Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Methyl tunnelling of adsorbed methoxy on alumina catalysts†‡
Stewart F.
Parker
*ab,
Victoria García
Sakai
a,
David
Lennon
bc,
Alice
Jackson
d,
Mark R.
Johnson
d and
Upali A.
Jayasooriya
e
aISIS Facility, STFC Rutherford Appleton Laboratory, Chilton, Didcot, Oxfordshire OX11 0QX, UK. E-mail: stewart.parker@stfc.ac.uk
bUK Catalysis Hub, Research Complex at Harwell, STFC Rutherford Appleton Laboratory, Chilton, Didcot, Oxfordshire OX11 0QX, UK
cSchool of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK
dInstitut Laue-Langevin, 71 Avenue des Martyrs, CS 20156, F – 38042 Grenoble Cedex 9, France
eSchool of Chemistry, Faculty of Science, University of East Anglia, Norwich Research Park, Norwich, Norfolk NR4 7TJ, UK
First published on 28th October 2015
Abstract
The aim of this paper is to investigate whether the methyl group of the adsorbed methoxy intermediate on industrial grade alumina catalysts undergoes rotational tunnelling on the wavenumber energy scale. The data show that this is clearly the case for a fraction of the methyl groups and potentially allows the subtle intermolecular interactions between adsorbed species and catalyst to be probed through the exponential dependence of the tunnel frequency on the rotational potential.
Heterogeneous catalysis is a key economic driver in advanced nations.1 It is integral to processes that range from crude oil refining to fine chemical and pharmaceutical production.2 Hence there is enormous interest and effort in optimising catalyst performance.
Alumina is ubiquitous in heterogeneous catalysis, where it is used as an actual catalytic material or as a catalyst support material.3 In fact, due to its general availability, structural stability and ability to be prepared in a variety of pore size distributions, alumina is the most widely used catalyst support material.
The interaction of methanol with alumina is important in the industrial manufacture of methyl chloride, where methanol and hydrogen chloride are combined over high surface area alumina catalysts:
| CH3OH + HCl → CH3Cl + H2O | (1) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) .
.
“Rotational tunnelling describes the phenomenon of the librational states of a molecule whose rotating atoms are indistinguishable, e.g., all protons, being multiplets. The splitting between the substates is called tunnel splitting”.7 Thus for a methyl group, the protons essentially ‘circulate’ between the equivalent positions. If the protons are labelled 1, 2, 3 then the methyl group rotation 123 → 231 → 312 → etc… between the equivalent orientations has a tunnel frequency, ωt, which is largely determined7 by the amplitude of the rotational potential, V:
 | (2) |
 | ||
| Fig. 1 The librational (a) and tunnelling (b) energies calculated as a function of the barrier height, V, for a 3-fold rotational potential. | ||
To our knowledge, this is the first time tunnelling has been looked for on a real catalyst, although Larese et al. have extensively studied physisorbed methane on (essentially) single crystal MgO.8
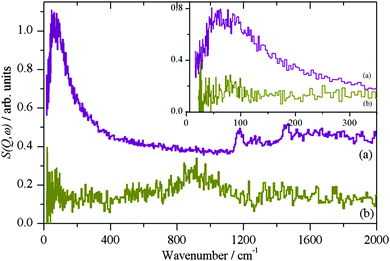
Fig. 2 shows the INS spectrum of η-alumina after reaction with methanol at 373 K. The peaks at 2990, 1456 and 1164 cm−1 are assigned as the C–H stretch, bend and rock of the methyl group and confirms the presence of chemisorbed methoxy.5 The peaks at 3600 and 872 cm−1 are assigned as the O–H stretch and bend of residual hydroxyls on the alumina.4
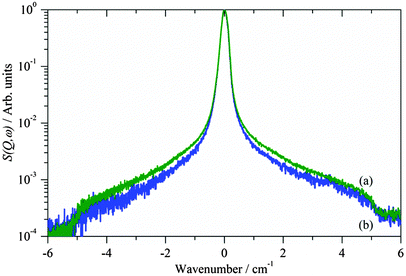
Fig. 3 shows the quasielastic neutron scattering (QENS) spectra at 2 K of clean γ-alumina and after reaction with methanol at 373 K to generate methoxy. It is apparent that there is a marked broadening of the central peak showing that there is additional motion in the system. Comparison of methoxy on η- and γ-alumina, Fig. 4, shows both materials behave similarly, the greater intensity of the η-Al2O3 spectrum arising partly because it has a larger surface area and a greater density of active sites for strong chemisorption, hence the quantity of adsorbed methoxy is greater for η-Al2O3 than for γ-Al2O3. As discussed below, the fraction of sites with low enough rotational barriers to give a tunnelling signal in this energy range must also be taken into consideration.
 | ||
| Fig. 3 QENS spectra at 2 K of γ-alumina (a) after reaction with methanol to generate methoxy (blue) and (b) clean (red). | ||
 | ||
| Fig. 4 Comparison of the QENS spectra at 2 K of (a) η-alumina (olive) and (b) γ-alumina (blue) after reaction with methanol to generate methoxy. | ||
As shown in Fig. 5a, in addition to the apparent quasielastic broadening, there is an additional feature at ∼24 cm−1. A feature at 45 cm−1, is also present in the clean γ-alumina, Fig. 5b, the same features are seen for η-Al2O3.
 | ||
| Fig. 5 QENS spectra at 2 K in an extended spectral range of γ-alumina (a) after reaction with methanol to generate methoxy (blue) and (b) clean (red). | ||
The intensity of neutron scattering spectra is strongly dependent on the incoherent cross section, σ, of the scattering atoms. This property is both element and isotope dependent and in particular σ(1H) = 80.26 barn (1 barn = 1 × 10−28 m2) while σ(2H) = 2.05 barn. Thus, by using selectively deuterated methanol, it is possible to determine the origin of the scattering. Fig. 6a shows the results for the reaction of CH3OD and Fig. 6b for CD3OH with η-alumina. While the profile from reaction with CH3OD (trace (a)) is very similar to those obtained with CH3OH, Fig. 3 and 5, reaction with CD3OH (trace (b)) results in almost no additional scattering, which demonstrates that all the features are due to methoxy, OH produced by the reaction has no effect in this energy region. (Chemisorption of methoxy and methoxy-D3 was confirmed by INS spectroscopy, see Fig. S3 and S4 of the ESI‡).
 | ||
| Fig. 6 QENS spectra at 2 K in a narrow (upper) and a wide (lower) energy range of the reaction products of selectively deuterated methanol with η-Al2O3: (a) CH3OD (purple) and (b) CD3OH (khaki). | ||
The data clearly show that chemisorption of methoxy on either alumina results in significant changes in the very low energy region. The peak at ∼24 cm−1 is assigned to surface modes of the alumina that acquire intensity because they result in motion of the methoxy – ‘riding modes’. A calculation5 at the Γ-point of methoxy on η-alumina predicts such modes around 16 cm−1, non-zone centre modes (which are observable by INS spectroscopy) are also a possible assignment. The feature at 45 cm−1, which is present in all the spectra before and after reaction with methanol must be assigned to a bulk alumina phonon.
The broadening present in Fig. 3–5a would usually be described as quasielastic scattering due to diffusional processes, however, this possibility can be discarded because it is still present at 2 K, see Fig. 3. Rather, we assign it as the signature of methyl tunnelling of the adsorbed methoxy species. The Lorentzian broadening is indicative of a distribution of adsorption sites giving rise to a continuous range of rotational potentials. From the data shown in Fig. 1, tunnelling resolved in the energy range from 0.5 to 6 cm−1 corresponds to rotational barriers, V < 120 cm−1. The fraction of sites with such low barriers can be estimated from the librational spectrum of the methyl groups shown in Fig. 7. Assuming, to a first approximation, that libration frequency varies linearly with V and that the inelastic structure factor for librations is independent of their frequency, the spectrum, up to ∼200 cm−1, reflects directly the distribution of rotational potentials. The tunnelling data corresponds to libration frequencies below 60 cm−1, which represents ∼15% of the spectral weight, and therefore absorbed species, up to 200 cm−1. This result is supported by the relative areas of the quasielastic contribution and the total scattering. For both CH3OH and CH3OD reacted with η-Al2O3, we find ∼15% undergoes rotational tunnelling.
It has been proposed that there are four types of Lewis acid sites on alumina.6 Local structure variation around each of these sites would give rise to a distribution of rotational barriers and therefore libration and tunnelling frequencies. According to the assumptions proposed above, the distribution of libration frequencies in Fig. 7 could therefore be fitted with, for example, four independent Gaussian distributions. Clearly the data does not allow this to be done in an unambiguous way, without additional information. Based on a model system of methoxy on η-Al2O3, see Fig. 8, which was used previously to analyse INS spectra in a broad frequency range, we have calculated the potential barrier (V in eqn (2)) as a function of the rotation of the methyl group. If the methyl group is kept rigid the barrier is 484–565 cm−1 (60–70 meV) (dark blue curve (a) in Fig. 8 lower panel), if the methyl group is allowed to relax the barrier is reduced to ∼320 cm−1 (∼40 meV) (purple curve (b) in Fig. 8 lower panel). Using the relaxed potential, the librational and tunnelling energies are calculated as a function of the barrier height, as shown in Fig. 1. The tunnel splitting is 0.025 cm−1, that is inside the elastic line and therefore not resolved in the quasielastic data, and the librational frequency is 110 cm−1, just beyond the maximum ∼75 cm−1 of the broad distribution of libration frequencies shown in Fig. 7.
 | ||
| Fig. 8 Upper: Model of methoxy on η-Al2O3 used to generate the rotational potential. (Magenta = Al, red = O, grey = C, white = H). Lower: Rotational potential when: (a) the methyl group is held rigid and (b) when it is allowed to relax. Computational details are given in the ESI.‡ | ||
In conclusion, we have shown unequivocally that the methyl group of the adsorbed methoxy intermediate on industrial grade alumina catalysts undergoes rotational tunnelling in the wavenumber energy range. The observed signal corresponds to about 15% of the total amount of methoxy being adsorbed on sites with low barriers for the methyl group rotation. 85% of adsorbed methoxy is on sites with higher rotational potentials for the methyl group, giving rise to the elastic signal in the tunnelling spectra and the distribution of libration frequencies up to ∼200 cm−1. Calculation of the rotational potential for an established model of an adsorption site of methoxy on alumina confirms the existence of sites with high rotational potentials. A comprehensive computational study is now required to identify and characterise energetically the full set of adsorption sites on alumina. Calculations establish the link between structure and excitations and demonstrate how the tunnelling data presented here can be used to probe the subtle intermolecular interactions between adsorbed species and catalyst. Using other backscattering spectrometers, with energy resolution of the order of 0.01 cm−1, the observable tunnelling spectrum could be sampled to lower energies.
This work was supported by the UK Catalysis Hub (EPSRC grant no. EP/K014714/1). We thank the STFC Rutherford Appleton Laboratory for access to neutron beam facilities. Experimental assistance and helpful discussions with Dr Franz Demmel and Dr Eugene A. Goremychkin (ISIS Facility) are gratefully acknowledged.
Notes and references
- M. Morbidelli, A. Gavriilidis and A. Varma, Catalyst Design, Cambridge University Press, Cambridge, 2001, p. 1 Search PubMed.
- H. J. Arpe, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 3rd edn, 2010 Search PubMed.
- R. J. Farrauto and C. H. Bartholomew, Fundamentals of Industrial Catalytic Processes, Blackie, London, 1997, p. 60 Search PubMed.
- A. R. McInroy, D. T. Lundie, J. M. Winfield, C. C. Dudman, P. Jones, S. F. Parker and D. Lennon, Catal. Today, 2006, 114, 403 CrossRef CAS.
- A. R. McInroy, D. T. Lundie, J. M. Winfield, C. Dudman, P. Jones, S. F. Parker, J. W. Taylor and D. Lennon, Phys. Chem. Chem. Phys., 2005, 7, 3093 RSC.
- D. Lennon and S. F. Parker, Acc. Chem. Res., 2014, 47, 1220–1227 CrossRef CAS PubMed.
- M. Prager and A. Heidemann, Chem. Rev., 1997, 97, 2934 CrossRef.
- J. Z. Larese, T. Arnold, A. Barbour and L. R. Frazier, Langmuir, 2009, 25, 4078–4083 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to the memory of Professor Norman Sheppard FRS 1922–2015. |
| ‡ Electronic supplementary information (ESI) available: Experimental details (sample preparation and instrumentation), computational methods and assignment of the spectrum of adsorbed methoxy. See DOI: 10.1039/c5cc08223d |
| This journal is © The Royal Society of Chemistry 2016 |