Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ruthenium- and palladium-catalyzed consecutive coupling and cyclization of aromatic sulfoximines with phenylboronic acids: an efficient route to dibenzothiazines†
Ravi Kiran
Chinnagolla
,
Arjun
Vijeta
and
Masilamani
Jeganmohan
*
Department of Chemistry, Indian Institute of Science Education and Research, Pune 411021, India. E-mail: mjeganmohan@iiserpune.ac.in
First published on 6th July 2015
Abstract
A ruthenium-catalyzed ortho arylation of aromatic sulfoximines with aromatic boronic acids followed by intramolecular cyclization in the presence of a palladium catalyst, providing dibenzothiazine derivatives in two consecutive steps, is described.
Sulfoximine is a pivotal structural motif which is present in various biologically active molecules, pharmaceuticals and agrochemicals (eqn (1)).1 The sulfoximine derivatives are also successfully used as chiral auxiliaries and ligands in asymmetric synthesis of various chiral organic molecules.2 Several methods are available in the literature to synthesize linear sulfoximine derivatives.3 But, the synthesis of cyclic sulfoximines is limited in the literature.4 Recently, the synthesis of cyclic sulfoximines has gained much attention in organic synthesis due to their usefulness as scaffolds in drug development and as chiral ligands in enantioselective reactions.5 Meanwhile, sulfoximine derivatives also serve as key synthetic intermediates in various organic transformations.5–7
 | (1) |
Harmata's group reported the synthesis of bicyclic sulfoximine derivatives such as 1,2-benzothiazine and 2,1-benzothiazine by palladium-catalyzed cyclization of 2-bromo benzaldehydes or 2-alkenylated aromatic bromides with sulfoximines, AlCl3-mediated cyclization of sulfonimidoyl chlorides with alkynes or alkenes and electrophilic cyclization of 2-bromophenyl substituted sulfoximines with terminal alkynes in the presence of palladium and copper catalysts.6 Very recently, Bolm's group reported the synthesis of bicyclic 1,2-benzothiazine derivatives via rhodium-catalyzed oxidative cyclization7a of phenyl sulfoximines with alkynes via a chelation-assisted C–H bond activation reaction.8–10 Subsequently, sulfoximine directed ortho alkenylation of phenyl sulfoximines with alkenes in the presence of a metal catalyst was also disclosed.7b–e In all these reports, sulfoximine containing bicyclic benzothiazine derivatives were synthesized efficiently.
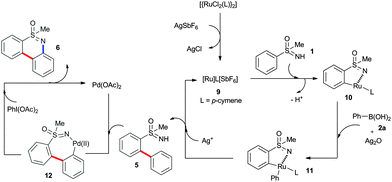
Herein, we report the synthesis of tricyclic dibenzothiazines by a ruthenium-catalyzed ortho arylation of phenyl sulfoximines with aromatic boronic acids followed by intramolecular cyclization in the presence of a palladium catalyst in two consecutive steps. The present reaction was compatible with various sensitive and useful functional group substituted phenyl sulfoximines and aromatic boronic acids. An enantioselective version of ortho arylation of phenyl sulfoximines with phenylboronic acids followed by cyclization, and this transformation leads to chiral dibenzothiazines with an excellent ee ratio of 99%.
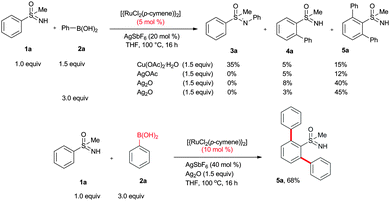
Initially, the ortho arylation of phenyl sulfoximine (1a) with phenylboronic acid (2a) (1.5 equiv.) in the presence of [{RuCl2(p-cymene)}2] (5 mol%), AgSbF6 (20 mol%) and Cu(OAc)2·H2O (1.5 equiv.) in THF at 100 °C for 16 h was investigated (Scheme 1). In the reaction, N-arylated phenyl sulfoximine 3a in 35% yield, mono ortho arylated phenyl sulfoximine 4a in 5% yield and bis ortho arylated phenyl sulfoximine 5a in 15% yield were observed, respectively (Scheme 1). It is known that the free N–H group of 1a is acidic in nature and smoothly undergoes N-arylation with aromatic electrophiles or organometallic reagents providing N-arylated sulfoximines 3 in the presence of metal catalysts.11 To successfully carry out the ortho arylation reaction, the suppression of product 3 is highly important. Next, the reaction was tested with other oxidants and acetate sources such as AgOAc, NaOAc, K2CO3, CsOAc and Ag2O. Among them, silver salts such AgOAc and Ag2O were active for the reaction and no N-arylated product 3a was observed. In AgOAc, product 4a in 5% and 5a in 12% yields were observed, respectively. In Ag2O, product 4a in 8% and 5a in 40% yields were observed, respectively. Other acetate sources were not active for the reaction. Next, the coupling reaction was tested with an excess amount of phenylboronic acid 2a (3.0 equiv.). In the reaction also, a mixture of products 4a and 5a were observed in 3% and 45% yields, respectively. To increase the yield of 5a, the coupling reaction was performed in the presence of 10 mol% of catalyst and 40 mol% of AgSbF6. Interestingly, in the reaction, only bis ortho arylated product 5a was observed in 68% isolated yield and no mono arylated product 4a was observed. For the detailed optimization studies, please see ESI.†
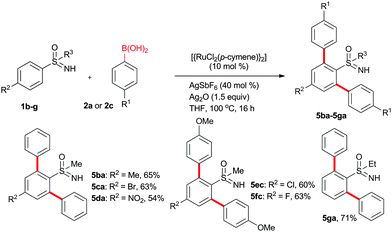
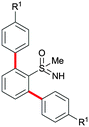
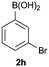
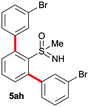
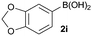
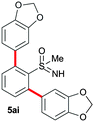
In addition to phenylboronic acid (2a), a wide range of aromatic boronic acids 2b–j also readily participates in the reaction with 1a. Table 1 summarizes the results of these reactions. Treatment of 4-phenyl substituted phenylboronic acid (2b) with 1a provided ortho bis arylated product 5ab in 72% yield (entry 1). Electron rich 4-methoxyphenyl boronic acid (2c) reacts smoothly with 1a, yielding the corresponding product 5ac in 66% yield (entry 2). Aromatic boronic acids having halogen groups I, Br, Cl and F 2d–g also undergo an ortho arylation reaction with 1a efficiently, giving products 5ad–ag in 65%, 62%, 64% and 60% yields, respectively (entries 3–6). However, 3-bromo phenylboronic acid (2h) yielded product 5ah only in 19% yield (entry 7). Benzo[d][1,3]dioxol-5-ylboronic acid (2i) and 2-naphthylboronic acid (2j) also efficiently participated in the reaction, affording coupling products 5ai and 5aj in 61% and 64% yields, respectively (entries 8 and 9).
| Entry | 2b–j | Product 5 | Yieldb (%) |
|---|---|---|---|
| a All reactions were carried out using 1a (100 mg), aromatic boronic acids (2b–j) (3.0 equiv.), [{RuCl2(p-cymene)}2] (10 mol%), AgSbF6 (40 mol%), Ag2O (1.5 equiv.) in THF (3.0 mL) at 100 °C for 16 h. b Isolated yield. | |||
|
|
||
| 1 | 2b: R1 = Ph | 5ab: R1 = Ph | 72 |
| 2 | 2c: R1 = OMe | 5ac: R1 = OMe | 66 |
| 3 | 2d: R1 = I | 5ad: R1 = I | 65 |
| 4 | 2e: R1 = Br | 5ae: R1 = Br | 62 |
| 5 | 2f: R1 = Cl | 5af: R1 = Cl | 64 |
| 6 | 2g: R1 = F | 5ag: R1 = F | 60 |
| 7 |
|
|
19 |
| 8 |
|
|
61 |
| 9 |
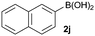
|
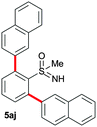
|
64 |
The arylation reaction was examined with substituted aromatic sulfoximines 1b–g (Scheme 2). Electron-rich, halogen and electron-deficient group substituted sulfoximines were compatible for the reaction. Methyl, Br and NO2 substituted sulfoximines 1b–d reacted with 2a, providing products 5ba–da in 65%, 63% and 54% yields, respectively. Similarly, Cl and F substituted aromatic sulfoximines 1e–f reacted with 2c, providing products 5ec–fc in 60% and 63% yields, respectively. Likewise, (ethylsulfonimidoyl)benzene (1g) afforded 5ga in 71% yield.
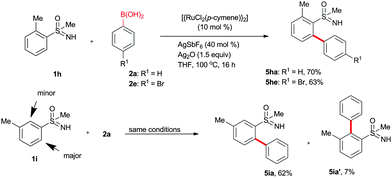
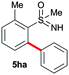
Apart from bis arylation, mono arylation of phenyl sulfoximines was also disclosed (Scheme 3). Treatment of 2-methyl phenylsulfoximine (1h) with 2a or 2f gave mono arylated sulfoximine derivatives 5ha and 5he in 70% and 63% yields, respectively. However, 3-methyl phenylsulfoximine (1i) afforded regioisomeric mono arylated products 5ia and 5ia′ in 62% and 7% yields, respectively.
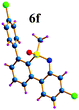
Next, we tried to couple the N–H bond of sulfoximine with one of the C–H bond of phenyl groups of compound 5via chelation-assisted remote C–H activation in order to prepare tricyclic dibenzothiazine derivatives. A Pd(OAc)2 catalyst along with an oxidant is the suitable condition for this type of cyclization.7c,12 The intramolecular cyclization of compound 5aa proceeded smoothly in the presence of Pd(OAc)2 (10 mol%) and PhI(OAc)2 (2.0 equiv.) in toluene at 120 °C for 10 h giving a tricyclic dibenzothiazine derivative 6a in 76% yield (Table 2, entry 1). The cyclization reaction also proceeded in the presence of PhI(OAc)2 without a palladium catalyst. However, product 6a was observed in a less amount of 25% yield. Under similar reaction conditions, products 5ab, 5ac, 5ad, 5ae, 5af and 5ag also efficiently participated in the reaction, providing cyclization products 6b–g in good to excellent yields (entries 2–7). Similarly, products 5ba, 5ca, 5da, 5ga and 5ha afforded dibenzothiazines 6h–l in 80%, 84%, 79%, 83% and 41% yields, respectively (entries 8–12). The structure of compound 6f was further confirmed by single crystal X-ray analysis (see ESI†).
| Entry | 5 | Product 6 | Yieldb (%) |
|---|---|---|---|
| a All reactions were carried out using 5 (100 mg), Pd(OAc)2 (10 mol%) and PhI(OAc)2 (2.0 equiv.) in toluene at 120 °C for 10 h. b Isolated yield. | |||
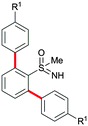
|
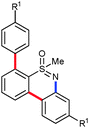
|
|
|
| 1 | 5aa: R1 = H | 6a: R1 = H | 76 |
| 2 | 5ab: R1 = Ph | 6b: R1 = Ph | 85 |
| 3 | 5ac: R1 = OMe | 6c: R1 = OMe | 65 |
| 4 | 5ad: R1 = I | 6d: R1 = I | 77 |
| 5 | 5ae: R1 = Br | 6e: R1 = Br | 85 |
| 6 | 5af: R1 = Cl | 6f: R1 = Cl | 79 |
| 7 | 5ag: R1 = F | 6g: R1 = F | 81 |
|
|
||
| 8 | 5ba: R2 = Me | 6h: R2 = Me | 80 |
| 9 | 5ca: R2 = Br | 6i: R2 = Br | 84 |
| 10 | 5da: R2 = NO2 | 6j: R2 = NO2 | 79 |
| 11 |
|
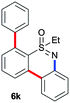
|
83 |
| 12 |
|
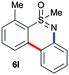
|
41 |
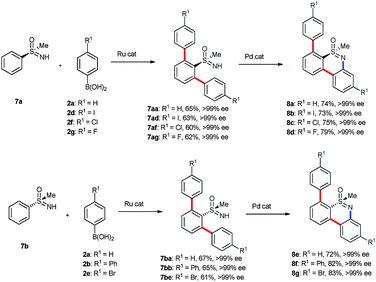
This result prompted us to explore the possibility of synthesis of chiral tricyclic dibenzothiazines by using chiral phenyl sulfoximines 7a–b (Scheme 4). Treatment of chiral (R)-(−)-S-methyl-S-phenylsulfoximine (7a) with substituted phenyl boronic acids 2a, 2d, 2f and 2g in the presence of [{RuCl2(p-cymene)}2], AgSbF6 and Ag2O in THF at 100 °C for 16 h gave chiral ortho arylated phenyl sulfoximines 7aa–ag in 65%, 63%, 60% and 62% yields, respectively (Scheme 4). Interestingly, the enantiomeric excess (ee) of products 7aa–ag did not drop and in all cases >99% ee ratios were observed. Later, compounds 7aa–ag were cyclized into chiral dibenzothiazines 8a–d in excellent 74%, 73%, 75% and 79% yields, respectively, in the presence of a palladium catalyst. In all these reactions, >99% ee ratios were observed. Furthermore, (S)-(−)-S-methyl-S-phenylsulfoximine (7b) underwent ortho arylation with aromatic boronic acids 2a, 2b and 2e in the presence of a ruthenium catalyst, providing chiral ortho arylated phenyl sulfoximines 7ba–be in 67%, 65% and 61% yields, respectively, with >99% ee ratios. Furthermore, 7ba–be were converted into chiral dibenzothiazines 8e–g in the presence of Pd(OAc)2 in 72%, 82% and 83% yields, respectively.
A possible reaction mechanism is proposed to account for the present reaction in Scheme 5. Two different catalytic reactions were involved in the reaction. In the first catalytic cycle, AgSbF6 likely removes all Cl− ligands from the ruthenium complex providing a cationic ruthenium complex 9.13 Coordination of the nitrogen atom of sulfoximine 1 into catalyst 9 followed by ortho-metalation provides a ruthenacycle intermediate 10. Transmetallation of phenyl boronic acid 2 into intermediate 10 in the presence of Ag2O affords intermediate 11. Subsequent reductive elimination of intermediate 11 in the presence of Ag+ source provides product 5 and regenerates the active ruthenium species 9 for the next catalytic cycle. Another ortho arylation also takes place in a similar fashion. In the second catalytic cycle, compound 5 reacts with Pd(OAc)2 giving palladacycle 12. Reductive elimination of intermediate 12 in the presence of PhI(OAc)2 provides cyclic product 6 and regenerates the active Pd(OAc)2 catalyst for the next catalytic cycle. The exact role of Ag2O is not clear to us, it could be possible that the AgO− anion acts as a base to accelerate the transmetallation of boronic acid 2 into intermediate 12 and the Ag+ ion acts as an oxidant to oxidize Ru(0) to Ru(II).
In conclusion, we have described a two-step synthesis of dibenzothiazines via a ruthenium-catalyzed ortho arylation of phenyl sulfoximines with phenyl boronic acids followed by intramolecular cyclization in the presence of Pd(OAc)2. Chiral dibenzothiazines were prepared efficiently by using chiral phenyl sulfoximine in a similar protocol.
We thank CSIR (02(0179)/14/EMR-II), India, for support of this research. R. K. thanks CSIR for a fellowship.
Notes and references
- Bioactive sulfoximines: (a) H. Yu, Z. Qin, H. Dai, X. Zhang, X. Qin, T. Wang and J. Fang, J. Agric. Food Chem., 2008, 56, 1135 Search PubMed; (b) D. Lu, Y. Y. Sham and R. Vince, Bioorg. Med. Chem., 2010, 18, 2037 CrossRef CAS PubMed; (c) X. Y. Chen, S. J. Park, H. Buschmann, M. De Rosa and C. Bolm, Bioorg. Med. Chem. Lett., 2012, 22, 4307 CrossRef CAS PubMed; (d) For a review, see: U. Lücking, Angew. Chem., Int. Ed., 2013, 52, 9399 CrossRef PubMed.
- Selected applications of chiral sulfoximines: (a) M. Haiza, J. Lee and J. K. Snyder, J. Org. Chem., 1990, 55, 5008 CrossRef CAS; (b) X. Shen, W. Zhang, C. Ni, Y. Gu and J. Hu, J. Am. Chem. Soc., 2012, 134, 16999 CrossRef CAS PubMed; (c) J. Brandt and H.-J. Gais, Tetrahedron: Asymmetry, 1997, 6, 909 CrossRef.
- (a) M. A. M. Capozzi, C. Cardellicchio, F. Naso and P. Tortorella, J. Org. Chem., 2001, 66, 5933 CrossRef CAS; (b) C. P. R. Hackenberger, G. Raabe and C. Bolm, Chem. – Eur. J., 2004, 10, 2942 CrossRef CAS PubMed; (c) S. Pyne, Sulfur Rep., 1992, 12, 57 CrossRef CAS PubMed; (d) C. R. Johnson, Acc. Chem. Res., 1973, 6, 341 CrossRef CAS; (e) M. Gerisch, J. R. Krumper, R. G. Bergman and T. D. Tilley, J. Am. Chem. Soc., 2001, 123, 5818 CrossRef CAS.
- (a) M. Harmata, Z. Cai and Y. Chen, J. Org. Chem., 2009, 74, 5559 CrossRef CAS PubMed; (b) M. Harmata, R. J. Claassen and C. L. Barnes, J. Org. Chem., 1991, 56, 5059 CrossRef CAS; (c) L. Wang, D. L. Priebbenow, X. Y. Chen, F.-F. Pan and C. Bolm, Eur. J. Org. Chem., 2015, 3338 CrossRef CAS PubMed; (d) M. Harmata and N. Pavri, Angew. Chem., Int. Ed., 1999, 38, 2419 CrossRef CAS.
- (a) H.-J. Gais, Heteroat. Chem., 2007, 18, 472 CrossRef CAS PubMed; (b) S. G. Pyne, Z. Dong, B. W. Skelton and A. H. White, J. Org. Chem., 1997, 62, 2337 CrossRef CAS PubMed; (c) B. M. Trost and R. T. Matuoka, Synlett, 1992, 27 CrossRef CAS; (d) M. Harmata and X. Hong, J. Am. Chem. Soc., 2003, 125, 5754 CrossRef CAS PubMed.
- (a) M. Harmata and N. Pavri, Angew. Chem., Int. Ed., 1999, 38, 2419 CrossRef CAS; (b) M. Harmata and X. Hong, Tetrahedron Lett., 2005, 46, 3847 CrossRef CAS PubMed; (c) M. Harmata, K. Rayanil, M. G. Gomes, P. Zheng, N. L. Calkins, S.-Y. Kim, Y. Fan, V. Bumbu, D. R. Lee, S. Wacharasindhu and X. Hong, Org. Lett., 2005, 7, 143 CrossRef CAS PubMed; (d) M. Harmata and E. O. Schlemper, Tetrahedron Lett., 1987, 28, 5997 CrossRef CAS; (e) M. Harmata, R. J. Claassen and C. L. Barnes, J. Org. Chem., 1991, 56, 5059 CrossRef CAS.
- (a) W. Dong, L. Wang, K. Parthasarathy, F. Pan and C. Bolm, Angew. Chem., Int. Ed., 2013, 52, 11573 CrossRef CAS PubMed; (b) K. Parthasarathy and C. Bolm, Chem. – Eur. J., 2014, 20, 4896 CrossRef CAS PubMed; (c) W. Dong, K. Parthasarathy, Y. Cheng, F. Pan and C. Bolm, Chem. – Eur. J., 2014, 20, 15732 CrossRef CAS PubMed; (d) R. M. Yadav, R. K. Rit, M. Shankar and A. K. Sahoo, J. Org. Chem., 2014, 79, 6123 CrossRef PubMed; (e) M. Bhanuchandra, M. R. Yadav, R. K. Rit, M. R. Kuram and A. K. Sahoo, Chem. Commun., 2013, 49, 5225 RSC.
- (a) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS PubMed; (b) G. P. McGlacken and L. M. Bateman, Chem. Soc. Rev., 2009, 38, 2447 RSC; (c) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; (d) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS PubMed; (e) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed; (f) D.-G. Yu, B.-J. Li and Z.-J. Shi, Tetrahedron, 2012, 68, 5130 CrossRef CAS PubMed; (g) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed.
- (a) S. Ueno, N. Chatani and F. Kakiuchi, J. Org. Chem., 2007, 72, 3600 CrossRef CAS PubMed; (b) F. Kakiuchi, S. Kan, K. Igi, N. Chatani and S. Murai, J. Am. Chem. Soc., 2003, 125, 1698 CrossRef CAS PubMed; (c) L. Ackermann, R. Vicente and A. Althammer, Org. Lett., 2008, 10, 2299 CrossRef CAS PubMed; (d) P. B. Arockiam, C. Fischmeister, C. Bruneau and P. H. Dixneuf, Angew. Chem., Int. Ed., 2010, 49, 6629 CrossRef CAS PubMed.
- (a) C. G. Ravi Kiran and M. Jeganmohan, Chem. Commun., 2014, 50, 2442 RSC , references therein; (b) C. G. Ravi Kiran and M. Jeganmohan, Org. Lett., 2012, 14, 5246 CrossRef PubMed; (c) J. Hubrich, T. Himmler, L. Rodefeld and L. Ackermann, Adv. Synth. Catal., 2015, 357, 474 CrossRef CAS PubMed; (d) V. K. Tiwari, N. Kamal and M. Kapur, Org. Lett., 2015, 17, 1766 CrossRef CAS PubMed Other metals: ; (e) S. Yang, B. Li, X. Wan and Z. Shi, J. Am. Chem. Soc., 2007, 129, 6066 CrossRef CAS PubMed; (f) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Commun., 2010, 46, 677 RSC; (g) J. Karthikeyan, R. Haridharan and C.-H. Cheng, Angew. Chem., Int. Ed., 2012, 51, 12343 CrossRef CAS PubMed; (h) N. Senthikumar, K. Parthasarathy, P. Gandeepan and C.-H. Cheng, Chem. – Asian J., 2013, 8, 2175 CrossRef PubMed; (i) S. Oi, S. Fukita and Y. Inoue, Chem. Commun., 1998, 2439 RSC; (j) K. Ueura, T. Satoh and M. Miura, Org. Lett., 2005, 7, 2229 CrossRef CAS PubMed.
- (a) C. Moessner and C. Bolm, Org. Lett., 2005, 7, 2667 CrossRef CAS PubMed; (b) J. Kim, J. Ok, S. Kim, W. Choi and P. H. Lee, Org. Lett., 2014, 16, 4602 CrossRef CAS PubMed; (c) N. Yongpruksa, N. L. Calkins and M. Harmata, Chem. Commun., 2011, 47, 7665 RSC; (d) M. Miyasaka, K. Hirano, T. Satoh, R. Kowalczyk, C. Bolm and M. Miura, Org. Lett., 2011, 13, 359 CrossRef CAS PubMed.
- (a) W. C. P. Tsang, R. H. Munday, G. Brasche, N. Zheng and S. L. Buchwald, J. Org. Chem., 2008, 73, 7603 CrossRef CAS PubMed; (b) P. Gandeepan, C.-H. Hung and C.-H. Cheng, Chem. Commun., 2012, 48, 9379 RSC; (c) B. Li, S. Tian, Z. Fang and Z. Shi, Angew. Chem., Int. Ed., 2008, 47, 1115 CrossRef CAS PubMed; (d) M.-L. Louillat and F. W. Patureau, Chem. Soc. Rev., 2014, 43, 901 RSC.
- (a) E. Ferrer-Flegeau, C. Bruneau, P. H. Dixneuf and A. Jutand, J. Am. Chem. Soc., 2011, 133, 10161 CrossRef PubMed; (b) S. Warratz, C. Kornhaab, A. Cajaraville, B. Niepotter, D. Stalke and L. Accermann, Angew. Chem., Int. Ed., 2015, 54, 5513 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and spectroscopic data. See DOI: 10.1039/c5cc04589d |
| This journal is © The Royal Society of Chemistry 2015 |