Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Less sensitive oxygen-rich organic peroxides containing geminal hydroperoxy groups†
Nipuni-Dhanesha H.
Gamage
a,
Benedikt
Stiasny
b,
Jörg
Stierstorfer
b,
Philip D.
Martin
a,
Thomas M.
Klapötke
*b and
Charles H.
Winter
*a
aDepartment of Chemistry, Wayne State University, Detroit, Michigan 48202, USA. E-mail: chw@chem.wayne.edu
bDepartment of Chemistry, Ludwig-Maximilians University, Butenandtstr. 5-13 (D), 81377 München, Germany. E-mail: tmk@cup.uni-muenchen.de
First published on 20th July 2015
Abstract
A series of oxygen-rich organic peroxide compounds each containing two bis(hydroperoxy)methylene groups is described. Energetic testing shows that these compounds are much less sensitive toward impact and friction than existing classes of organic peroxides. The compounds are highly energetic, which may lead to practical peroxide-based explosives.
Organic energetic materials usually contain carbon, hydrogen, nitrogen, and oxygen, and tend to be nitrogen rich to increase the energy content through formation of highly stable dinitrogen upon detonation.1 While the explosive nature of organic peroxides is widely recognized, due to the presence of weak O–O bonds (45–50 kcal mol−1),2–5 detailed energetic materials properties have only been reported for triacetone triperoxide (TATP), diacetone diperoxide (DADP), hexamethylene triperoxide diamine (HMTD), and methyl ethyl ketone peroxide (MEKP).2–4 The high sensitivities of TATP, DADP, HMTD, and MEKP toward impact, friction, and other stimuli have precluded civilian and military energetic materials applications due to safety concerns.2 These high sensitivities, coupled with the widely publicized use of TATP by terrorists,2 have likely discouraged broader exploration of organic peroxides as energetic materials. To allow practical applications, it will be necessary to identify organic peroxides that combine high energy contents with reduced sensitivities toward stimuli. In this direction, a recent report demonstrated that cocrystals of DADP and 1,3,5-triiodo-2,4,6-trinitrobenzene (TITNB) have reduced impact sensitivity compared to both pure DADP and TITNB, because of stabilizing I⋯O close contacts in the cocrystals.6
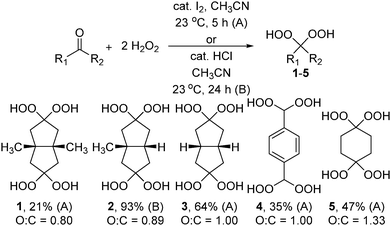
Herein, we describe the synthesis, structure, and energetic materials properties of five new organic compounds (1–5) that each contain two geminal methylene bis(hydroperoxy) moieties. These compounds have oxygen to carbon ratios ranging from 0.80 to 1.33. Four of the new compounds are significantly less sensitive toward impact and friction than TATP, and the detonation velocity and detonation pressure of one compound are higher than those of 2,4,6-trinitrotoluene.
Geminal hydroperoxides 1–5 were synthesized by treating the corresponding ketones or aldehyde with 30–50 wt% H2O2 in the presence of iodine (method A, 1, 3–5) or concentrated HCl (method B, 2) as a catalyst using published general procedures for geminal hydroperoxides (Scheme 1).7,8 Compounds 1–5 were characterized with 1H and 13C NMR spectroscopy, infrared spectroscopy, and elemental analyses. Additionally, X-ray crystal structures of 4 and 5·H2O were determined. A low resolution X-ray crystal structure of 1·Et2O confirmed the molecular structure. Solvates 1·Et2O and 5·H2O were used only for the crystallography experiments; unsolvated 1–5 were used for all other measurements. Attempts to prepare the geminal hydroperoxides derived from cyclohexane-1,3,5-trione, cyclohexane-1,2,3,4,5,6-hexaone, and benzene-1,3,5-tricarbaldehyde led to violent gas evolution, likely due to the instability of the products.
The thermal behaviour was studied with thermogravimetry. Compounds 1–5 show onsets of thermal decomposition between 98 and 117 °C (Table 1). CBS-4M electronic enthalpies were calculated with the Gaussian09 software package to obtain heat of formation values.9 The heat of formation values are all exothermic, ranging from −703.6 to −418.2 kJ mol−1. Compound 4 has the most positive heat of formation.
| 1 | 2 | 3 | 4 | 5 | TNTl | TATPl | |
|---|---|---|---|---|---|---|---|
| a BAM drophammer. b BAM friction. c Electrostatic discharge sensitivity. d Oxygen balance for CO2. e Decomposition temperature from DTA (5 °C min−1). f Room temperature density estimation without solvent. g Calculated molar enthalpy of formation. h Total energy of detonation. i Detonation pressure. j Detonation velocity. k Volume of detonation products. l Values from ref. 3h and 16. m Values from ref. 10. | |||||||
| Formula | C10H18O8 | C9H16O8 | C8H14O8 | C8H10O8 | C6H12O8 | C7H5N3O6 | C9H18O6 |
| FW (g mol−1) | 266.28 | 252.25 | 238.22 | 234.18 | 212.18 | 227.14 | 222.24 |
| ISa (J) | 2 | 1 | 2 | 3 | <1 | 15 | 0.3 |
| FSb (N) | 5 | 5 | 5 | <5 | <5 | 353 | 0.1 |
| ESDc (J) | 0.2 | 0.5 | 0.1 | 0.25 | 0.6 | 0.57 | 0.16 |
| Ω CO2 (%) | −126.20 | −114.18 | −100.76 | −88.83 | −75.41 | −73.96 | −151.19 |
| T Dec (°C) | 117 | 98 | 100 | 105 | 117 | 240 | 150–160 |
| ρ (g cm−3) | 1.35 | 1.375 | 1.40 | 1.60 | 1.40 | 1.704, 1.713m | 1.18 |
ΔfH°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g (kJ mol−1) g (kJ mol−1) |
−703.6 | −660.8 | −617.0 | −418.2 | −627.1 | −70.6 | −583.8 |
| EXPLO5 V6.02 | |||||||
ΔExU°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h (kJ kg−1) h (kJ kg−1) |
−4636 | −4875 | −5083 | −5498 | −5329 | −2732 | −2745 |
| P CJ (kbar) | 117 | 126 | 138 | 195 | 155 | 190 | — |
| V Det (m s−1) | 6150 | 6250 | 6428 | 7130 | 6700 | 6900 | 5300 |
| V o (L kg−1) | 829 | 831 | 808 | 688 | 847 | 825 | 855 |
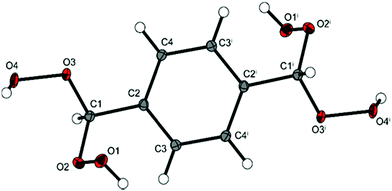
A perspective view of 4 is shown in Fig. 1. This is the only compound among 1–5 for which unsolvated single crystals could be grown. Compound 4 has a crystalline density (1.648 g cm−3 at 100 K) that is slightly lower than those of orthorhombic (1.704 g cm−3 at 123 K) and monoclinic (1.713 g cm−3 at 100 K) 2,4,6-trinitrotoluene (TNT).10 Since the formula weights of 4 and TNT are similar (Table 1), 4 packs nearly as efficiently as TNT in the solid state. TNT does not contain any strong hydrogen bonds, and only van der Waals forces are present.10 By contrast, the lattice of 4 contains intermolecular O–H⋯O hydrogen bonds, where the hydrogen atom on O1 is donated to O4′ and the hydrogen atom on O4 is donated to O1′. The oxygen–oxygen distance in this interaction is 2.701 Å. This configuration results in O1 and O4 being both hydrogen bond donors and acceptors. Additionally, there are close contacts between O2 and O2′ (2.912 Å) and C3–H5′ (2.896 Å). These contacts are within or at the edge of the van der Waals radii for O⋯O (3.04 Å) and C⋯H (2.80 Å).11 Recent studies of energetic materials have shown that such close contacts are attractive because the dispersion forces are larger than the repulsive Coulombic forces.12 Dissociation energies of O⋯O close contacts are similar to those of weak hydrogen bonds (3–13 kJ mol−1).12
Table 1 gives energetic test results for 1–5, with TNT and TATP for comparison. Impact, friction, and electrostatic discharge sensitivities were determined with a BAM drop hammer, a BAM friction tester, and an electrostatic discharge tester using standard test methods.13 Sensitivity classifications are based on the “UN Recommendations on the Transport of Dangerous Goods”.14 Energetic performance was calculated using the EXPLO5 V6.02 software.15 Compounds 1–5 are “very sensitive” toward impact,14 with values ranging from <1 to 3 J. They are “extremely sensitive” toward friction,14 with values of 5 N for 1–3 and <5 N for 4 and 5. The electrostatic discharge sensitivity values for 1–5 are much greater than electrical discharges that can be created by the human body (≤0.02 J1), so they can be safely handled.
The calculated detonation velocities of 1–5 range from 6150 to 7130 m s−1 (Table 1). The increase in detonation velocities in going from 1 to 3 parallels the increasing oxygen to carbon ratios and increasing crystalline density. Compound 4 has the highest detonation velocity (7130 m s−1) and the highest crystalline density (1.648 g cm−3 at 100 K) among 1–5.
This work demonstrates that 1–4 have impact and friction sensitivities that are much lower than those of the known peroxide explosives TATP, DADP, HMTD, and MEKP.2–4 Compound 5 is much more sensitive than 1–4, with values similar to those of TATP. The higher sensitivity of 5 may arise from its high oxygen to carbon ratio of 1.33. The calculated detonation velocities for 1–5 are much higher than that of TATP, most likely due to the higher crystalline densities and greater oxygen to carbon ratios. The calculated detonation velocity and detonation pressure of 4 are higher than those of TNT (Table 1). Thus, 4 is highly energetic. Typical primary energetic materials have impact and friction sensitivities of ≤4 J and ≤10 N, respectively, but must be safe enough to handle.1 Compounds 1–4 have sensitivity values in this range, and are the first organic peroxides that might be safely used as primary explosives. For comparison, the impact and friction sensitivities of 5, TATP, DADP, HMTD, and MEKP are too high for safe use.2–4 Despite their less sensitive nature, the thermal decomposition temperatures of 1–4 will need to be increased to allow use as primary energetic materials. Interestingly, the impact (1–3 J) and friction (∼5 N) sensitivity values for 1–4 are similar, and do not vary with the nature of the organic framework and increasing O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios from 1–4. This lack of a trend is consistent with the O–O linkages being the “trigger bonds” that initiate decomposition upon cleavage. The solid state structure of 4 reveals intermolecular O–H⋯O hydrogen bonds, as well as several O⋯O and C⋯H close contacts. The hydrogen bonds and attractive close contacts may serve to stabilize the labile O–O bonds and buffer them toward shock, thereby reducing the sensitivities of 1–4. Intermolecular I⋯O close contacts also lead to reduced sensitivity in cocrystals of DADP and TITNB.6 For comparison, the solid state structures of highly sensitive DADP and TATP lack O–H⋯O hydrogen bonds and O⋯O close contacts, and contain only very weak O⋯H and C⋯H interactions.3b The stronger hydrogen bonds and close contacts are likely important stabilizing features in 1–4. Finally, this work demonstrates that careful manipulation of organic peroxide structures can lead to compounds with useful energetic materials properties.
C ratios from 1–4. This lack of a trend is consistent with the O–O linkages being the “trigger bonds” that initiate decomposition upon cleavage. The solid state structure of 4 reveals intermolecular O–H⋯O hydrogen bonds, as well as several O⋯O and C⋯H close contacts. The hydrogen bonds and attractive close contacts may serve to stabilize the labile O–O bonds and buffer them toward shock, thereby reducing the sensitivities of 1–4. Intermolecular I⋯O close contacts also lead to reduced sensitivity in cocrystals of DADP and TITNB.6 For comparison, the solid state structures of highly sensitive DADP and TATP lack O–H⋯O hydrogen bonds and O⋯O close contacts, and contain only very weak O⋯H and C⋯H interactions.3b The stronger hydrogen bonds and close contacts are likely important stabilizing features in 1–4. Finally, this work demonstrates that careful manipulation of organic peroxide structures can lead to compounds with useful energetic materials properties.
The authors acknowledge generous support from the Office of Naval Research (Grant No. N00014-12-1-0526 to C.H.W., Grant No. N00014-12-1-0538 to T.M.K.).
Notes and references
- T. M. Klapötke, Chemistry of High-Energy Materials, de Gruyter, Berlin/Boston, 2nd edn, 2012 Search PubMed.
- T. M. Klapötke and T. Wloka, Peroxide Explosives, Patai's Chemistry of Functional Groups, S. Patai Ed, Wiley, 2014, pp. 1–28 Search PubMed.
- (a) J. C. Oxley, J. L. Smith and H. Chen, Propellants, Explos., Pyrotech., 2002, 27, 209–216 CrossRef CAS; (b) F. Dubnikova, R. Kosloff, J. Almog, Y. Zeiri, R. Boese, H. Itzhaky, A. Alt and E. Keinan, J. Am. Chem. Soc., 2005, 127, 1146–1159 CrossRef CAS PubMed; (c) C. Denekamp, L. Gottlieb, T. Tamiri, A. Tsoglin, R. Shilav and M. Kapon, Org. Lett., 2005, 7, 2461–2464 CrossRef CAS PubMed; (d) O. Reany, M. Kapon, M. Botoshansky and E. Keinan, Cryst. Growth Des., 2009, 9, 3661–3670 CrossRef CAS; (e) R. Matyáš and J. Šelešovský, J. Hazard. Mater., 2009, 165, 95–99 CrossRef PubMed; (f) A. E. Contini, A. J. Bellamy and L. N. Ahad, Propellants, Explos., Pyrotech., 2012, 37, 320–328 CrossRef CAS PubMed; (g) G. R. Peterson, W. P. Bassett, B. L. Weeks and L. J. Hope-Weeks, Cryst. Growth Des., 2013, 13, 2307–2311 CrossRef CAS; (h) V. P. Sinditskii, V. I. Kolesov, V. Y. Egorshev, D. I. Patrikeev and O. V. Dorofeeva, Thermochim. Acta, 2014, 585, 10–15 CrossRef CAS PubMed.
- J. C. Oxley, J. L. Smith, H. Chen and E. Cioffi, Thermochim. Acta, 2002, 388, 215–225 CrossRef CAS.
- R. D. Bach, P. Y. Ayala and H. B. Schlegel, J. Am. Chem. Soc., 1996, 118, 12758–12765 CrossRef CAS.
- K. B. Landenberger, O. Bolton and A. Matzger, J. Am. Chem. Soc., 2015, 137, 5074–5079 CrossRef CAS PubMed.
- (a) V. I. Tropina, O. V. Krivykh, N. P. Sadchikova, A. O. Terent'ev and I. B. Krylov, Pharm. Chem. J., 2010, 44, 248–250 CrossRef CAS; (b) K. Žmitek, M. Zupan, S. Stavber and J. Iskra, Org. Lett., 2006, 8, 2491–2494 CrossRef PubMed; (c) P. Ghorai and P. H. Dussault, Org. Lett., 2008, 10, 4577–4579 CrossRef CAS PubMed.
- K. Žmitek, M. Zupan, S. Stavber and J. Iskara, J. Org. Chem., 2007, 72, 6534–6540 CrossRef PubMed.
- M. J. Frisch, et al., Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- R. M. Vreclj, J. N. Sherwood, A. R. Kennedy, H. G. Gallagher and T. Gelbrich, Cryst. Growth Des., 2003, 3, 1027–1032 Search PubMed.
- M. Mantina, A. C. Chamberlain, R. Valero, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. A, 2009, 113, 5806–5812 CrossRef CAS PubMed.
- (a) C. J. Eckhardt and A. Gavezzotti, J. Phys. Chem. B, 2007, 111, 3430–3437 CrossRef CAS PubMed; (b) Y. Ma, A. Zhang, X. Zue, D. Jiang, Y. Zhu and C. Zhang, Cryst. Growth Des., 2014, 14, 6101–6114 CrossRef CAS; (c) Y. Ma, A. Zhang, C. Zhang, D. Jiang, Y. Zhu and C. Zhang, Cryst. Growth Des., 2014, 14, 4703–4713 CrossRef CAS.
- (a) NATO Standardization Agreement (STANAG) on Explosives, Impact Sensitivity Tests, No. 4489, 1st edn, Sept. 17, 1999; (b) WIWEB-Standardarbeitsanweisung 4-5.1.02, Ermittlung der Explosionsgefähr-lichkeit, hier der Schlagempfindlichkeit mit dem Fallhammer, Nov. 8, 2002; (c) http://www.bam.de ; (d) NATO Standardization Agreement (STANAG) on Explosives, Friction Sensitivity Tests, No. 4487, 1st edn, Aug. 22, 2002.
- (a) Test Methods According to the UN Manual of Tests and Criteria, Recommendations on the Transport of Dangerous Goods, United Nations Publications, New York, Geneva, 4th revised edn, 2003 Search PubMed; (b) http://www.reichel-partner.de .
- M. Sućeska, EXPLO5 V6.02 Program, Brodarski Institute, Zagreb, Croatia, 2014 Search PubMed.
- (a) R. Meyer, J. Kohler and A. Homburg, Explosives, Wiley-VCH, Weinheim, 6th edn, 2007 Search PubMed; (b) D. Skinner, D. Olson and A. Block-Bolten, Propellants, Explos., Pyrotech., 1998, 23, 34–42 CrossRef CAS; (c) V. S. Mishra, S. R. Vadali, R. K. Garg, V. S. Joshi, R. D. Wasnik and S. Asthana, Cent. Eur. J. Energ. Mater., 2013, 10, 569–580 CAS; (d) H. Muthurajan, R. Sivabalan, M. B. Talawar, M. Anniyappan and S. Venugopalan, J. Hazard. Mater., 2006, A133, 30–45 CrossRef PubMed; (e) M. S. Keshavarz, Indian J. Eng. Mater. Sci., 2007, 14, 77–80 CAS; (f) R. Matyas and J. Pachman, Primary Explosives, Springer-Verlag, Berlin, 2013, vol. 10 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, structural figures, and crystallographic data. CCDC 1406377 and 1406378. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5cc05015d |
| This journal is © The Royal Society of Chemistry 2015 |