 |
| 18 | 4-OMePh | Et | 4hb | 93 | 96 |
| 19 | 4-CH(CH3)2Ph | Et | 4ib | 92 | 93 |
| 20 | 2,4,6-(CH3)3Ph | Et | 4jb | 90 | 91 |
| 21 | n-Pr | Me | 4ka | 93 | 94 |
| 22 | n-Pr | Et | 4kb | 91 | 95 |
Biginelli reactions of urea with aromatic aldehydes and isobutyrylacetate were also tested on the basis of the optimal conditions and with adjusted reaction conditions (solvent, temperature and feed ratio), but no corresponding products were obtained.
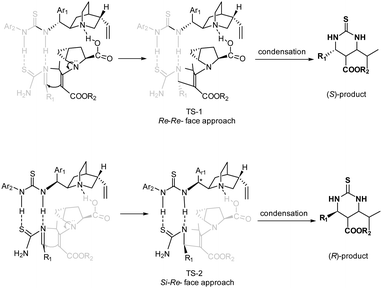
The opposite senses of the enantioselectivity for the assemblies of 5a with 6a and 6c may be rationalized by the proposed transition states, as shown in Scheme 1. Based on relevant reports,9m,12c there are three important interactions among the substrates and the catalysts: (1) the carboxylic group of trans-4,5-methano-L-proline 5a undergoes proton exchange with the quinoline moiety of quinidine thiourea 6a, thus bringing the electronic and steric environment closer to the reaction center; (2) two NH groups of quinidine thiourea engage themselves in hydrogen bonding with imine by a condensation of the aldehyde and thiourea to activate the electrophilic nature and the benzylideneurea is restricted by the quinidine thiourea scaffold of the catalyst; (3) the secondary amine group of 5a forms an enamine intermediate with β-keto esters 3 to activate the nucleophilic nature. In the case of quinidine thiourea 6a (TS-1), in which the Re-face of the imine is predominantly approached by the enamine intermediate, the Re, Re-attack of the hydrogen-bonded imine on the enamine intermediate leads to the major S-configured product. In contrast, in the case of quinidine thiourea 6c (TS-2), and the Re, Si-attack of the hydrogen-bonded imine on the enamine intermediate leads to the major R-configured product.
In summary, we have designed a new and efficient self-assembled methanoproline–thiourea organocatalyst for the asymmetric Biginelli reaction, which is superior to the individual precatalyst. Under the optimal reaction conditions, a wide range of optically active 6-isopropyl-3,4-dihydropyrimidines with remarkable pharmacological interest was obtained in high yields with excellent enantioselectivities (up to 99% ee) using this practical method under mild conditions. A plausible transition state has been proposed to explain the origin of the activation and the asymmetric induction. Further exploration of the catalytic mechanism and the applications of the novel self-assembled methanoproline–thiourea organocatalysts in asymmetric catalysis is in progress in our laboratory.
Financial support by the NSFC (21402065) and the start-up fund of Shanghai Institute of Technology is gratefully acknowledged.
Notes and references
- (a) C. O. Kappe, Eur. J. Med. Chem., 2000, 35, 1043 CrossRef CAS PubMed; (b) C. O. Kappe, in Multicomponent Reactions, ed. J. Zhu and H. Bienayme, Wiley-VCH, Weinheim, 2005, p. 95 Search PubMed.
- (a) K. S. Atwal, G. C. Rovnyak, B. C. O'Reilly and J. Schwartz, J. Org. Chem., 1989, 54, 5898 CrossRef CAS; (b) K. S. Atwal, B. N. Swanson, S. E. Unger, D. M. Floyd, S. Moreland, A. Hedberg and B. C. O'Reilly, J. Med. Chem., 1991, 34, 806 CrossRef CAS PubMed; (c) G. C. Rovnyak, S. D. Kimball, B. Beyer, G. Cucinotta, J. D. DiMarco, J. Gougoutas, A. Hedberg, M. Malley and J. P. McCarthy, J. Med. Chem., 1995, 38, 119 CrossRef CAS PubMed; (d) C. O. Kappe, W. M. Fabian and M. A. Semones, Tetrahedron, 1997, 53, 2803 CrossRef CAS.
- (a) D. Nagarathnam, S. W. Miao, B. Lagu, G. Chiu, J. Fang, T. Murali Dhar, J. Zhang, S. Tyagarajan, M. R. Marzabadi and F. Zhang, J. Med. Chem., 1999, 42, 4764 CrossRef CAS PubMed; (b) T. Murali Dhar, D. Nagarathnam, M. R. Marzabadi, B. Lagu, W. C. Wong, G. Chiu, S. Tyagarajan, S. W. Miao, F. Zhang and W. Sun, J. Med. Chem., 1999, 42, 4778 CrossRef CAS PubMed; (c) J. C. Barrow, P. G. Nantermet, H. G. Selnick, K. L. Glass, K. E. Rittle, K. F. Gilbert, T. G. Steele, C. F. Homnick, R. M. Freidinger and R. W. Ransom, J. Med. Chem., 2000, 43, 2703 CrossRef CAS PubMed; (d) M. Yarım, S. Saraç, F. S. Kılıç and K. Erol, Il Farmaco, 2003, 58, 17 CrossRef.
- C. Blackburn, B. Guan, J. Brown, C. Cullis, S. M. Condon, T. J. Jenkins, S. Peluso, Y. Ye, R. E. Gimeno and S. Punreddy, Bioorg. Med. Chem. Lett., 2006, 16, 3504 CrossRef CAS PubMed.
- Y. Huang, F. Yang and C. Zhu, J. Am. Chem. Soc., 2005, 127, 16386 CrossRef CAS PubMed.
- X.-H. Chen, X.-Y. Xu, H. Liu, L.-F. Cun and L.-Z. Gong, J. Am. Chem. Soc., 2006, 128, 14802 CrossRef CAS PubMed.
- J. Xin, L. Chang, Z. Hou, D. Shang, X. Liu and X. Feng, Chem. – Eur. J., 2008, 14, 3177 CrossRef CAS PubMed.
- For a recent review of the asymmetric Biginelli reaction, see: (a) M. M. Heravi, S. Asadi and B. M. Lashkariani, Mol. Diversity, 2013, 17, 389 CrossRef CAS PubMed; (b) Y. Wang, H. Yang, J. Yu, Z. Miao and R. Chen, Adv. Synth. Catal., 2009, 351, 3057 CrossRef CAS; (c) D. Ding and C. G. Zhao, Eur. J. Org. Chem., 2010, 3802 CrossRef CAS PubMed; (d) Y. F. Cai, H. M. Yang, L. Li, K. Z. Jiang, G. Q. Lai, J. X. Jiang and L. W. Xu, Eur. J. Org. Chem., 2010, 4986 CrossRef CAS; (e) Y. Wang, J. Yu, Z. Miao and R. Chen, Org. Biomol. Chem., 2011, 9, 3050 RSC; (f) D.-Z. Xu, H. Li and Y. Wang, Tetrahedron, 2012, 68, 7867 CrossRef CAS; (g) S. Saha and J. N. Moorthy, J. Org. Chem., 2010, 76, 396 CrossRef PubMed; (h) Y. Y. Wu, Z. Chai, X. Y. Liu, G. Zhao and S. W. Wang, Eur. J. Org. Chem., 2009, 904 CrossRef; (i) L. D. S. Yadav, A. Rai, V. K. Rai and C. Awasthi, Tetrahedron, 2008, 64, 1420 CrossRef CAS; (j) N. Li, X.-H. Chen, J. Song, S.-W. Luo, W. Fan and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 15301 CrossRef CAS PubMed; (k) F. Xu, D. Huang, X. Lin and Y. Wang, Org. Biomol. Chem., 2012, 10, 4467 RSC; (l) D. An, Y. S. Fan, Y. Gao, Z. Q. Zhu, L. Y. Zheng and S. Q. Zhang, Eur. J. Org. Chem., 2014, 301 CrossRef CAS.
- For examples, see: (a) M. L. Clarke and J. A. Fuentes, Angew. Chem., Int. Ed., 2007, 46, 930 CrossRef CAS PubMed; (b) D.-Q. Xu, H.-D. Yue, S.-P. Luo, A.-B. Xia, S. Zhang and Z.-Y. Xu, Org. Biomol. Chem., 2008, 6, 2054 RSC; (c) D. Uraguchi, Y. Ueki and T. Ooi, Science, 2009, 326, 120 CrossRef CAS PubMed; (d) M. Bella, D. M. S. Schietroma, P. P. Cusella, T. Gasperi and V. Visca, Chem. Commun., 2009, 597 RSC; (e) O. Reis, S. Eymur, B. Reis and A. S. Demir, Chem. Commun., 2009, 1088 RSC; (f) A. S. Demir and S. Eymur, Tetrahedron: Asymmetry, 2010, 21, 112 CrossRef CAS; (g) N. El-Hamdouni, X. Companyó, R. Rios and A. Moyano, Chem. – Eur. J., 2010, 16, 1142 CrossRef CAS PubMed; (h) F. Rodríguez-Llansola, J. F. Miravet and B. Escuder, Chem. – Eur. J., 2010, 16, 8480 CrossRef PubMed; (i) W.-H. Wang, T. Abe, X.-B. Wang, K. Kodama, T. Hirose and G.-Y. Zhang, Tetrahedron: Asymmetry, 2010, 21, 2925 CrossRef CAS; (j) A. B. Xia, D. Q. Xu, S. P. Luo, J. R. Jiang, J. Tang, Y. F. Wang and Z. Y. Xu, Chem. – Eur. J., 2010, 16, 801 CrossRef CAS PubMed; (k) J. A. Fuentes, T. Lebl, A. M. Slawin and M. L. Clarke, Chem. Sci., 2011, 2, 1997 RSC; (l) G. Ma, A. Bartoszewicz, I. Ibrahem and A. Córdova, Adv. Synth. Catal., 2011, 353, 3114 CrossRef CAS; (m) D. B. Ramachary, R. Sakthidevi and K. S. Shruthi, Chem. – Eur. J., 2012, 18, 8008 CrossRef CAS PubMed.
- For reviews on self-assembled organocatalysts, see: (a) J.-F. Brière, S. Oudeyer, V. Dalla and V. Levacher, Chem. Soc. Rev., 2012, 41, 1696 RSC; (b) J. Meeuwissen and J. N. Reek, Nat. Chem., 2010, 2, 615 CrossRef CAS PubMed; (c) S. Piovesana, D. M. Scarpino Schietroma and M. Bella, Angew. Chem., Int. Ed., 2011, 50, 6216 CrossRef CAS PubMed.
- T. Mandal and C.-G. Zhao, Angew. Chem., Int. Ed., 2008, 47, 7714 CrossRef CAS PubMed.
- (a) S. Muramulla and C.-G. Zhao, Tetrahedron Lett., 2011, 52, 3905 CrossRef CAS; (b) D. Sinha, T. Mandal, S. Gogoi, J. J. Goldman and J. C. g. Zhao, Chin. J. Chem., 2012, 30, 2624 CAS; (c) D. Sinha, S. Perera and J. C.-G. Zhao, Chem. – Eur. J., 2013, 19, 6976 CrossRef CAS PubMed; (d) S. Perera, D. Sinha, N. K. Rana, V. Trieu-Do and J. C.-G. Zhao, J. Org. Chem., 2013, 78, 10947 CrossRef CAS PubMed; (e) S. Muramulla, J.-A. Ma and J. C.-G. Zhao, Adv. Synth. Catal., 2013, 355, 1260 CrossRef CAS; (f) N. K. Rana, H. Huang and J. C. G. Zhao, Angew. Chem., Int. Ed., 2014, 126, 7749 CrossRef.
- C. O. Kappe, J. Org. Chem., 1997, 62, 7201 CrossRef CAS PubMed.
- (a) H. Yu, M. Liu; and S. Han, Tetrahedron, 2014, 70, 8380 CrossRef CAS; (b) Y. Zhang, J. Zhu, N. Yu and H. Yu, Chin. J. Chem., 2015, 33, 171 CrossRef CAS; (c) N. Yu, S. Han and H. Yu, Tetrahedron, 2015, 71, 4665 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc07880f |
| This journal is © The Royal Society of Chemistry 2016 |