Open Access Article
Open Access ArticleEnantioselective hydrogenation of cyclic imines catalysed by Noyori–Ikariya half-sandwich complexes and their analogues†
B.
Vilhanová
*a,
J.
Václavík
bc,
P.
Šot
a,
J.
Pecháček
a,
J.
Zápal
c,
R.
Pažout
d,
J.
Maixner
d,
M.
Kuzma
c and
P.
Kačer
*a
aDepartment of Organic Technology, University of Chemistry and Technology, Technická 5, CZ-166 28 Prague, Czech Republic. E-mail: vilhanob@vscht.cz; kacerp@vscht.cz
bInstitute of Organic Chemistry and Biochemistry, v.v.i., Academy of Sciences of the Czech Republic, Flemingovo nám. 2, CZ-166 10 Prague, Czech Republic
cLaboratory of Molecular Structure Characterization, Institute of Microbiology, v.v.i., Academy of Sciences of the Czech Republic, Vídeňská 1083, CZ-142 20 Prague, Czech Republic
dCentral Laboratories, University of Chemistry and Technology, Technická 5, CZ-166 28 Prague, Czech Republic
First published on 19th October 2015
Abstract
A method for enantioselective hydrogenation of cyclic imines with gaseous hydrogen has been developed. Easily accessible Noyori–Ikariya Ru(II) and Rh(III) complexes can be used directly without an inert atmosphere. Substrate activation has been achieved by trifluoroacetic acid. A new hydroxyl-functionalized complex is reported, showing high activity in transfer hydrogenation.
Efficient methods toward the construction of optically enriched amines are in the forefront of modern synthetic chemistry.1 One such method is the asymmetric transfer hydrogenation (ATH) of imines catalysed by chiral half-sandwich Ru(II),2 Rh(III)3,4 and Ir(III)3 complexes. Asymmetric hydrogenation (AH) reactions directly using gaseous hydrogen are often preferred, and the existing ATH catalytic systems have thus been modified to be applicable under AH conditions.5 The first use of complexes [Ru(II)Cl(η6-arene)(N-R-sulfonyl-DPEN)] (where DPEN = 1,2-diphenylethylene-1,2-diamine and R = aryl or alkyl) in AH was reported by Ohkuma et al. in 2006 in the reduction of ketones.5a The key difference from ATH was a switch from basic to mildly acidic conditions (i.e., methanol as the solvent). The authors found autodissociation of the Ru–Cl bond to be essential.5c To facilitate this, a Ru–triflate complex was used.5a–e Wills's5f and Ikariya's5g tethered complexes proved useful in AH of ketones without modification, i.e. as Ru–chloride complexes.
The first AH of cyclic imines using this catalytic system was shown by Li et al. on a Cp*–Rh(III) (Cp* = 1,2,3,4,5-pentamethylcyclopentadienyl) catalyst.6a They generated a [Rh]+SbF6− complex in situ by reacting the Rh–chloride complex with AgSbF6. For acyclic imines, a Cp*–Ir(III) catalyst with a chiral phosphate anion was employed.6b Ikariya and co-workers developed an alternative strategy for the AH of acyclic imines with an Ir(III) catalyst and AgSbF6, proposing that Ag+ can activate the substrate.6c Imine AH was further screened by Chen et al. by testing various counteranions.6d,e They also synthesized enantioenriched 1,2,3,4-tetrahydroquinolines via AH of quinolines using the Ru–triflate complex under a variety of reaction conditions.6f–i
In this work, we present a method for the AH of cyclic imines with the aim of simplifying the reaction conditions: standard metal–chloride complexes are employed and the necessity of an inert atmosphere is avoided.
For the initial experiments, catalyst [RuCl(η6-p-cymene)(S,S)-TsDPEN] (A) and substrate 6,7-dimethoxy-1-methyl-3,4-dihydroisoquinoline (6,7-dimethoxy-1-methyl-DHIQ, 1) were selected because both substances are very often used to benchmark ATH.7 Screening of solvents (Table S1, ESI†) revealed that the reaction did not proceed in acetonitrile whilst only minimal reactivity was observed in DMSO (<5% conversion) and methanol (10% conversion). In this catalytic system, it is presumed that imines require activation by polarization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in order to undergo reduction.8 This activation has been achieved by Brønsted8a or Lewis acids,8a,9 the electron-withdrawing effect of a CF3 group,10 or conversion of the imine to a quarternary iminium salt.11 Therefore, we envisaged that we could activate the C
N bond in order to undergo reduction.8 This activation has been achieved by Brønsted8a or Lewis acids,8a,9 the electron-withdrawing effect of a CF3 group,10 or conversion of the imine to a quarternary iminium salt.11 Therefore, we envisaged that we could activate the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond by adding a suitable acid (Table S2, ESI†). Using methanol as the solvent, tetrafluoroboric acid (48% wt solution in water, 1 equiv.) enhanced the reaction only moderately (19% conversion) and trifluoromethanesulfonic acid (1 equiv.) had no effect (9% conversion). Gratifyingly, trifluoroacetic acid (1 equiv.) increased the conversion to 57%. Smaller amounts were found to be insufficient, probably because the substrate could not be fully protonated.‡
N bond by adding a suitable acid (Table S2, ESI†). Using methanol as the solvent, tetrafluoroboric acid (48% wt solution in water, 1 equiv.) enhanced the reaction only moderately (19% conversion) and trifluoromethanesulfonic acid (1 equiv.) had no effect (9% conversion). Gratifyingly, trifluoroacetic acid (1 equiv.) increased the conversion to 57%. Smaller amounts were found to be insufficient, probably because the substrate could not be fully protonated.‡![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 Excess trifluoroacetic acid gave no improvement, but could be detrimental by causing partial or full protonation of the TsDPEN ligand. The conversion was further improved to 96% by increasing the temperature to 40 °C. At this point, it was found that the reaction was equally feasible in dichloromethane under the conditions developed for methanol. However, this avenue was not pursued any further as it is not a preferred solvent in the pharmaceutical industry, mainly for environmental reasons. Dried and/or degassed solvents were not necessary since we obtained identical results both with and without paying attention to this aspect.
12 Excess trifluoroacetic acid gave no improvement, but could be detrimental by causing partial or full protonation of the TsDPEN ligand. The conversion was further improved to 96% by increasing the temperature to 40 °C. At this point, it was found that the reaction was equally feasible in dichloromethane under the conditions developed for methanol. However, this avenue was not pursued any further as it is not a preferred solvent in the pharmaceutical industry, mainly for environmental reasons. Dried and/or degassed solvents were not necessary since we obtained identical results both with and without paying attention to this aspect.
We still could not achieve full conversion. Neither the addition of acid or catalyst, nor increasing the reaction temperature, nor prolongation of the reaction time led to significant improvement. Eventually, it was discovered that the order of addition of the reaction mixture components played a critical role. The original order was as follows: (1) substrate, (2) methanol, (3) catalyst, and (4) acid – after switching the catalyst and acid, the reaction proceeded to full conversion. Given that the substrate must be protonated, it is advisable for it to react with the acid first. Subsequently, the catalyst can be added with a significantly lower risk of deactivation.
Under optimized conditions, the method was tested on a mini-library containing six catalysts and twelve substrates (Fig. 1). Aside from complex A, its derivative, the 16e− amido complex B, was selected to examine the role of the Ru–Cl bond autodissociation5c and capability of B to react with hydrogen – e.g. in the AH of quinolines it was reported that B was catalytically inactive.6i Complex C was chosen as an alternative to A, bearing a different η6-arene. D and E were studied as representatives of the newer tethered complexes, and Rh(III) analogue F was included in order to show the applicability of the method on another metal. As substrates we tested nine DHIQs differing by substitutions in positions 1, 6 and 7 (1–9), 3,4-dihydro-β-carbolines harmalane (10) and harmaline (11), and cyclic N-sulfonyl imine 12.
| Imine | Complex | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |||||||||
| cnv | ee | cnv | ee | cnv | ee | cnv | ee | cnv | ee | cnv | ee | cnv | ee | cnv | ee | |
| a Amount of substrate n = 44 μmol, concentration of substrate c = 88 mM, catalyst loading 1 mol%, TFA-to-substrate molar ratio A/S = 1, p(H2) = 15 bar, 40 °C, 6 h. Order of addition: imine-solvent-acid-catalyst. | ||||||||||||||||
| 1 | >99 | 96 | >99 | 96 | >99 | 96 | >99 | 78 | 44 | 94 | >99 | 89 | 98 | 95 | 95 | 96 |
| 2 | >99 | 87 | >99 | 85 | >99 | 81 | >99 | 59 | >99 | 84 | >99 | 76 | >99 | 87 | >99 | 87 |
| 3 | >99 | 92 | >99 | 92 | >99 | 89 | 96 | 68 | 84 | 90 | >99 | 90 | >99 | 92 | 90 | 91 |
| 4 | >99 | 93 | >99 | 92 | >99 | 90 | >99 | 74 | >99 | 91 | >99 | 75 | >99 | 91 | >99 | 91 |
| 5 | 1 | n.d. | 4 | n.d. | 3 | n.d. | 24 | 11 | 0 | n.d. | >99 | 7 | 2 | n.d. | 6 | n.d. |
| 6 | 26 | 5 | 2 | n.d. | 1 | n.d. | 12 | n.d. | 0 | n.d. | >99 | 9 | 2 | n.d. | 3 | n.d. |
| 7 | >99 | 97 | >99 | 96 | 98 | 97 | 70 | 83 | 31 | 95 | 98 | 96 | 98 | 97 | 99 | 97 |
| 8 | >99 | 98 | >99 | 98 | 98 | 98 | 90 | 96 | 27 | 98 | >99 | 99 | 99 | 98 | 99 | 96 |
| 9 | 39 | 72 | 35 | 70 | 38 | 39 | >99 | 66 | 23 | 61 | >99 | 84 | 88 | 82 | >99 | 81 |
| 10 | >99 | 97 | >99 | 97 | >99 | 97 | 70 | 93 | 69 | 95 | >99 | 95 | 99 | 96 | 99 | 96 |
| 11 | >99 | 95 | >99 | 95 | 98 | 92 | 71 | 88 | 90 | 91 | >99 | 93 | 99 | 93 | 98 | 90 |
Full conversion was achieved with substrates 1–4 in 6 hours with complexes A–D and F (Table 1). A and B showed comparable activity and enantioselectivity, implying that the Ru–Cl autodissociation is not rate limiting and that B can react with molecular hydrogen under such conditions. Interesting differences emerged with E – the 6-methoxy substituted substrates 1 and 3 showed lower reactivity than 2 and 4, which agrees with our previous ATH study on this set of substrates.13
1-Aryl-DHIQs 5 and 6 were poorly reactive with the exception of Rh(III) complex F, delivering nearly racemic products, which is in agreement with previously reported findings from ATH.3 Otherwise, maximum conversions (26% and 24%) were achieved with combinations 6-A and 5-D, respectively. 1-Aryl-substituted DHIQs thus require different reaction conditions and most likely different, more reactive catalysts. Experiments towards the efficient extension of our methodology to these substrates are currently underway.
6,7-Diethoxy-substituted imine 7 performed similarly to those described above, except that with D and E we observed lower reactivity. Imines 8 and 9, bearing an isopropyl group in position 1, showed quite dissimilar performance: just like 7, the 6,7-dimethoxy derivative 8 gave very high conversions with all complexes apart from D and E. On the contrary, 9 exhibited sluggish reactivity and surprisingly, only the tethered complex D afforded full conversion out of the A–E Ru(II) series. β-Carbolines 10 and 11 behaved in line with the previously described substrates. Apparently, the structurally more complex imines (containing bulkier substituents in positions 1, 6 or 7, or having the β-carboline scaffold) are less reactive when using tethered catalysts D and E. Eventually we attempted the AH of N-sulfonyl imine 12 – unfortunately, it was not soluble under the reaction conditions in methanol, and did not react in dichloromethane.
Good-to-excellent enantioselectivity was achieved in most cases (Table 1). Lower ee values were typically obtained with complexes D and F, and imine 9 gave only moderate enantioselectivity.
One reaction was performed on a ten-fold scale with complex A and substrate 1 (0.44 mmol of 1; all other components scaled up accordingly) to show the synthetic utility of these reactions. The product was obtained at 92% yield, 97% ee and >95% purity.
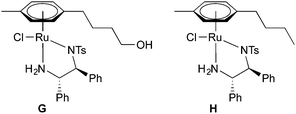
In the course of this project, we also synthesized a complex bearing a 4-hydroxybutyl group at the η6-arene (G, see Fig. 2). Such a functionalization offers the possibility of its immobilization – unlike heterogenization via the DPEN ligand, which has been shown on many examples,7 the arene ligand has been utilized much less often.14
We employed the [4+2] cycloaddition reaction5g,15,16 of a terminal alkyne (5-hexyn-1-ol) with a 1,3-diene (isoprene) to conveniently synthesize cyclohexadiene 13 (Scheme S1, ESI†). In the original procedure,5g the authors suggested that along with 1,4-substituted cyclohexa-1,4-diene (13a), only analogous 1,3-substituted cyclohexa-1,4-diene (13c) was formed (>80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio). However, with the aid of 2D NMR spectroscopy (Fig. S2, ESI†), we identified that three regioisomers 13a, 13b (being a 1,4-substituted cyclohexa-1,3-diene) and 13c were formed in a ratio of 74
20 ratio). However, with the aid of 2D NMR spectroscopy (Fig. S2, ESI†), we identified that three regioisomers 13a, 13b (being a 1,4-substituted cyclohexa-1,3-diene) and 13c were formed in a ratio of 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. As these could not be separated, the mixture served for the synthesis of Ru(II) dimer (14a),17 which further afforded G by complexation with the (S,S)-TsDPEN ligand (Scheme S1, ESI†). As expected, dimer 14a contained around 7% of the meta-substituted analogue (14b) originating from 13c. In the spectra of G this could no longer be resolved due to signal broadening. Complex G was further characterized by single-crystal X-ray diffraction (Fig. S3 and S4, ESI).†
7. As these could not be separated, the mixture served for the synthesis of Ru(II) dimer (14a),17 which further afforded G by complexation with the (S,S)-TsDPEN ligand (Scheme S1, ESI†). As expected, dimer 14a contained around 7% of the meta-substituted analogue (14b) originating from 13c. In the spectra of G this could no longer be resolved due to signal broadening. Complex G was further characterized by single-crystal X-ray diffraction (Fig. S3 and S4, ESI).†
In the context of this study, we were interested in both AH and ATH with G to evaluate its eligibility for its use as a modular substitute for A. Monitoring the ATH of imines 1–4, 8–10 and 12 by 1H NMR,18 we observed enhanced reactivity in comparison to complex A (Table 2, Fig. S1, ESI†). To probe whether the hydroxyl group of G was responsible for this, we synthesized complex H (Fig. 2, Scheme S1, ESI†), which does not contain any heteroatom on the η6-arene. Surprisingly, ATH using H revealed similar or only slightly lower performance compared to G, except for N-sulfonyl imine 12, which can form an O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bond with G that may increase the reactivity. Therefore, the hydroxyl group was not the key parameter responsible for the enhanced reactivity of G. The reaction kinetics (one example in Fig. 3, complete set of results in Fig. S1, ESI†) suggest the hydrogenations with A to be first order, while G and H are closer to zero-order kinetics. Systematic studies to clarify this phenomenon are currently underway in our laboratories.
S hydrogen bond with G that may increase the reactivity. Therefore, the hydroxyl group was not the key parameter responsible for the enhanced reactivity of G. The reaction kinetics (one example in Fig. 3, complete set of results in Fig. S1, ESI†) suggest the hydrogenations with A to be first order, while G and H are closer to zero-order kinetics. Systematic studies to clarify this phenomenon are currently underway in our laboratories.
| ee (%) | TONb | TOFc (h−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | G | H | A | G | H | A | G | H | |
a Amount of substrate n = 55 μmol, concentration of substrate c = 75 mM, catalyst loading 0.5 mol%, hydrogen source HCOOH/Et3N (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 30 °C.
b Turnover number calculated after 50 min.
c Turnover frequency calculated at 20% conversion. 2), 30 °C.
b Turnover number calculated after 50 min.
c Turnover frequency calculated at 20% conversion.
|
|||||||||
| 1 | 93 | 94 | 92 | 142 | 174 | 154 | 178 | 195 | 175 |
| 2 | 85 | 86 | 85 | 108 | 138 | 135 | 136 | 155 | 157 |
| 3 | 90 | 91 | 90 | 110 | 138 | 126 | 146 | 163 | 162 |
| 4 | 87 | 88 | 87 | 125 | 134 | 114 | 160 | 153 | 145 |
| 8 | 93 | 95 | 95 | 78 | 160 | 150 | 111 | 207 | 198 |
| 9 | 50 | 70 | 68 | 29 | 82 | 68 | 32 | 102 | 83 |
| 10 | 92 | 93 | 93 | 148 | 200 | 146 | 183 | 209 | 187 |
| 12 | 92 | 92 | 92 | 37 | 76 | 39 | 45 | 97 | 46 |
 | ||
Fig. 3 ATH of imine 1 catalysed by complexes A, G and H using HCOOH/Et3N (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in CD3CN at 0.5 mol% catalyst loading and a temperature of 30 °C. 2) in CD3CN at 0.5 mol% catalyst loading and a temperature of 30 °C. | ||
Both G and H were also tested in AH under optimised conditions (Table 1), and their activity was comparable to or higher than that of Ru(II) complexes A–E. In particular, the poorly reactive imine 9 was hydrogenated with high conversion. The AH method was thus extended to a complex containing a hydroxyl, and has the potential to be operable with heterogenized catalysts derived from G, which are being developed in our laboratories.
The ees delivered by G and H were very similar to those obtained with A–F in both AH (Table 1) and ATH (Table 2) for all substrates, again with the exception of 9, in which case we observed a significant increase. This means that replacing the isopropyl group of p-cymene with butyl or hydroxybutyl had no negative effect on the ee. A similar observation was made earlier for a complex bearing a 2-hydroxyethoxy group on the η6-arene.19
In conclusion, we present a simple method for the AH of cyclic imines catalysed by Ru(II) and Rh(III) half-sandwich complexes using trifluoroacetic acid for substrate activation. The catalysts were used in the standard Ru–chloride forms, which are easily accessible and non-air sensitive. In contrast to other reported approaches to imine AH with hydrogen gas, this method does not require air-sensitive additives or an inert atmosphere. New hydroxybutyl-arene-functionalized Ru(II) catalyst was synthesized – such functionalization gives the possibility of modular heterogenization. This complex (and its congener lacking the hydroxyl group) showed enhanced reactivity in imine ATH and performed similarly to the existing catalysts under the newly-developed AH conditions. Asymmetric hydrogenation of imines is still a discussed topic. By this work, we wish to add one more option to the highly versatile collection of possibilities offered by the Noyori–Ikariya class of catalysts.
This work was financially supported by the Czech Science Foundation (P106/12/1276 and 15-08992S), grant for long-term conceptual development of the Institute of Microbiology (RVO: 61388971) and the National Program of Sustainability (NPU I (LO-2015) MSMT – 34870/2013). The research was conducted within the infrastructure built up from the support of the Operational Program Prague – Competitiveness (projects CZ.2.16/3.1.00/22197, CZ.2.16/3.1.00/24501 and CZ.2.16/3.1.00/24023).
Notes and references
- T. C. Nugent and M. El-Shazly, Adv. Synth. Catal., 2010, 352, 753–819 CrossRef CAS.
- R. Noyori and S. Hashiguchi, Acc. Chem. Res., 1997, 30, 97–102 CrossRef CAS.
- J. Mao and D. C. Baker, Org. Lett., 1999, 1, 841–843 CrossRef CAS PubMed.
- K. Murata and T. Ikariya, J. Org. Chem., 1999, 64, 2186–2187 CrossRef CAS.
- (a) T. Ohkuma, N. Utsumi, K. Tsutsumi, K. Murata, C. Sandoval and R. Noyori, J. Am. Chem. Soc., 2006, 128, 8724–8725 CrossRef CAS PubMed; (b) C. A. Sandoval, T. Ohkuma, N. Utsumi, K. Tsutsumi, K. Murata and R. Noyori, Chem. – Asian J., 2006, 1, 102–110 CrossRef CAS PubMed; (c) C. A. Sandoval, F. Bie, A. Matsuoka, Y. Yamaguchi, H. Naka, Y. Li, K. Kato, N. Utsumi, K. Tsutsumi, T. Ohkuma and R. Noyori, Chem. – Asian J., 2010, 5, 806–816 CrossRef CAS PubMed; (d) T. Ohkuma, K. Tsutsumi, N. Utsumi, N. Arai, R. Noyori and K. Murata, Org. Lett., 2007, 9, 255–257 CrossRef CAS PubMed; (e) T. Ohkuma, N. Utsumi, M. Watanabe, K. Tsutsumi, N. Arai and K. Murata, Org. Lett., 2007, 9, 2565–2567 CrossRef CAS PubMed; (f) K. E. Jolley, A. Zanotti-Gerosa, F. Hancock, A. Dyke, D. M. Grainger, J. A. Medlock, H. G. Nedden, J. J. M. Le Paih, S. J. Roseblade, A. Seger, V. Sivakumar, I. Prokes, D. J. Morris and M. Wills, Adv. Synth. Catal., 2012, 354, 2545–2555 CrossRef CAS; (g) T. Touge, T. Hakamata, N. Hideki, T. Kobayashi, N. Sayo, T. Saito, Y. Kayaki and T. Ikariya, J. Am. Chem. Soc., 2011, 133, 14960–14963 CrossRef CAS PubMed.
- (a) C. Li and J. Xiao, J. Am. Chem. Soc., 2008, 130, 13208–13209 CrossRef CAS PubMed; (b) C. Li, C. Wang, B. Villa-Marcos and J. Xiao, J. Am. Chem. Soc., 2008, 130, 14450–14451 CrossRef CAS PubMed; (c) S. Shirai, H. Nara, Y. Kayaki and T. Ikariya, Organometallics, 2009, 28, 802–809 CrossRef CAS; (d) F. Chen, T. Wang, Y. He, Z. Ding, Z. Li, L. Xu and Q.-H. Fan, Chem. – Eur. J., 2011, 17, 1109–1113 CrossRef CAS PubMed; (e) F. Chen, Z. Ding, Y. He, J. Qin, T. Wang and Q.-H. Fan, Tetrahedron, 2012, 68, 5248–5257 CrossRef CAS; (f) H. Zhou, Z. Li, Z. Wang, T. Wang, L. Xu, Y. He, Q.-H. Fan, J. Pan, L. Gu and A. S. C. Chan, Angew. Chem., Int. Ed., 2008, 47, 8464–8467 CrossRef CAS PubMed; (g) Z.-J. Wang, H.-F. Zhou, T.-L. Wang, Y.-M. He and Q.-H. Fan, Green Chem., 2009, 11, 767–769 RSC; (h) T. Wang, L.-G. Zhuo, Z. Li, F. Chen, Z. Ding, Y. He, Q.-H. Fan, J. Xiang, Z.-X. Yu and A. S. C. Chan, J. Am. Chem. Soc., 2011, 133, 9878–9891 CrossRef CAS PubMed; (i) Z.-Y. Ding, T. Wang, Y.-M. He, F. Chen, H.-F. Zhou, Q.-H. Fan, Q. Guo and A. S. C. Chan, Adv. Synth. Catal., 2013, 355, 3727–3735 CrossRef CAS.
- J. Václavík, P. Kačer, M. Kuzma and L. Červený, Molecules, 2011, 16, 5460–5495 CrossRef PubMed.
-
(a) J. B. Åberg, J. S. M. Samec and J.-E. Bäckvall, Chem. Commun., 2006, 2771–2773 Search PubMed;
(b) J. E. D. Martins, G. J. Clarkson and M. Wills, Org. Lett., 2009, 11, 847–850 CrossRef CAS PubMed;
(c) J. Václavík, M. Kuzma, J. P
![[q with combining caron]](https://www.rsc.org/images/entities/char_0071_030c.gif) ech and P. Kačer, Organometallics, 2011, 30, 4822–4829 CrossRef.
ech and P. Kačer, Organometallics, 2011, 30, 4822–4829 CrossRef. - L. Evanno, J. Ormala and P. M. Pihko, Chem. – Eur. J., 2009, 15, 12963–12967 CrossRef CAS PubMed.
- X. Dai and D. Cahard, Adv. Synth. Catal., 2014, 356, 1317–1328 CrossRef CAS.
- J. Wu, F. Wang, Y. Ma, X. Cui, L. Cun, J. Zhu, J. Deng and B. Yu, Chem. Commun., 2006, 1766–1768 RSC.
- Z.-W. Li, T.-L. Wang, Y.-M. He, Z.-J. Wang, Q.-H. Fan, J. Pan and L.-J. Xu, Org. Lett., 2008, 10, 5265–5268 CrossRef CAS PubMed.
- J. Václavík, J. Pecháček, B. Vilhanová, P. Šot, J. Januščák, V. Matoušek, J. Přech, S. Bártová, M. Kuzma and P. Kačer, Catal. Lett., 2013, 143, 555–562 CrossRef.
- (a) M. A. N. Virboul and R. J. M. K. Gebbink, Organometallics, 2012, 31, 85–91 CrossRef CAS; (b) S. B. Wendicke, E. Burri, R. Scopelliti and K. Severin, Organometallics, 2003, 22, 1894–1897 CrossRef CAS; (c) G. J. Sherborne, M. R. Chapman, A. J. Blacker, R. A. Bourne, T. W. Chamberlain, B. D. Crossley, S. J. Lucas, P. C. McGowan, M. A. Newton, T. E. O Screen, P. Thompson, C. E. Willans and B. N. Nguyen, J. Am. Chem. Soc., 2015, 137, 4151–4157 CrossRef CAS PubMed.
- V. Parekh, A. Ramsden and M. Wills, Catal. Sci. Technol., 2012, 2, 406–414 CAS.
- (a) G. Hilt and F.-X. du Mesnil, Tetrahedron Lett., 2000, 41, 6757–6761 CrossRef CAS; (b) F. K. Cheung, A. M. Hayes, D. J. Morris and M. Wills, Org. Biomol. Chem., 2007, 5, 1093–1103 RSC.
- T. Reiner, M. Waibel, A. N. Marziale, D. Jantke, F. J. Kiefer, T. F. Fässler and J. Eppinger, J. Org. Chem., 2010, 695, 2667–2672 CrossRef CAS.
- J. Václavík, J. Pecháček, J. Přech, M. Kuzma, P. Kačer and L. Červený, Chem. Listy, 2012, 106, 206–210 Search PubMed.
- J. Soleimannejad, A. Sisson and C. White, Inorg. Chim. Acta, 2003, 352, 121–128 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental; hydrogenation results, NMR and X-ray data. CCDC 1021406. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc06712j |
| ‡ Fan et al.12 found that the addition of trifluoroacetic acid promoted the AH of quinolines with Ir(III)–triflate complexes. However, Ir(III) complexes are much more reactive and their activity is further enhanced by the triflate counteranion. Therefore, we could not employ these reaction conditions in our work. |
| This journal is © The Royal Society of Chemistry 2016 |