Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
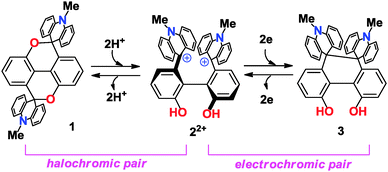
Two-way chromic interconversion of the 2,2′-biphenol-6,6′-diyl dication with 5H,10H-dioxapyrene or 9H,10H-4,5-dihydroxyphenanthrene†
Yuto
Sakano
,
Ryo
Katoono
,
Kenshu
Fujiwara
and
Takanori
Suzuki
*
Department of Chemistry, Faculty of Science, Hokkaido University, Sapporo, Hokkaido 060-0810, Japan. E-mail: tak@sci.hokudai.ac.jp; Fax: +81-11-706-2714; Tel: +81-11-706-2714
First published on 10th August 2015
Abstract
Two-proton or two-electron transfer of the title biphenolic dication proceeds nearly simultaneously to induce 2,6′/2′,6- or 6,6′-bond formation to give dioxapyrene or dihydrophenanthrene derivatives, respectively, with vivid changes in color (halochromism and electrochromism).
5H,10H-Dioxapyrene (diopy; 5H,10H-[1]benzopyrano[5,4,3-cde][1]benzopyran) is a less-studied heterocyclic skeleton1 in contrast to its 5,10-dione analogue. We envisaged that the flattened framework of diopy provides a unique opportunity for the development of new bistable molecular response systems, in which an external stimulus induces the cleavage of two C(sp3)–O bonds to transform into the corresponding biphenyl derivatives with a twisted conformation (Scheme 1). Upon acid treatment of diopy (A) with cation-stabilizing substituents at 5,10-positions, the biphenolic dication (C2+) would be generated via the monocationic intermediate (B+). When B+ suffers from severe steric repulsion at the bay-region, this amphiprotic species easily undergoes acid–base disproportionation to A and C2+, so that double protonation/deprotonation between A and C2+ would occur nearly simultaneously. Such a simplified pseudo-two-state switching is favored for the construction of promising molecular response systems with a sharp ON/OFF threshold.2 When the cationic part in C2+ is endowed with a strong absorption in the visible region, interconversion between A and C2+ is accompanied by halochromism,3 since diopy A shows absorptions only in the UV region.
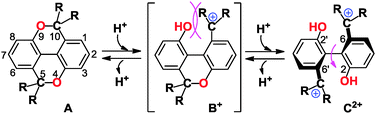 | ||
| Scheme 1 Interconversion of diopy A and biphenolic dication C2+ upon double protonation/deprotonation via intermediate B+. | ||
To generate and isolate the dicationic state as a stable entity despite the presence of hydroxy groups within the molecule, the cationic subunit should have a large pKR+ value, which prompted us to select the 10-methylacridinium chromophore4,5 (Scheme 2). Due to the bulkiness of the chromophore, the biphenol skeleton in 22+ would have a large torsion angle, whereas the diopy skeleton in 1 would be nearly planar, since the spiro(10-methylacridan) units do not induce any steric hindrance. Such a drastic structural change would realize the two-state halochromic interconversion between 1 and 22+. Another interesting point is that, upon reduction, dication 22+ would be transformed into a dihydrophenanthrene (DHP) derivative 3 accompanied by C(sp3)–C(sp3) bonding through “dynamic redox (dyrex)” behavior,6 and the interconversion between 22+ and 3 would also exhibit characteristic color and structural changes. Thus, 1, 22+ and 3 can serve as a novel motif for multi-input molecular response systems.7
Here we report the preparation and X-ray structures of 1 and 22+ along with their chromic behavior during the interconversion between 1 and 22+ (halochromic3 pair) as well as 22+ and 3 (electrochromic8 pair).
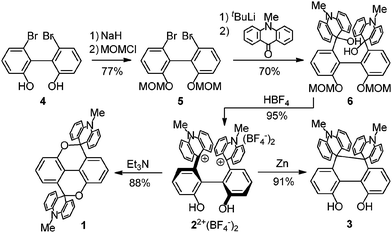
6,6′-Dibromo-2,2′-biphenol 49 was first reacted with methoxymethyl (MOM) chloride/NaH in DMF to give MOM-protected biphenol 510 in 77% yield. The dilithio derivative derived from 5 and 4 equiv. of tBuLi in THF was then reacted with 10-methyl-9(10H)-acridone to give bis(hydroxy)base 610 in 70% yield. Upon treatment of 6 with HBF4 in MeOH–CHCl3 at reflux afforded the desired 22+(BF4−)210 as yellow-orange crystals in 95% yield. The reaction of 22+(BF4−)2 with Et3N in MeCN gave colorless crystals of diopy 110 in 88% yield (Scheme 3).
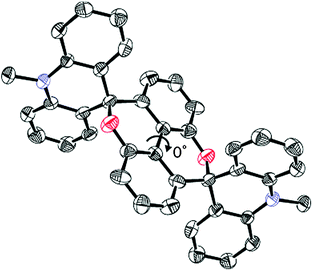
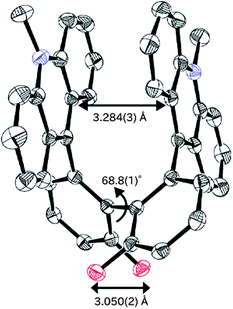
Based on the results of an X-ray analysis11 at 150 K, the diopy core in 1 is nearly planar (largest deviation of an atom from the mean plane: 0.23 Å), although the pyran rings adopt a very shallow twist-chair form (Fig. 1 and Fig. S1, ESI†). The two benzene rings are coplanar (dihedral angle: 0°). To this core are attached the spiro(10-methylacridan) units, which are slightly deformed into a butterfly-shape [dihedral angle between two benzene nuclei of acridan: 21.3(2)°], as found in other structurally-related molecules.12 In contrast, the two molecular halves in dication 22+ are largely twisted in the crystal of (BF4−)2 salt11 (Fig. 2 and Fig. S2, ESI†). The dihedral angle of the biphenyl unit is 68.8(1)° (syn-form), and there are no signs to indicate coordination of the hydroxy groups to the acridinium chromophores. If we consider that the two oxygen atoms at the 2,2′-positions are separated by a distance of 3.050(2) Å, intermolecular H-bonding is not effective in 22+ (typical distance for the H-bonded O⋯O: 2.75 ± 0.2 Å). The π–π interaction between two acridinium units must be the major directing force to give the observed syn-form (Fig. S2 and S3, ESI†),13 and thus the chromophores are stacked nearly in parallel [dihedral angle: 3.92(3)°] with the shortest C⋯C contact of 3.284(3) Å (sum of van der Waals radii: 3.40 Å).
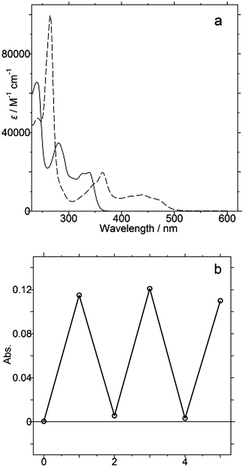
Diopy 1 is colorless, with absorptions only in the UV region [λmax/nm: 339 (4.30) in CH2Cl2], whereas 22+ exhibits a yellow-orange color [358(3.92) in MeCN] due to the characteristic absorptions of acridinium (Fig. 3a). Although 10-methylacridinium itself is highly fluorescent, 22+ is non-fluorescent due to the charge-shift-type quenching of the excited state by the electron-donating biphenol unit. Upon the aliquot addition of TfOH to a DMSO-d6 solution of 1, a clean conversion to 22+ was observed (Fig. S4, ESI†). The resulting spectra showed the presence of only two species (1 and 22+), which demonstrated that the steady-state concentration of the intermediary monocationic derivatives is negligible. The halochromic response was examined by the repeated addition of TfOH (100 microL) to a DMSO solution of 1 (1.2 × 10−5 M), followed by the addition of Et3N (200 microL) to the solution of as-generated 22+. By monitoring the color change using UV-vis spectroscopy, we could confirm the reversibility of the present halochromism (Fig. 3b and Fig. S5, ESI†).
According to the results of a voltammetric analysis,143 undergoes irreversible two-electron oxidation at an anodic peak potential (Epa) of +0.32 V in CH2Cl2/MeCN (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
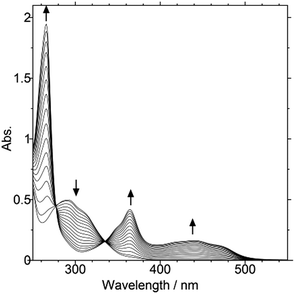
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) vs. SCE (Fig. S6a, ESI†). The return peak was observed in the far cathodic region (Epc = −0.23 V), which corresponds to the reduction process of dication 22+ (Fig. S6b, ESI†). In fact, Zn-reduction of 22+(BF4−)2 induced C(sp3)–C(sp3) bonding at the C6 and C6′ positions to give DHP 3. Colorless crystals of 3 [λmax/nm: 285 (4.37) in CH2Cl2] were isolated in 91% yield, and regenerated 22+(BF4−)2 in 87% yield upon treatment with 2 equiv. of ferrocenium tetrafluoroborate in CH2Cl2/MeCN. In this way, reversible redox interconversion between 22+ and 3 accompanied by C–C bond formation/cleavage (“dyrex” behavior) was confirmed. Due to the dynamic geometrical changes,15 two-electron transfer occurs nearly simultaneously, which was confirmed by the negligible steady-state concentration of the intermediary cation radical upon the electrochemical conversion of 3 to 22+ (Fig. 4).
1) vs. SCE (Fig. S6a, ESI†). The return peak was observed in the far cathodic region (Epc = −0.23 V), which corresponds to the reduction process of dication 22+ (Fig. S6b, ESI†). In fact, Zn-reduction of 22+(BF4−)2 induced C(sp3)–C(sp3) bonding at the C6 and C6′ positions to give DHP 3. Colorless crystals of 3 [λmax/nm: 285 (4.37) in CH2Cl2] were isolated in 91% yield, and regenerated 22+(BF4−)2 in 87% yield upon treatment with 2 equiv. of ferrocenium tetrafluoroborate in CH2Cl2/MeCN. In this way, reversible redox interconversion between 22+ and 3 accompanied by C–C bond formation/cleavage (“dyrex” behavior) was confirmed. Due to the dynamic geometrical changes,15 two-electron transfer occurs nearly simultaneously, which was confirmed by the negligible steady-state concentration of the intermediary cation radical upon the electrochemical conversion of 3 to 22+ (Fig. 4).
In this work, we have demonstrated the reversible halochromic and electrochromic interconversion of 2,2′-biphenyl-6,6′-diyl dication with two kinds of neutral molecules (diopy and DHP). This is the first example of concomitant but independent two-proton or two-electron transfer with a negligible concentration of the intermediates. A drastic structural change is the key to this novel feature, which may represent a new molecular design concept for multi-input response systems with advanced features.
Notes and references
- (a) P. S. Dewar, A. R. Forrester and R. H. Thomson, J. Chem. Soc. C, 1971, 3950–3959 RSC; (b) A. M. Costero and M. Pitarch, J. Chem. Res., Miniprint, 1994, 761–769 Search PubMed; (c) M. Mazzega, F. Fabris, S. Cossu, O. De Lucchi, V. Lucchini and G. Valle, Tetrahedron, 1999, 55, 4427–4440 CrossRef CAS.
- Reviews: (a) M. Takeuchi, M. Ikeda, A. Sugasaki and S. Shinkai, Acc. Chem. Res., 2001, 34, 865–873 CrossRef CAS PubMed; (b) L. Kovbasyuk and R. Krämer, Chem. Rev., 2004, 104, 3161–3187 CrossRef CAS PubMed; (c) N. C. Gianneschi, M. S. Masar, III and C. A. Mirkin, Acc. Chem. Res., 2005, 38, 825–837 CrossRef CAS PubMed; (d) Y. Kubo and Y. Ishii, J. Nanosci. Nanotechnol., 2006, 6, 1489–1509 CrossRef CAS PubMed; (e) C. Kremer and A. Luetzen, Chem. – Eur. J., 2013, 19, 6162–6196 CrossRef CAS PubMed; (f) H. Kawai, Bull. Chem. Soc. Jpn., 2015, 88, 399–409 CrossRef CAS.
- P. Bamfield and M. G. Hutchings, Chromic Phenomena: Technological Applications of Colour Chemistry, RSC, Cambridge, 2nd edn, 2010 Search PubMed.
- The pKR+ value of 10-methyl-9-phenylacridinium is 11.0: J. W. Bunting and W. G. Meathrel, Can. J. Chem., 1973, 51, 1965–1972 CrossRef CAS.
- T. Suzuki, T. Takeda, E. Ohta, K. Wada, R. Katoono, H. Kawai and K. Fujiwara, Chem. Rec., 2015, 15, 280–294 CrossRef CAS PubMed.
- Review on “dyrex” systems undergoing drastic structural changes upon electron transfer: T. Suzuki, E. Ohta, H. Kawai, K. Fujiwara and T. Fukushima, Synlett, 2007, 851 CrossRef CAS.
- (a) F. M. Raymo, Adv. Mater., 2002, 14, 401–414 CrossRef CAS; (b) T. Suzuki, S. Tanaka, H. Kawai and K. Fujiwara, Chem. – Asian J., 2007, 2, 171–177 CrossRef CAS PubMed; (c) T. Suzuki, T. Iwai, E. Ohta, H. Kawai and K. Fujiwara, Tetrahedron Lett., 2007, 48, 3599–3603 CrossRef CAS; (d) T. Suzuki, K. Ohta, T. Nehira, H. Higuchi, E. Ohta, H. Kawai and K. Fujiwara, Tetrahedron Lett., 2008, 49, 772–776 CrossRef CAS; (e) V. Luxami and S. Kumar, New J. Chem., 2008, 32, 2074–2079 RSC; (f) T. Suzuki, Y. Ishigaki, T. Iwai, H. Kawai, K. Fujiwara, H. Ikeda, Y. Kano and K. Mizuno, Chem. – Eur. J., 2009, 15, 9434–9441 CrossRef CAS PubMed; (g) T. Suzuki, Y. Umezawa, Y. Sakano, H. Tamaoki, R. Katoono and K. Fujiwara, Chem. Lett., 2015, 44, 905–907 CrossRef CAS.
- (a) P. M. S. Monk, R. J. Mortimer and D. R. Rosseinsky, Electrochromism: Fundamentals and Applications, VCH, Weinheim, 1995 Search PubMed; (b) P. M. S. Monk, R. J. Mortimer and D. R. Rosseinsky, Electrochromism and Electrochromic Devices, Cambridge Univ. Press, Cambridge, 2007 Search PubMed.
- Y. Zou, H. Geng, W. Zhang, S. Yu and X. Zhang, Tetrahedron Lett., 2009, 50, 5777–5779 CrossRef CAS.
- Experimental details and selected spectral data are given in the ESI†.
- CCDC deposition numbers are as follows: 1 [P21/c, Z = 2] 1061368; 22+(BF4−)2 [Pbca, Z = 8] 1061367; MOM-22+(BF4−)2-(CH3CN)0.5 [P21/c, Z = 8] 1061369.
- (a) T. Suzuki, A. Migita, H. Higuchi, H. Kawai, K. Fujiwara and T. Tsuji, Tetrahedron Lett., 2003, 44, 6837–6840 CrossRef CAS; (b) K. Wada, T. Takeda, H. Kawai, R. Katoono, K. Fujiwara and T. Suzuki, Chem. Lett., 2013, 42, 1194–1196 CrossRef CAS.
- A quite similar molecular geometry was observed in the structurally related dication salt of MOM-22+(BF4−)2,11 which was selectively obtained upon treatment of diol 6 with HBF4 in MeOH–CHCl3 at ambient temperature. The crystal of the MOM-22+ salt contains two independent molecules (mol-1, -2), in which the main difference is the geometry around the MOM groups. The torsion angle of the biphenyl unit is 64.7(1)° or 63.2(1)° in mol-1 and -2, respectively. In both molecules, the two acridinium chromophores are stacked nearly in parallel [dihedral angle: 2.04(7)° or 4.99(7)°] with the shortest C⋯C contact of 3.315(6) Å or 3.266(6) Å, respectively.
- Cyclic voltammetry was conducted in CH2Cl2/MeCN (4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing 0.1 M Bu4NBF4 as a supporting electrolyte (E/V vs. SCE, Pt electrode, scan rate 100 mV s−1). Ferrocene undergoes 1e-oxidation at +0.53 V under similar conditions.
1) containing 0.1 M Bu4NBF4 as a supporting electrolyte (E/V vs. SCE, Pt electrode, scan rate 100 mV s−1). Ferrocene undergoes 1e-oxidation at +0.53 V under similar conditions. - (a) M. R. Bryce, A. J. Moore, M. Hasan, G. J. Ashwell, A. T. Fraser, W. Clegg, M. B. Hursthouse and A. I. Karaulov, Angew. Chem., Int. Ed., 1990, 29, 1450–1452 CrossRef; (b) K. Hu and D. H. Evans, J. Phys. Chem., 1996, 100, 3030–3036 CrossRef CAS; (c) M. Guerro, R. Carlier, K. Boubekeur, D. Lorcy and P. Hapiot, J. Am. Chem. Soc., 2003, 125, 3159–3167 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure and characterization data. Supplementary figures of X-ray single-crystal structure analyses, halochromic titration, and cyclic voltammograms. CCDC 1061367, 1061368 and 1061369. For crystallographic data in CIF or other electronic format, see DOI: 10.1039/c5cc06338h |
| This journal is © The Royal Society of Chemistry 2015 |