Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stabilizing bijels using a mixture of fumed silica nanoparticles†
Dongyu
Cai
and
Paul S.
Clegg
*
School of Physics and Astronomy, University of Edinburgh, Peter Guthrie Tait Road, Edinburgh, EH9 3FD, UK. E-mail: dongyu.cai@hotmail.com; paul.clegg@ed.ac.uk
First published on 8th October 2015
Abstract
Bijels are typically prepared by arresting the phase separation of two liquids using interfacial particles. The surface treatment of the particles is challenging but can be overcome at a cost (Cui et al., Science, 2013, 342, 460–463). Here, we use mixed commercial fumed-silica nanoparticles, giving a facile route to bijel production.
Bicontinuous materials are of great interest because they are desirable for application in solar cells, fuel cells, batteries, supercapacitors, tissue engineering scaffolds, separation processes, sensors, catalyst supports, etc.1 Using bicontinuous emulsions as templates is a popular route for designing multiphase materials with controlled bicontinuity, beginning two decades ago with bicontinuous microemulsions stabilized by surfactants.2 Very recently, bicontinuous interfacially jammed emulsion gels, known as ‘bijels’, have become attractive surfactant-free templates.3,4 They have two inter-penetrating continuous fluid domains sharing a layer of colloidal particles forced into contact (jammed) at the interface.3 Unlike microemulsions, bijels are static with an elastic modulus; the structure and porosity are tuneable, and the liquid ingredients remain in contact via interstitial spaces. Furthermore, the fluid domains can be used for hosting versatile precursor monomers to form solid scaffolds when required.4
Following initial fabrication via spinodal decomposition in 2007,3 bijels have been developed by several groups: (1) water–water bijels were prepared via the phase separation of biopolymers;5 (2) a pair of organic liquids were employed to make non-aqueous bijels including demonstrating smaller (1 μm) interface separations;6 (3) non-spherical particles (rods7 and graphene8) with high aspect ratio led to bijels with smaller domain size relative to the volume of particles; (4) bijels with large domain size (up to 450 μm) were stabilized using bridged droplets formed in one fluid domain, which showed the potential application in tissue engineering;9 (5) very recently, the fluids for realizing bijels were extended to pairs of non-polar liquids.10
In order to stabilize a bijel the particles must have symmetric wettability with the two fluid phases, also called neutrally wetting (contact angle ≈90°). This condition avoids the particles imposing a preferred curvature on the liquid–liquid interface during phase separation. The coarsening of the spinodal structure is then arrested as a result of the neutrally wetting particles jamming together on the interface. Monodisperse Stöber silica particles have been used for revealing the fundamental connections to particle wettability,3,10,11 particle size6,12 and particle–particle interactions.11 With these particles, the surface properties are tuned by a skilled practitioner for each new batch.6,11 Just like every coin has two sides, these model particles turn out to be a major impediment to this phase-separation route: the choice of tailored particles with tuned properties hinders the further adoption of bijels for large-scale applications.
There are two problems associated with the use of Stöber silica particles. First, they are not cost-effective for bijel fabrication since they need to be either sourced at high price for a small quantity or synthesized specially. Second, Stöber silica particles are initially dispersed in mixture of water, ethanol and ammonia, which necessitate post-processing in order to obtain neutrally wetting particles i.e. removing ammonia, chemical modification and careful drying. Among these multi-step treatments, chemical modification, either through thermal annealing3,11 or surface silanization,6,7 demands significant care because the reproducibility is adversely affected by minor variations from batch to batch. This problem has been addressed by Cui and co-workers.13 They combined a bespoke surfactant and nanoparticles self-assembled at an interface so as to arrest elongated water droplets in the oil phase via interfacial jamming, resulting in a “glass wool”-type (or “bijel”-like) structure. However, the use of surfactant to tune the wettability of the nanoparticles (is expensive and) does not suit the phase-separation route since the addition of surfactant would modify the phase diagram of the binary liquids.
In this Communication, we address these problems by introducing fumed silica nanoparticles for stabilizing bijels. Fumed silica has been commercially available for decades, and is an affordable alternative to Stöber silica particles. However, fumed silica nanoparticles normally exists as secondary particles in the form of branched chains and clusters,14 and it is not obvious that these particle clusters can be blindly treated like monodisperse Stöber particles for the purpose of arresting bijels. Here, we take a pair of partially miscible binary liquids, ethylene carbonate (EC) and p-xylene for our studies. They have a symmetric phase diagram that is shown in Fig. 2. We have reported that HDK®H30 fumed silica (a commercial grade of hydrophobic fumed silica with 50% silanol groups, Wacker-Chemie AG) have appropriate surface chemistry to become interfacial particles for this pair of liquids although they are preferentially wet by EC-rich phase.15 Through silanization of the silica surface using hexamethyldisilazane (HMDS), the H30 silica particles are tuned to be more hydrophobic so as to have higher affinity with xylene-rich phase.
For comparison, we begin by precisely tuning the wettability of H30 silica particles with the aim of achieving neutrally wet particles by varying the amount of HMDS. As stated above, the particles here are more likely secondary particle clusters rather than single particles. The confocal image in Fig. 1 shows an EC/xylene bijel stabilized by these particle clusters treated with appropriate amount of HMDS (see ESI†). This is a typical bicontinuous arrangement with EC-rich and xylene-rich phases, in which the xylene-rich phase (grey region) is dyed using Nile Red. However, pinpointing the optimal amount of HMDS is not straightforward but is a process of trial and error. Fig. 2 shows the protocol for this process. It commences with treating H30 particles using HMDS through a wet chemistry approach. The TEM image in Fig. 2 shows the typical morphology of particle aggregates of H30 fumed silica. Sonication is a common tool to break down these chain-like aggregates into smaller particle clusters composed of several primary silica particles. To introduce fumed silica particles into the binary liquids, dried particles are directly dispersed in a single-fluid phase of EC and xylene mix at a critical composition (45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 mol/mol) by using strong tip sonication. The liquid mixture is heated to 100 °C to give full miscibility (see phase diagram, Fig. 2). After phase separation induced by a fast temperature quench (with the rate and final temperature chosen to avoid crystallization, see ESI†), confocal microscopy is used to identify if the arrest of bijels occurred. The confocal image on the top right in Fig. 2 demonstrates an example of a “bad” bijel with a lot of EC-rich droplets, indicating the particle clusters are over-modified to have slightly higher affinity with the xylene-rich phase. Therefore, less HMDS is required to create neutrally wetting fumed silica particles. H30 particles are manufactured on an industrial scale with constant surface chemistry which is helpful for minimizing the impact of changes of batch. However, a significant effort is still required for optimizing the conditions for reproducibly obtaining particles with neutral wettability.
55 mol/mol) by using strong tip sonication. The liquid mixture is heated to 100 °C to give full miscibility (see phase diagram, Fig. 2). After phase separation induced by a fast temperature quench (with the rate and final temperature chosen to avoid crystallization, see ESI†), confocal microscopy is used to identify if the arrest of bijels occurred. The confocal image on the top right in Fig. 2 demonstrates an example of a “bad” bijel with a lot of EC-rich droplets, indicating the particle clusters are over-modified to have slightly higher affinity with the xylene-rich phase. Therefore, less HMDS is required to create neutrally wetting fumed silica particles. H30 particles are manufactured on an industrial scale with constant surface chemistry which is helpful for minimizing the impact of changes of batch. However, a significant effort is still required for optimizing the conditions for reproducibly obtaining particles with neutral wettability.
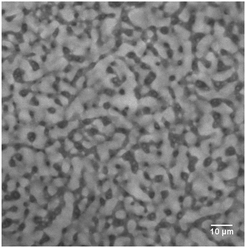 | ||
| Fig. 1 Showing a confocal image of a bijel with bicontinuous EC-rich and xylene-rich phases. In this case the particle wettability has been tuned using HMDS. Note: xylene-rich phase is dyed. | ||
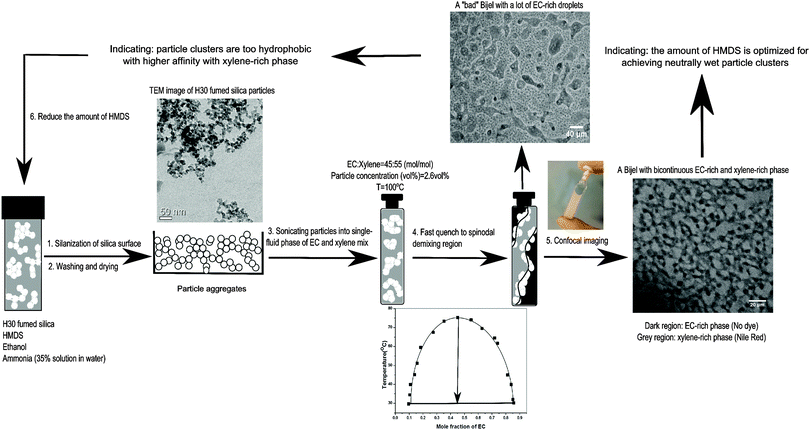 | ||
| Fig. 2 Showing the sequence of steps leading to an optimal amount of HMDS for neutrally wet particle clusters. | ||
Now we demonstrate that the bijel can be made without painstaking tuning of surface chemistry. This approach was hinted at by a study fifteen years ago, in which Binks and Lumsdon studied emulsions with equal volumes of toluene and water stabilized by mixtures of two particles types with different wettability (hydrophilic and hydrophobic fumed silica particles), and demonstrated that varying the ratio between two types of particles could result in a transitional inversion of the emulsions, from oil-in-water to water-in-oil and vice versa.16 Since we have shown that secondary particle clusters can be used to arrest bijels in the same manner as monodisperse particles, it is of great interest to explore whether the wettability of these particle clusters could be tuned by just mixing two grades of fumed silica together instead of tuning the particle surface chemistry.
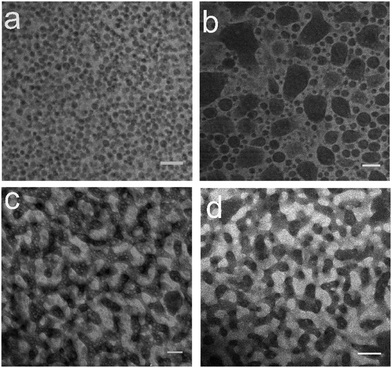
We now empirically find an appropriate combination of fumed silica nanoparticles. We know that H30 silica particles are more easily wet by EC-rich phase and need to be more hydrophobic for bijel fabrication. Based on this, we select two other grades of fumed silica to pair with H30: Aerosil®R972 and Aerosil®R812 are manufactured by Evonik Industries and have 31% and 18% silanol groups, respectively. Hence, both of them are more hydrophobic than H30, with R812 more hydrophobic than R972. Bijel fabrication follows the protocol illustrated in Fig. 2 starting directly from step 3. The total volume fraction of particles is fixed at 2.6 vol%. Fig. 3 shows the confocal images of phase-separated samples at different R972/H30 ratio by weight. As shown in Fig. 3(a), the use of pure R972 leads to the formation of an EC-in-xylene Pickering emulsion. When H30 accounts for 26.7% of the particles, we observe the formation of some EC-rich droplets of non-spherical shape in Fig. 3(b). For single-type particles, this phenomenon can indicate that the surface of particles is close to being equally wet by both liquid phases. When the proportion of H30 is increased to 33.3%, Fig. 3(c) confirms the formation of the bijel with bicontinuous EC-rich and xylene-rich phases. However, this bijel is not complete “clean” as the droplets are spotted in both continuous phases, and we find that the “quality” of this bijel cannot be improved by changing the R972/H30 ratio. With increasing the volume fraction of the particle mixture to 3.9 vol%, Fig. 3(d) shows that an improved bijel is formed and, as expected, the size of continuous liquid phase domains is also reduced. The need for a higher volume fraction of particles presumably reflects improved packing of the secondary clusters for the mixed particle system. For comparison, we also attempt to achieve bijels by using the pair of R812 and H30, but no bijels were formed no matter how we change R812/H30 ratio, which indicates that R812 is too hydrophobic in this case.
It is difficult to reveal directly what happens to the particle mixtures at the interface during spinodal phase separation via imaging techniques. Binks and Lumsdon's work16 suggests a possible scenario for the arrest of bijels using mixed particles as illustrated in Scheme 1. After breaking down R972 and H30 aggregates co-existing in the single-fluid phase of EC and xylene by tip sonication, new hetero-particle clusters are formed through the interaction between R972 and H30 particle clusters. The wettability of these hetero-particle clusters is generally tuned by changing the proportion of R972 and H30. The arrest of bijels takes place once the hetero-particle clusters become neutrally wetting particles and jam the interface formed during phase separation.
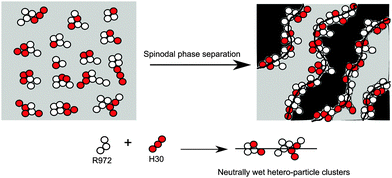 | ||
| Scheme 1 Illustrating a possible mechanism for interfacial jamming by a mixture of R972 and H30 fumed silica particles. | ||
To conclude: solid particles are the key ingredients for bijels. Fumed silica particles exist as secondary particle clusters, and are less ideal than monodisperse Stöber particles for understanding the physics of bijels. However, they are very useful for facile mass production. In this work, we demonstrate that particle clusters with proper surface treatment are suitable for arresting bijels in a similar way to monodisperse particles. We show that the same result can be accomplished without surface treatment via mixing different grades of commercial particles. The success of the mixed particle approach is of particular interest as it is the first time that surface chemistry modification has been removed from bijel fabrication. This simplifies production, enhances reproducibility and is an important step for scaling-up bijels for industrial applications.
We thank EPSRC (UK) for funding this work (Grant No. EP/J007404/1).
Notes and references
- W. L. Ma, C. Y. Yang, X. Gong, K. Lee and A. J. Heeger, Adv. Funct. Mater., 2005, 15, 1617 CrossRef CAS PubMed
; J. R. Wilson, J. S. Cronin, A. T. Duong, S. Rukes, H. Y. Chen, K. Thornton, D. R. Mumm and S. Barnett, J. Power Sources, 2010, 195, 1829 CrossRef PubMed
; H. Zhang, X. Yu and P. V. Braun, Nat. Nanotechnol., 2011, 6, 277 CrossRef PubMed
; M. N. Lee, M. A. Santiago-Cordoba, C. E. Hamilton, N. K. Subbaiyan, J. G. Duque and K. A. D. Obrey, J. Phys. Chem. Lett., 2014, 5, 809 CrossRef PubMed
; N. Shirshova, A. Bismarck, S. Carreyette, Q. P. V. Fontana, E. S. Greenhalgh, P. Jacobsson, P. Johansson, M. J. Marczewski, G. Kalinka, A. R. J. Kucernak, J. Scheers, M. S. P. Shaffer, J. H. G. Steinke and M. Wienrich, J. Mater. Chem. A, 2013, 1, 15300 Search PubMed
; S. G. Levesque, R. M. Lim and M. S. Shoichet, Biomaterials, 2005, 26, 7436 CrossRef PubMed
; M. Martina, G. Subramanyam, J. C. Weaver, D. W. Hutmacher, D. E. Morse and S. Valiyaveettil, Biomaterials, 2005, 26, 5609 CrossRef PubMed
; N. Tanaka, H. Kobayashi, N. Ishizuka, H. Minakuchi, K. Nakanishi, K. Hosoya and T. Ikegami, J. Chromatogr. A, 2002, 965, 35 CrossRef
; M. Motokawa, H. Kobayashi, N. Ishizuka, H. Minakuchi, K. Nakanishi, H. Jinnai, K. Hosoya, T. Ikegami and N. Tanaka, J. Chromatogr. A, 2002, 961, 53 CrossRef
; C. A. L. Colard, R. A. Cave, N. Grossiord, J. A. Covington and S. A. F. Bon, Adv. Mater., 2009, 21, 2894 CrossRef PubMed
; A. N. Pestryakov, V. V. Lunin, A. N. Devochkin, L. A. Petrov, N. E. Bogdanchikova and V. P. Petranovskii, Appl. Catal., A, 2002, 227, 125 CrossRef
.
- C. H. Chew, T. D. Li, L. H. Gan, C. H. Quek and L. M. Gan, Langmuir, 1998, 14, 6068 CrossRef CAS
; F. Gao, C. C. Ho and C. C. Co, Macromolecules, 2006, 39, 9467 CrossRef
; B. H. Jones and T. P. Lodge, Polym. J., 2012, 44, 131 CrossRef PubMed
.
- M. E. Cates and P. S. Clegg, Soft Matter, 2008, 4, 2132 RSC
; K. Stratford, R. Adhikari, I. Pagonabarraga, J. C. Desplat and M. E. Cates, Science, 2005, 309, 2198 CrossRef CAS PubMed
; E. M. Herzig, K. A. White, A. B. Schofield, W. C. K. Poon and P. S. Clegg, Nat. Mater., 2007, 6, 966 CrossRef PubMed
.
- M. N. Lee and A. Mohraz, Adv. Mater., 2010, 22, 4836 CrossRef CAS PubMed
; M. N. Lee and A. Mohraz, J. Am. Chem. Soc., 2011, 133, 6945 CrossRef PubMed
; M. N. Lee, J. H. J. Thijssen, J. A. Witt, P. S. Clegg and A. Mohraz, Adv. Funct. Mater., 2013, 23, 417 CrossRef PubMed
.
- H. Firoozmand, B. S. Murray and E. Dickinson, Langmuir, 2009, 25, 1300 CrossRef CAS PubMed
.
- J. W. Tavacoli, J. H. J. Thijssen, A. B. Schofield and P. S. Clegg, Adv. Funct. Mater., 2011, 21, 2020 CrossRef CAS PubMed
.
- N. Hijnen, D. Cai and P. S. Clegg, Soft Matter, 2015, 11, 4351 RSC
.
- L. Imperiali, C. Clasen, J. Fransaer, C. W. Macosko and J. Vermant, Mater. Horiz., 2014, 1, 139 RSC
.
- J. A. Witt, D. R. Mumm and A. Mohraz, Soft Matter, 2013, 9, 6773 RSC
.
- L. Bai, J. W. Fruehwirth, X. Cheng and C. W. Macosko, Soft Matter, 2015, 11, 5282 RSC
.
- K. A. White, A. B. Schofield, P. Wormald, J. W. Tavacoli, B. P. Binks and P. S. Clegg, J. Colloid Interface Sci., 2011, 359, 126 CrossRef CAS PubMed
.
- M. Reeves, A. T. Brown, A. B. Schofield, M. E. Cates and J. H. J. Thijssen, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2015, 92, 032308 CrossRef
.
- M. Cui, T. Emrick and T. P. Russell, Science, 2013, 342, 460 CrossRef CAS PubMed
.
- J. Nordström, A. Matic, J. Sun, M. Forsyth and D. R. MacFarlane, Soft Matter, 2010, 6, 2293 RSC
.
- D. Cai, J. H. T. Thijssen and P. S. Clegg, ACS Appl. Mater. Interfaces, 2014, 6, 9214 CAS
.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2000, 16, 3748 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc07346d |
| This journal is © The Royal Society of Chemistry 2015 |