Stacking interactions of hydrogen-bridged rings – stronger than the stacking of benzene molecules†
Abstract
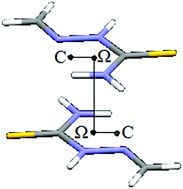
Analysis of crystal structures from the Cambridge Structural Database showed that 27% of all planar five-membered hydrogen-bridged rings, possessing only single bonds within the ring, form intermolecular stacking interactions. Interaction energy calculations show that interactions can be as strong as −4.9 kcal mol−1, but dependent on ring structure.

 Please wait while we load your content...
Please wait while we load your content...