Open Access Article
Open Access ArticleSolution and air stable host/guest architectures from a single layer covalent organic framework†
D.
Cui
a,
J. M.
MacLeod
 *ab,
M.
Ebrahimi
a,
D. F.
Perepichka
c and
F.
Rosei
*acd
*ab,
M.
Ebrahimi
a,
D. F.
Perepichka
c and
F.
Rosei
*acd
aCentre Énergie, Matériaux et Télécommunications, Institut National de la Recherche Scientifique, 1650 Boulevard Lionel-Boulet, Varennes, Québec J3X 1S2, Canada. E-mail: jennifer.macleod@qut.edu.au; rosei@emt.inrs.ca
bSchool of Chemistry, Physics, and Mechanical Engineering, Queensland University of Technology (QUT), Brisbane, 4000 QLD, Australia
cCenter for Self-Assembled Chemical Structures, McGill University, 801 Sherbrooke Street West, Montreal, Quebec H3A 0B8, Canada
dInstitute for Fundamental and Frontier Science, University of Electronic Science and Technology of China, Chengdu 610054, P. R. China
First published on 24th September 2015
Abstract
We show that the surface-supported two-dimensional covalent organic framework (COF) known as COF-1 can act as a host architecture for C60 fullerene molecules, predictably trapping the molecules under a range of conditions. The fullerenes occupy the COF-1 lattice at the solution/solid interface, and in dried films of the COF-1/fullerene network that can be synthesized through either drop-deposition of fullerene solution or by a dipstick-type synthesis in which the surface-supported COF-1 is briefly dipped into the fullerene solution.
Covalent organic frameworks (COFs) have gained considerable attention, since first being synthesized in 2005,1 as robust porous crystalline solids that can be synthesized to incorporate application-specific functionalization.2,3 By employing precursor molecules with different functional groups, boronic acid (BA)-based COFs can be tailored for specific applications, for example the storage of hydrogen4,5 and other gases,6,7 or photoconductivity.8,9 A number of investigations, both experimental10,11 and theoretical,12,13 have focused on bulk heterojunctions formed by COFs with ordered charge donors and acceptors, targeting applications in photovoltaics.
The layered structure of COFs is analogous to the packing of graphene into graphite, with non-covalent (dispersive and electrostatic) interactions stabilizing the basal planes defined by the 2D COFs14 into 3D crystals. There is hence considerable interest is isolating 2D sheets of COF, motivated by analogy with the exceptional materials properties of graphene.15,16 COF platelets several layers thick can be produced by delaminating 3D COFs through sonication17,18 or mechanical exfoliation.19 The direct synthesis of single layer 2D COFs on a substrate addresses the advantage of defining the orientation of the COFs, which is necessary for most device applications,20,21 and allows for the use of tailored vapor-solid reactions to produce 2D COFs.22
The surface-confined synthesis of COFs has previously been carried out by self-condensation of 1,4-benzenediboronic acid (BDBA) under ultrahigh vacuum (UHV). Under these conditions, the rapid removal of the by-product water molecules precludes reversibility and leads to a relatively disordered network of poly(BDBA),23,24 a single layer of COF-1.1 When synthesized on-surface under ambient pressure and elevated humidity, defect correction within the COF lattice leads to improved structural order.25,26 As has been demonstrated in 3D COFs,27 the range of available BA derivatives allows to tune the structural properties of surface-synthesized COFs.28
On the other hand, extensive work has been carried out on surface-confined, single layer host/guest (H/G) architectures where the host is a noncovalent porous molecular network stabilized by,29e.g., hydrogen bonds,30–32 van der Waals interactions,33–35 or metal–organic coordination.36 The formation and characterization of these H/G networks has been performed under both UHV conditions31,35–37 and at the solution/solid interface.30,32,34 Compared with these non-covalent networks, the robustness of COFs presents an advantage for preparation of systems with covalent guest molecules, opening the door to a range of new functional host–guest materials.
Here, we report the synthesis of a single-layer H/G structure that uses highly-oriented pyrolytic graphite (HOPG)-supported COF-1 as the host template. This template stabilizes fullerene guests at the solution/solid interface; in turn, the solution/solid structure can be dried to produce an air-stable H/G architecture. The insertion of guest molecules can also be implemented using a “dipstick” method, wherein substrate-supported COF-1 is dipped into a solution of fullerene molecules and allowed to dry. These results indicate that single-layer COFs may be suitable for applications in molecular sensing or trapping as H/G architectures, and reveal a simple synthesis route that can be applied to the fabrication of 2D donor/acceptor networks.
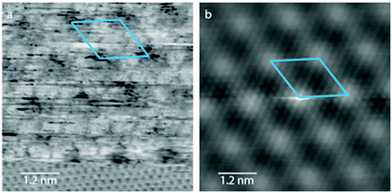
The COF-1 template was prepared on HOPG according to methods previously described in the literature (see Experimental methods section in ESI†).25,28 Boroxine (B3O3) rings are formed through cyclocondensation of three BDBA monomers to produce an extended structure of COF-1, which consists of hexagonal cavities (see S1 in ESI†). STM measurements that simultaneously reveal the atomic lattice of HOPG and the COF-1 mesh indicate that COF-1 aligned with HOPG with a 6 × 6 epitaxial unit cell, as shown in Fig. 1. This corresponds to a lattice parameter of 1.476 nm, and suggests a slight compression with respect to X-ray diffraction measured periodicity of 1.542 nm for bulk COF-1 solid.1 In all experiments, the presence of COF-1 on the HOPG surface was confirmed using scanning tunneling microscopy (STM), which revealed the distinctive honeycomb lattice extending over domains tens of nanometers in size. A typical image of COF-1 is shown in Fig. 3b.
After confirming the presence of COF-1 on the surface, a solution of C60 fullerene in heptanoic acid was applied drop-wise to the COF. Following the application of the fullerene solution, COF-1 was still evident in STM images, and stable adsorption of fullerenes was observed.
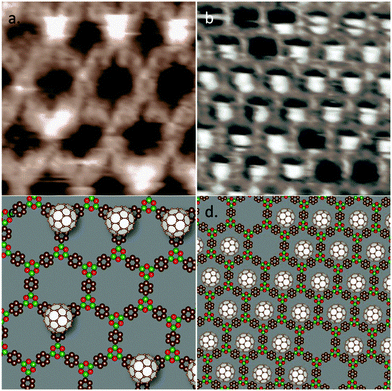
Using a dilute fullerene solution (2 × 10−5 M), a relatively low fullerene guest occupation density was obtained, allowing the position of the fullerene guest molecules to be discerned clearly, as shown in Fig. 2a and b. Two different fullerene adsorption sites can be identified. In Fig. 2a, COF-1 is discernible as a low-contrast hexagonal template, whereas bright protrusions indicate the positions of the fullerene molecules. In this image, the fullerene molecules do not lie within the pores of the host structure, as is typically observed for other systems;30,35,38 instead, each fullerene molecule is adsorbed on top of a boroxine ring of the COF. We denote this site, shown schematically in Fig. 2c, as a top site. A similar adsorption on top of the host framework was observed for C60 adsorption on oligothiophene macrocycles, where a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 donor–acceptor complex is formed between the C60 and the electron rich oligothiophene macrocycle.34 Electrostatic forces are also significant in COF-1 as evident from the bulk 3D crystal structure of COF-1. The latter exhibits ABAB (staggered) packing of the 2D basal planes, which positions a benzene ring above each boroxine ring.1
1 donor–acceptor complex is formed between the C60 and the electron rich oligothiophene macrocycle.34 Electrostatic forces are also significant in COF-1 as evident from the bulk 3D crystal structure of COF-1. The latter exhibits ABAB (staggered) packing of the 2D basal planes, which positions a benzene ring above each boroxine ring.1
The fullerene guests can also adsorb within the pores of the BDBA mesh. We denote this adsorption site as the pore site and assign it as slightly off-center within the BDBA pore (Fig. 2d). In Fig. 2b, the fullerene guests appear localized at the lower side of the COF-1 pores. Since this image was acquired by scanning in an upward direction, we interpret the asymmetric appearance of the guests as resulting from an off-centre adsorption (rather than, e.g., attractive tip-sample interaction, which we would expect to lead to bright contrast at the top of the pores).‡ The adsorption site appears to occupy the same position within the pore for all fullerene guests observed in Fig. 2b. We previously observed similar domain-wide positioning of fullerene guests in hydrogen-bonded oligothiophene host networks, where we attributed the domain-wide organization to the electrostatic ordering resulting from a partial charge transfer to the fullerene.39
We did not observe neighbouring domains of top site adsorption and pore site adsorption within the same image, but did routinely observe domains of each adsorption geometry within the same local (2500 nm2) region of the same sample. Although we did not perform a systematic study, we anecdotally observed a preference for top-site adsorption of fullerenes (top-site adsorption is observed approximately ten times more frequently than pore-site adsorption). Adsorption of heptanoic acid within the pore, as has been postulated for other porous 2D nanostructures at the solution/solid interface,40 could affect the adsorption kinetics of pore-site fullerene, making top-site adsorption favourable. Our molecular mechanics calculations (see S3 in ESI†) suggest that pore-site fullerene adsorption is energetically preferred with respect to adsorption on the COF. Between the possible adsorption sites on the COF, adsorption over a boroxine ring is favoured with respect to adsorption over a phenyl ring. Gas-phase density functional theory calculations also indicate an energetic preference for fullerene adsorption over the boroxine compared to the benzene.
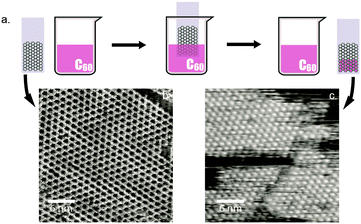
We were able to obtain and image dried films of the COF-1/fullerene H/G system by allowing the solvent to evaporate in ambient (see Experimental methods and S4 in ESI†). The dried H/G monolayer could also be conveniently obtained through dipstick method: following the preparation of COF-1 on HOPG, the HOPG substrate was dipped vertically into a suspension of fullerene in heptanoic acid with a nominal concentration of 10−3 M. The substrate was held in the solution for less than five seconds, and was subsequently left to dry under ambient conditions for 12 hours (Fig. 3). Images acquired after air drying (Fig. 3c) revealed near complete occupancy of the COF-1 host by C60 guest molecules. We conducted control experiments in which a HOPG substrate without COF-1 was dipped into a fullerene suspension of the same nominal concentration (10−3 M) and allowed to dry. In this case, STM imaging did not reveal any molecular structures at the surface. Instead, only typical HOPG features were present, suggesting that the stabilization of the fullerene molecules on the HOPG is dependent on the formation of a COF-1/fullerene H/G structure.
We have shown that COF-1 can act as a robust host for C60 fullerene guest molecules at the solution/solid interface and under ambient conditions, demonstrating the possibility for COF-based host/guest architectures at monolayer thickness. At both the solution/solid interface and in dried films, COF-1 presents a lattice with two distinct fullerene adsorption sites: a pore site, where the C60 molecule is adsorbed on the underlying HOPG, and a top site, where the fullerene is adsorbed on the boroxine ring. Dried COF-1/fullerene films can be produced either through drop deposition or by dipping the HOPG-supported COF-1 into the fullerene solution. Since boronic acid derivatives can form a wide variety of template architectures with different functionalizations, this approach has an inherent flexibility that could be exploited to produce surface-mounted sensors amenable to integration into device architectures.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) through the Discovery Grants program, as well as the Fonds de Recherche du Québec-Nature et Technologies (FRQNT) through a Team Grant and the Ministère du Développement Économique, de l'Innovation et de l'Exportation (MDEIE) through an international collaboration grant. F.R. thanks the Alexander von Humboldt Foundation for an F. W. Bessel Award and acknowledges NSERC for an EWR Steacie Memorial Fellowship. Computational resources and services used in this work were provided by the HPC and Research Support Group, Queensland University of Technology, Brisbane, Australia.
Notes and references
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed.
- S.-Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- X. Zou, H. Ren and G. Zhu, Chem. Commun., 2013, 49, 3925–3936 RSC.
- R. W. Tilford, S. J. Mugavero, P. J. Pellechia and J. J. Lavigne, Adv. Mater., 2008, 20, 2741–2746 CrossRef CAS PubMed.
- J.-T. Yu, Z. Chen, J. Sun, Z.-T. Huang and Q.-Y. Zheng, J. Mater. Chem., 2012, 22, 5369–5373 RSC.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS PubMed.
- S. S. Han, H. Furukawa, O. M. Yaghi and W. A. Goddard III, J. Am. Chem. Soc., 2008, 130, 11580–11581 CrossRef CAS PubMed.
- S. Wan, J. Guo, J. Kim, H. Ihee and D. Jiang, Angew. Chem., Int. Ed., 2009, 48, 5439–5442 CrossRef CAS PubMed.
- X. Ding, L. Chen, Y. Honsho, X. Feng, O. Saengsawang, J. Guo, A. Saeki, S. Seki, S. Irle, S. Nagase, V. Parasuk and D. Jiang, J. Am. Chem. Soc., 2011, 133, 14510–14513 CrossRef CAS PubMed.
- L. Chen, K. Furukawa, J. Gao, A. Nagai, T. Nakamura, Y. Dong and D. Jiang, J. Am. Chem. Soc., 2014, 136, 9806–9809 CrossRef CAS PubMed.
- M. Dogru, M. Handloser, F. Auras, T. Kunz, D. Medina, A. Hartschuh, P. Knochel and T. Bein, Angew. Chem., Int. Ed., 2013, 125, 2992–2996 CrossRef PubMed.
- B. T. Koo and P. Clancy, Mol. Simul., 2014, 40, 58–70 CrossRef CAS PubMed.
- B. T. Koo, P. G. Berard and P. Clancy, J. Chem. Theory Comput., 2015, 11, 1172–1180 CrossRef CAS.
- B. Lukose, A. Kuc and T. Heine, Chem. – Eur. J., 2011, 17, 2388–2392 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang, S. a. Dubonos, I. Grigorieva and A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- D. F. Perepichka and F. Rosei, Science, 2009, 323, 216–217 CrossRef CAS PubMed.
- I. Berlanga, M. L. Ruiz-González, J. M. González-Calbet, J. L. G. Fierro, R. Mas-Ballesté and F. Zamora, Small, 2011, 7, 1207–1211 CrossRef CAS PubMed.
- I. Berlanga, R. Mas-Ballesté and F. Zamora, Chem. Commun., 2012, 48, 7976–7978 RSC.
- S. Chandra, S. Kandambeth, B. P. Biswal, B. Lukose, S. M. Kunjir, M. Chaudhary, R. Babarao, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 17853–17861 CrossRef CAS PubMed.
- J. W. Colson and W. R. Dichtel, Nat. Chem., 2013, 5, 453–465 CrossRef CAS PubMed.
- S.-L. Cai, W.-G. Zhang, R. N. Zuckermann, Z.-T. Li, X. Zhao and Y. Liu, Adv. Mater., 2015 DOI:10.1002/adma.201500124.
- X.-H. Liu, C.-Z. Guan, S.-Y. Ding, W. Wang, H.-J. Yan, D. Wang and L.-J. Wan, J. Am. Chem. Soc., 2013, 135, 10470–10474 CrossRef CAS PubMed.
- S. Clair, O. Ourdjini, M. Abel and L. Porte, Chem. Commun., 2011, 47, 8028–8030 RSC.
- S. Clair, M. Abel and L. Porte, Chem. Commun., 2014, 50, 9627–9635 RSC.
- J. r. F. Dienstmaier, A. M. Gigler, A. J. Goetz, P. Knochel, T. Bein, A. Lyapin, S. Reichlmaier, W. M. Heckl and M. Lackinger, ACS Nano, 2011, 5, 9737–9745 CrossRef CAS PubMed.
- C.-Z. Guan, D. Wang and L.-J. Wan, Chem. Commun., 2012, 48, 2943–2945 RSC.
- A. P. Cote, H. M. El-Kaderi, H. Furukawa, J. R. Hunt and O. M. Yaghi, J. Am. Chem. Soc., 2007, 129, 12914–12915 CrossRef CAS PubMed.
- J. r. F. Dienstmaier, D. D. Medina, M. Dogru, P. Knochel, T. Bein, W. M. Heckl and M. Lackinger, ACS Nano, 2012, 6, 7234–7242 CrossRef CAS PubMed.
- F. Cicoira, C. Santato and F. Rosei, Top. Curr. Chem., 2008, 285, 203–267 CrossRef CAS.
- S. J. Griessl, M. Lackinger, F. Jamitzky, T. Markert, M. Hietschold and W. M. Heckl, J. Phys. Chem. B, 2004, 108, 11556–11560 CrossRef CAS.
- J. A. Theobald, N. S. Oxtoby, M. A. Phillips, N. R. Champness and P. H. Beton, Nature, 2003, 424, 1029–1031 CrossRef CAS PubMed.
- D. den Boer, G. D. Han and T. M. Swager, Langmuir, 2014, 30, 762–767 CrossRef CAS PubMed.
- G.-B. Pan, X.-H. Cheng, S. Höger and W. Freyland, J. Am. Chem. Soc., 2006, 128, 4218–4219 CrossRef CAS PubMed.
- E. Mena-Osteritz and P. Bäuerle, Adv. Mater., 2006, 18, 447–451 CrossRef CAS PubMed.
- H. Spillmann, A. Kiebele, M. Stöhr, T. A. Jung, D. Bonifazi, F. Cheng and F. Diederich, Adv. Mater., 2006, 18, 275–279 CrossRef CAS PubMed.
- S. Stepanow, M. Lingenfelder, A. Dmitriev, H. Spillmann, E. Delvigne, N. Lin, X. Deng, C. Cai, J. V. Barth and K. Kern, Nat. Mater., 2004, 3, 229–233 CrossRef CAS PubMed.
- S. J. Griessl, M. Lackinger, F. Jamitzky, T. Markert, M. Hietschold and W. M. Heckl, Langmuir, 2004, 20, 9403–9407 CrossRef CAS PubMed.
- M. Li, K. Deng, S. B. Lei, Y. L. Yang, T. S. Wang, Y. T. Shen, C. R. Wang, Q. D. Zeng and C. Wang, Angew. Chem., Int. Ed., 2008, 120, 6819–6823 CrossRef PubMed.
- J. M. MacLeod, O. Ivasenko, C. Fu, T. Taerum, F. Rosei and D. F. Perepichka, J. Am. Chem. Soc., 2009, 131, 16844–16850 CrossRef CAS PubMed.
- R. Gutzler, T. Sirtl, J. R. F. Dienstmaier, K. Mahata, W. M. Heckl, M. Schmittel and M. Lackinger, J. Am. Chem. Soc., 2010, 132, 5084–5090 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Methods, details of DFT calculations, additional STM images. See DOI: 10.1039/c5cc07059g |
| ‡ Tip-sample interaction has been observed for this system, and, for example, enables the demonstration of a transfer of a C60 molecule from one cavity of the host structure to an adjacent one (see S4 in ESI†). |
| This journal is © The Royal Society of Chemistry 2015 |